Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Good judgement comes from experience. Experience comes from bad judgement. ANONYMOUS
Half of what I'm teaching you is wrong. The problem is that I don't know what half it is. ANONYMOUS
Some of the most challenging forms of congenital heart disease (CHD) fall into the category of those with functionally one pumping chamber, colloquially known as “single ventricle” defects, although we prefer the term functionally univentricular heart (fUVH). A fUVH is defined as a heart in which division into separate pulmonary and systemic ventricular pumping chambers is not possible. This includes malformations such as hypoplastic left heart syndrome, tricuspid atresia, double-inlet left ventricle, severe forms of pulmonary atresia and intact septum, unbalanced atrioventricular (AV) septal defects in which one of the ventricles is prohibitively hypoplastic or absent, as well as other malformations where two pumping chambers are present but division is not practical due to AV valve straddling or arrangement of the great vessels and remote location of a ventricular septal defect.
In the past 3 decades survival beyond the neonatal period has become a reality due to a broad range of innovations, including the use of prostaglandin to maintain ductal patency; improved diagnostic capabilities, particularly with echocardiography; innovations in surgery such as the Norwood procedure; improvements in postoperative management; improved postdischarge management; and the development of a standardized three-stage approach to completion of the Fontan procedure. Favorable short- and long-term outcomes are now the expectation and survival into adulthood is anticipated. With improved survival, the focus is now being directed at optimizing neurodevelopmental outcome, functional outcome, and quality of life among survivors of with fUVH anatomy.
The ultimate anatomic goal for the patient with a fUVH is a Fontan circulation in which the venous return through the superior and inferior caval veins is connected directly to the pulmonary arteries. With this arrangement, blood circulates as closely as possible to the normal pattern. A successful Fontan is more likely in individuals with normal pulmonary arteriolar resistance, no pulmonary artery stenoses, no obstruction to pulmonary venous return, good systemic ventricular function, and adequate function of the AV and semilunar valves and AV sequential rhythm. The goal of surgical palliation of the infant with a fUVH is the creation of a good Fontan candidate. Over time, based on the increasing knowledge of risk factors for the Fontan procedure, a number of surgical tenets have emerged ( Box 71.1 ; see also Chapter 70 ).
Unobstructed systemic blood flow
Approximately limited pulmonary blood flow
Undistorted pulmonary arteries
Unobstructed pulmonary venous return
Unobstructed systemic venous return
This chapter presents the combined experience of multiple centers in the United States (the Cincinnati Children's Hospital Medical Center, Texas Children's Hospital, Boston Children's Hospital, Children's Hospital of Philadelphia, and Children's National Medical Center). They are not meant to be all-inclusive, and strategies that are successful in one institution may not be as successful in others. Multiple reports have shown considerable variability in practice; these are beyond the scope of this chapter. Where controversies exist, we attempt to describe areas of future research that may differentiate best practices.
The general physiologic factors influencing adequate systemic oxygen delivery (DO 2 ) in neonates with a “multidistribution” circulation are discussed in detail in Chapter 70 . It is beyond the scope of this chapter to discuss palliative care and nonintervention for infants born with any of the complex anomalies in the spectrum of a fUVH. Detailed discussions of the ethics and controversies may be found elsewhere. In addition, this chapter focuses on surgical strategies leading toward a planned Fontan operation. Cardiac transplantation is discussed in Chapter 67 .
The principal goal of preoperative management and stabilization in the neonate with a fUVH is to minimize the risk of future surgical management . This general principle contains a number of key components ( Box 71.2 ).
Monitoring and vascular access
Presentation in shock
Effects of positive-pressure ventilation
Nursing considerations and family support
Feeding and nutrition
Timing of surgery
Despite significant variability in practice, monitoring of the neonate with a fUVH has similar fundamental goals. Physiologic monitoring gives the bedside clinician an overview of the dynamic physiologic state of the preoperative multidistribution physiology. Stable vascular access allows for infusions of prostaglandin E 1 (PGE 1 ), vasoactive medications, and parenteral nutrition. In addition, appropriate vascular devices access provide crucial information regarding intravascular pressures, including systolic and diastolic arterial and central venous pressures. Co-oximetry data from indwelling vascular catheters can provide valuable estimates of cardiac output and mixing. Finally, continuous tracings from vascular lines can provide clues to the physiologic consequences of important residual functional or anatomic abnormalities.
The risks and benefits of individual monitoring systems and vascular access devices must be carefully considered. Although basic devices such as electrocardiography (ECG) and pulse oximetry pose little risk to the patient, invasive devices such as intravascular lines carry the risk of vascular injury, thromboembolic complications, bloodstream infection, and future vascular occlusions. Devices must be carefully considered in the context of patient acuity and future vascular needs ( Table 71.1 ).
| Monitoring Device | Location | Indication | Risks |
|---|---|---|---|
| Electrocardiograph | Right shoulder, left shoulder, left leg | All | Skin irritation |
| Pulse oximetry | Right hand, lower extremity | All | None |
| Noninvasive blood pressure | Right upper extremity, lower extremity calf | All | Irritation of the extremity, peripheral nerve damage |
| Invasive arterial catheter | Umbilical artery, femoral artery, radial artery | Need for continuous assessment of blood pressure and frequent arterial blood sampling | Vascular injury, bleeding, infection |
| Invasive central venous catheter | Umbilical vein, femoral vein, internal jugular vein | Central venous pressure monitoring, hyperosmolar infusions, frequent blood sampling | Vascular injury, bleeding, infection |
| Near infrared spectroscopy | Multisite | Adjunctive assessment of adequate oxygen delivery | Skin irritation |
| iDO2 index | N/A | Evolving | None |
| Electroencephalography | Head | Evaluation of epileptiform activity | Skin irritation |
Continuous ECG monitoring should be used in all patients with congenital heart disease admitted to the cardiac intensive care unit (CICU). A standard three-electrode system with leads on the right arm, left arm, and left leg (right and left shoulder and left abdomen in neonates) can provide a single-lead (lead I, II, or III) continuous ECG tracing that accurately detects changes in the heart rate and rhythm along with ischemic changes. Continuous monitoring of the ECG tracing can also be utilized in the early detection of electrolyte disturbances, including potassium and calcium.
An occlusive cuff around the arm and leg of a preoperative neonate is an essential noninvasive monitoring device. In neonates, an appropriately sized cuff on the upper arm and calf may be used to monitor arm and calf pressures; these should be essentially identical. Noninvasive blood pressure measurements can be made by auscultatory methods or by the use of automated cuffs that use oscillometric methods. It should be noted that oscillometric automated cuffs measure systolic and mean arterial blood pressures directly and calculate the diastolic blood pressure. Thus interpretation of the diastolic blood pressure calculated by these automated cuffs should be interpreted with this in mind, particularly in with patients where there is diastolic runoff into the pulmonary artery via a patent arterial duct.
One of the main advantages of an indwelling vascular catheter is the monitoring of pressure in an arterial or venous vascular structure. Pressure monitoring requires the introduction of an end-hole catheter into the vessel to be interrogated. Expert technical skill is necessary to place these catheters in preoperative neonates to avoid injury to vascular structures. Such catheters are commonly inserted into the umbilical vein, femoral vein, jugular vein, umbilical artery, femoral artery, or radial artery. Once placed, the catheter is connected to a pressure transducer via a coupling system that is subsequently connected to the monitor. Coupling systems contain tubing filled with a saline solution and a port for zeroing the catheter to atmospheric pressure and withdrawing blood samples. Modern transducers contain silicon crystals that change resistance in proportion to the changes in pressure from the coupling system's fluid. These systems must be properly calibrated for the accurate assessment of intravascular pressures.
The pressure waveforms themselves are valuable sources of data. In arterial tracings, the slope of the upstroke can be used to generally assess contractility. A steep upstroke indicates adequate cardiac contractility, whereas a blunted upstroke may indicate poor contractility, aortic valve disease, or peripheral vasoconstriction from vasoactive drugs. The position of the dicrotic notch of the ventricular end-systolic pressure is an important indicator of peripheral vascular resistance. A low notch indicates low systemic vascular resistance (SVR) and can be a useful clinical indicator in a neonate with a patent arterial duct. Significant respiratory variation in the arterial waveform may indicate hypovolemia. Finally, one of the most important indications for placement of an invasive arterial catheter is the need for accurate assessment of the pulse width. A wide pulse width in the preoperative neonate is an important indicator of diastolic runoff into the pulmonary circulation and may be an early indicator of inadequate systemic blood flow. The reliability of these invasive data is improved by the use of a central arterial catheter as opposed to the noninvasive assessment of blood pressure.
Another advantage of an indwelling catheter is the ability to draw blood samples for serial laboratory monitoring. This is particularly useful for patients in whom there is concern for an evolving deficiency in DO 2 . For example, the development of a metabolic acidosis or a rise in serum lactate levels may be an early indicator of inadequate DO 2 . In neonates requiring diuretic therapy, an indwelling catheter allows for the daily assessment of serum electrolytes and safer intravenous replacement of any derangements. Indwelling central venous catheters also allow for the administration of parenteral nutrition.
It is important to consider that the majority of preoperative neonates with a fUVH may be managed adequately with a peripheral intravenous line rather than an indwelling central venous catheter. The peripheral catheter can be used to administer infusions of PGE 1 and low-concentration glucose. Peripheral intravenous catheters carry low insertion complication rates and minimize the risk for thromboembolism, vascular occlusion, and infection complications.
Continuous pulse oximetry is utilized in all neonates with a fUVH. The probe is often placed on the distal extremity. In patients with fUVH with anatomic obstruction to systemic blood flow (see Chapter 69 ), a “preductal” and “postductal” saturation measurement is typically monitored by a probe on the right hand and either foot.
A near infrared spectroscopy (NIRS) probe is used in many centers as a surrogate measure of the adequacy of DO 2 . The probe is attached with a noninvasive self-adhesive pad applied to the forehead, abdomen, and/or lumbar area. It emits light in the near infrared spectrum, which is then measured by sensors at specific distances from the light source. Oxyhemoglobin and deoxyhemoglobin have unique peak absorption in the near infrared spectrum. Total hemoglobin concentration is measured by the absorbance of light at 800 nm. Analogous to the calculation of systemic saturation by the pulse oximeter, the NIRS monitor reports the ratio of oxyhemoglobin to total hemoglobin concentration. This value is often used as a surrogate for “mixed venous” oxygen saturation. Commercially available NIRS devices use proprietary algorithms to subtract oxygen saturation from overlying tissue such as bone. It is also important to note that NIRS does not distinguish between arterial and venous blood. Owing to these limitations, the NIRS monitor is used primarily as an adjunct to other indicators of cardiac output.
Increasing numbers of centers now employ solutions to linearly display high-fidelity continuous physiologic data at the patient's bedside. These systems are capable of presenting in a single longitudinal view any continuous physiologic data stream connected to the bedside monitor. Continuous data inputs include heart rate, respiratory rate, arterial and venous pressures, pulse oximetry, temperature, ventilator data, and medication infusion data. These data are typically displayed at 5-second intervals. Modern systems are also capable of overlaying serial pertinent laboratory data (including blood gas and lactate levels) on the physiologic data streams. Longitudinal displays can be used to quickly visualize important clinical trends in pre- versus postductal oxygen saturations, blood pressures, and pulse width.
Continuous data streams have recently been used to develop complex mathematical algorithms to detect the risk for inadequate DO 2 . One such algorithm, the iDO 2 index, approved by the US Food and Drug Administration, uses physiologic data streams to display the probability of the mixed venous saturation below 40% and thus inadequate DO 2 . This algorithm was validated in a large cohort of neonates with a fUVH and a multidistribution circulation. A sustained period with an iDO 2 index below 40% has been associated with an increased incidence of cardiac arrest and a protracted stay in the CICU.
Continuous EEG monitoring may provide information on cortical brain activity. In standard EEG monitoring, leads are placed over regions of the skull, and a cart with a CPU and video camera is placed at the foot of the bed. Changes in the electrical tracings are correlated with abnormal movements or changes in vital signs. Preoperative EEG monitoring is not routinely undertaken in most centers unless there is specific concern for a structural brain abnormality, genetic syndrome, or clinical seizure.
Neonates with a fUVH and hypoplasia/atresia of the systemic AV valve and/or ventricle as well as hypoplasia/atresia of the aorta valve (see Chapter 69 ) may develop and grow adequately in utero; following birth, however, their systemic blood flow is dependent on a patent arterial duct and unobstructed systemic and pulmonary venous return (see Chapter 70 ). In the current era, with the advent of fetal echocardiography and the increasing prevalence of prenatal diagnosis, many neonates with a fUVH are delivered following a prenatal diagnosis with informed families, physiologic stability, and an expectant cardiovascular team. However, there are still neonates who are not detected prenatally and, as the arterial duct constricts, there is systemic hypoperfusion with a relative increase in pulmonary blood flow. Clinically this presents as tachypnea and poor feeding, progressing to listlessness and finally cardiovascular shock. The clinical presentation mimics sepsis and the onus on the clinician is to have a high index of suspicion for CHD. On laboratory testing there is profound metabolic acidosis with an elevated lactate and end-organ dysfunction with elevated hepatic enzymes and creatinine.
Shock occurs when oxygen demand exceeds systemic DO 2 . In this instance, oxygen consumption (VO 2 ) is less than O 2 demand and the neonate relies increasingly on anaerobic metabolism to maintain energy substrate. Accordingly, shock results from either impaired oxygenation, O 2 -carrying capacity, or delivery, all of which is often compounded by an elevated O 2 demand. Oxygenation and O 2 content (CaO 2 ) are compromised due to the mixing of systemic and pulmonary venous return; thus systemic blood flow (Qs) must compensate to maintain adequate tissue oxygenation.
The health of the neonate with a fUVH and shock will be compounded by an elevated O 2 demand. The primary factors responsible for an increase in metabolism include increased ventilatory demand and impaired respiratory mechanics, resulting in a marked increase in respiratory muscle VO 2 and corresponding DO 2 ; elevated myocardial O 2 demand; and elevated total body VO 2 due to catecholamine exposure (endo- and exogenous sources).
Standard “hemodynamic” parameters such as blood pressure, heart rate, and arterial oxygen saturations (SaO 2 ), and the perfusion exam may not provide an accurate indication of the adequacy of DO 2 . The SaO 2 may be elevated, indicating a generous Qp, but that does not necessarily have to occur at the expense of Qs and DO 2 if the total cardiac output (CO) is adequate. Conversely, an “acceptable” SaO 2 (e.g., 80%) does not indicate adequate DO 2 if the total CO is limited. A normal or elevated blood pressure does not indicate adequate DO 2 as SVR may be compensating for a low Qs. This is a particularly important consideration in patients with a fUVH and multidistribution physiology, as an elevated and rising SVR/pulmonary vascular resistance (PVR) ratio creates a positive feedback cycle that culminates in tissue hypoxia and shock. As Qs falls and becomes limited, neurohormonal activation ensues, which—if systolic function is impaired—causes stroke volume and CO to decrease while increasing the SVR/PVR ratio, thus increasing the Qp/Qs. And in this instance the increase in Qp is occurring at the expense of the systemic circulation.
One strategy that may be used to assess Qs and DO 2 is the use of venous and or cerebral NIRS oximetry, which relies on the use of a manipulated and simplified Fick equation:
where ScvO 2 is the central venous O 2 saturation obtained from the jugular vein, superior vena cava, or inferior vena cava–right atrial juncture and SaO 2 − ScvO 2 /SaO 2 is the oxygen extraction ratio (O 2 ER). A cerebral NIRS O 2 saturation may be used a surrogate for regional (cerebral) and global (ScvO 2 ) VO 2 /DO 2 analysis; however, there are important factors to consider, which are discussed elsewhere. DO 2 is coupled to metabolism, which is accomplished by viscera modulating vasomotor tone, blood flow, and regional DO 2 . The O 2 ER increases from normal (25% to 30% or so) when DO 2 becomes limited. A O 2 ER of 50% to 60% is consistent with impending shock and the critical O 2 ER (defined by the onset of anaerobic metabolism and fall in VO 2 without a decrement in O 2 demand) is 60% or so. Clinical findings consistent with impending or existing shock include an elevated O 2 ER that is approaching if not exceeding the “critical” O 2 ER and lactic acidosis. An important limitation of lactate levels is that the O 2 ER must exceed critical levels for lactate production to exceed its clearance.
The principles of initial simultaneous assessment and management are outlined in Table 71.2 . The top priority should be to establish access for the initiation of prostaglandin, correction of acidosis, and initiation of inotropic support. The dose of prostaglandin needed to open the constricted ductus arteriosus is 0.1 to 0.2 µg/kg per minute, which is 10 to 20 times higher than that needed to maintain patency typically 0.01 µg/kg per minute. Apnea can occur with the initiation of high-dose prostaglandin; therefore the patient should be prepared for intubation. The caveat to intubating the neonate in shock is that tachypnea may be a response to a profound metabolic acidosis; therefore minute ventilation should be high until the metabolic component has been corrected. Partial correction of the acidosis with sodium bicarbonate should be considered prior to intubation. Supplemental oxygen should be administered with caution, as once the patency of the ductus arteriosus has been reestablished, oxygen can decrease PVR and increase maldistribution of flow into the pulmonary vascular bed. It is generally recommended not to “intubate for transport,” as preoperative intubation carries risk during the procedure; it also increases the mortality risk for subsequent surgery, the likelihood of earlier extubation, and the hospital length of stay.
| Assessment | Management |
|---|---|
| A irway/ A ccess/ A ntibiotics |
|
| B reathing |
|
| C irculation |
|
Once the neonate has been stabilized, a comprehensive evaluation for end-organ function should occur. This includes assessment for intracranial hemorrhage and ischemic brain injury, necrotizing enterocolitis, and testing of hepatic and renal function. However, if the neonate remains unstable without end-organ recovery, worsening end-organ injury, and ongoing acidosis, bilateral pulmonary artery banding can be performed to increase systemic blood flow and decrease flow to the pulmonary vascular bed, thus allowing for end-organ recovery. This strategy has been shown to improve systolic blood pressure, renal function, and acidosis with recovery of end organ function, allowing for more definitive surgical palliations including the Norwood operation and transplantation.
In addition to ductal restriction, one etiology of shock in a neonate with a fUVH with ductal-dependent systemic blood flow deserves special mention. The neonate with hypoplastic left heart syndrome and a restrictive atrial septum (usually less than 2 to 3 mm) who will appear well in the first few hours of life. But as PVR falls and left atrial hypertension progresses, such a neonate will develop tachypnea, hypoxemia, and eventually hypotension, low cardiac output, and shock. This physiology requires emergent catheter-based or surgical atrial decompression.
In our experience, an unrestrictive atrial septum and low PVR as causes of shock preoperatively are rare. In the few days after birth, symptoms of congestive heart failure (tachypnea, tachycardia, hepatomegaly) may occur; however, circulatory collapse is exceedingly unlikely. In babies with a more indolent picture of congestive heart failure, manipulation of the PVR to mimic the fetal circulation is sometimes undertaken with subatmospheric oxygen by using nitrogen blended with room air to induce hypoxic pulmonary vasoconstriction). More invasive strategies including intubation, sedation, paralysis, and hypoventilation with hypercapnia or inspired carbon dioxide have also been used. However, we generally recommend that babies with surgical heart disease do not undergo extensive medical management—babies with early symptoms generally undergo an early first- stage operation, whereas those with circulatory collapse undergo bilateral pulmonary artery banding (with either a stent or prostaglandin to maintain ductal patency).
The presentation of the neonate with a multidistribution physiology (see Chapter 70 ) can be quite varied, as discussed earlier. The anatomy and physiology must be delineated and an assessment made of the determinants of VO 2 and DO 2 in order to identify next steps in management.
Noninvasive and invasive positive airway pressure may be used to recruit lung volume and improve oxygenation (continuous positive airway pressure and positive end-expiratory pressure, respectively) and provide ventilatory assistance (bilevel positive airway pressure [BiPAP] and tidal volume ventilation, respectively). In addition, mechanical ventilation unloads the respiratory pump, allowing for the redistribution of a limited Qs to other vital organs. Under normal conditions, respiratory muscle VO 2 is a small fraction of global VO 2 . Accordingly, the respiratory pump receives less than 5% of total CO. However, as respiratory work increases—due to metabolic acidosis–induced hyperventilation, for example, or impaired respiratory mechanics—respiratory muscle VO 2 may approach 40% to 50% of total body VO 2 . When this occurs and CO is limited, respiratory pump perfusion may occur at the expense of other vital organs, including the brain. Also, in the neonate with an increased minute ventilation and work of breathing, by eliminating exaggerated negative intrathoracic pressure (ITP) and increasing ITP above atmospheric with positive-pressure ventilation (PPV), afterload for the systemic ventricle decreases significantly (ventricular systolic transmural pressure decreases). The net effect of these changes is a marked improvement in the respiratory, myocardial, and global VO 2 -DO 2 relationship. In summary, intubation and PPV is helpful in neonates—usually with a postnatal diagnosis—who present with low systemic blood flow and/or circulatory collapse, whereas “elective” PPV (1) is usually not indicated and (2) can increase surgical risk and contribute to hospital morbidity (see earlier).
Nurses at the bedside of this particularly tenuous patient population must be mindful of the impact of routine care on DO 2 /VO 2 balance, and individual items should be clustered whenever possible. When patients present in shock, nonessential care should be limited to avoid excessive VO 2 in the setting of inadequate DO 2 .
Admission of a neonate to a specialized intensive care unit requires attention to and support from the entire family, particularly in the case of complex CHD when surgery is expected in the neonatal period. Both acute and posttraumatic stress disorders are common, having both short- and long-term implications for the family and the neonate. In a meta-analysis of over 37,000 healthy mothers (of healthy infants), the prevalence of postpartum depression ranges from 8% (Europe) to 26% (Middle East) and is considered the most common complication of pregnancy. The prevalence of short- and long-term depression in mothers of infants with CHD has been reported much more frequently than that in fathers and, not surprisingly, the rate of significant maternal depression is higher than that in controls. In addition, the frequency is higher in mothers whose children have more complex CHD, such as those with a fUVH versus milder forms of CHD. The recognition of maternal anxiety and depression is particularly important for the bedside staff, so that adequate support and referrals may be made.
With the increasing prevalence of prenatal diagnosis, the timing of this initial support has shifted from the CICU to the fetal heart programs; nonetheless, the reality of the upcoming surgery and impending threat to the infant's survival may be overwhelming. Even families who are well supported through diagnosis in the prenatal period are likely to require high levels of continued support following birth, as the severity of the diagnosis is often fully realized only once the child has been born. Challenges include family and marital stress, sibling mental health, financial considerations, and much more beyond the scope of this chapter. It is important for the entire care team, in particular the bedside nurse, to be aware of these short- and long-term challenges. Efforts to facilitate parental bonding should be considered a routine part of care in the preoperative period. Holding of the infant, particularly skin to skin, should be recommended and encouraged unless contradicted by clinical status. Parents should be proactively engaged in bedside care and empowered to ask clarifying questions when the plan of care is being discussed with the medical team.
Finally, an important component of preoperative support and education is to prepare the family as well as possible for the visual appearance of a neonate following cardiac surgery. Although the medical and nursing staff are quite familiar with all of the medical equipment, drainage catheters, monitors, and so on, the shock of the appearance of the baby in the middle of all this technology can be overwhelming. Delayed sternal closure, in particular, should be discussed, and pictures made available if possible.
The nutritional status of the neonate should be assessed prior to palliating the neonate with a fUVH. Feeding prior to surgery remains controversial. The risk of impaired systemic perfusion leading to necrotizing enterocolitis may outweigh the benefit of enteral feeding. However, several studies have indicated that initiation of preoperative enteral feeds improves oral-motor coordination, decreases infection rates, and prevents bacterial translocation. Unless there is profound evidence of inadequate DO 2 or an anatomic gastrointestinal comorbidity, most centers will allow the preoperative neonate with single-ventricle physiology the opportunity to feed orally on demand without forced gastric tube supplementation. This is the case even when indwelling umbilical arterial catheters are in situ or prostaglandin infusions are being given. In cases where enteral nutrition is established, serial and careful monitoring of the abdominal exam and stool is paramount. The clinician must have a low threshold for discontinuation of enteral feeds if there is any evidence of shock or poor intestinal perfusion. Although human milk is the preferred option for enteral feeding, other options include donor milk or a standard-calorie neonatal formula. If there is a contraindication to enteral feeding or any delay in the initial palliation, one should consider initiation of total parenteral nutrition (TPN) early and advance to full fluid (100 to 120 mL/kg per day), calorie (90 to 100 kcal/kg per day), and protein (1.5 to 3 g/kg per day) goals. Elemental supplements of sodium, potassium, calcium, phosphorus, magnesium, zinc, and carnitine are also common.
Survival to birth of the individual with a fUVH is possible due to the presence of the arterial duct, which can allow the dominant ventricle to perform systemic work. The diagnoses included in the general category of fUVH can be divided into several categories that correspond to surgical interventions. The patient may have obstruction to pulmonary blood flow (such as tricuspid atresia with normally related great vessels), obstruction to systemic blood flow (such as, hypoplastic left heart syndrome), or absence of obstruction to either circulation. Neonatal palliation is different for each of these conditions.
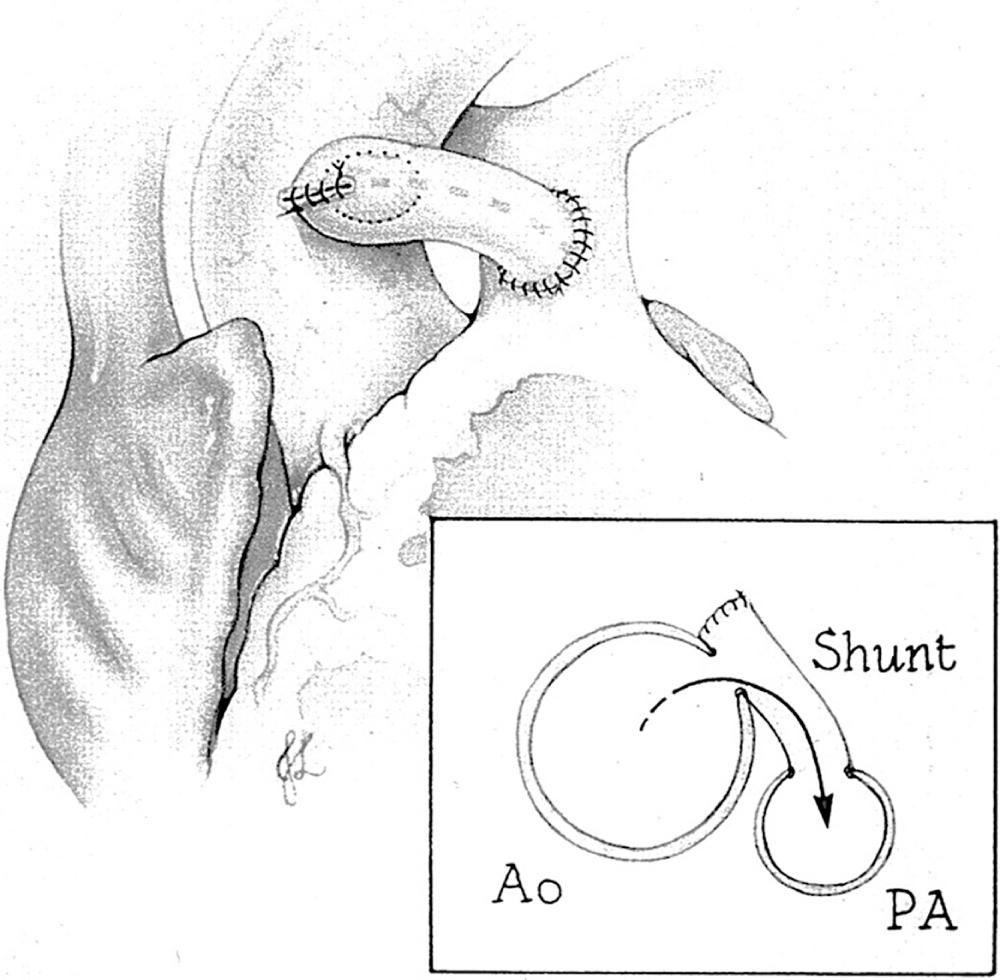
Patients with pulmonary atresia or severe obstruction to pulmonary blood flow will require a stable source of pulmonary blood flow that will not result in excessive hypoxemia or excessive pulmonary blood flow at the expense of systemic blood flow. Excessive pulmonary blood flow will lead to heart failure or elevation of PVR, which will complicate subsequent surgical palliation. Options include a systemic-to-pulmonary artery shunt or ductal stenting. The most common surgically created systemic-to-pulmonary artery shunt is the modified Blalock-Taussig shunt described by DeLeval and colleagues ( Fig. 71.1 ). Here an interposition graft of expanded polytetrafluoroethylene (ePTFE) is used to connect a systemic artery, most commonly the innominate artery, to the ipsilateral branch pulmonary. Other options may include a central shunt in which the ePTFE graft is taken from the ascending aorta to the main or a proximal-branch pulmonary artery ( Fig. 71.2 ). Other shunts include direct connection of the ascending aorta to the right pulmonary artery (Waterston shunt) and direct connection of the left pulmonary artery to the descending thoracic aorta (Potts shunt). These are uncommonly used for surgical palliation, although the Potts shunt has found new life in the treatment of end-stage pulmonary hypertension. The decision regarding shunt construction depends on the patient's specific anatomy. Patients with normally related great vessels and typical arch branching anatomy could undergo a modified Blalock-Taussig shunt. Individuals with pulmonary atresia with an intact septum and small branch pulmonary arteries or those with abnormal arch branching such as anomalous origin of the right subclavian artery with an isolated right carotid artery as the first vessel may benefit from construction of a central shunt from the aorta to the main pulmonary artery.
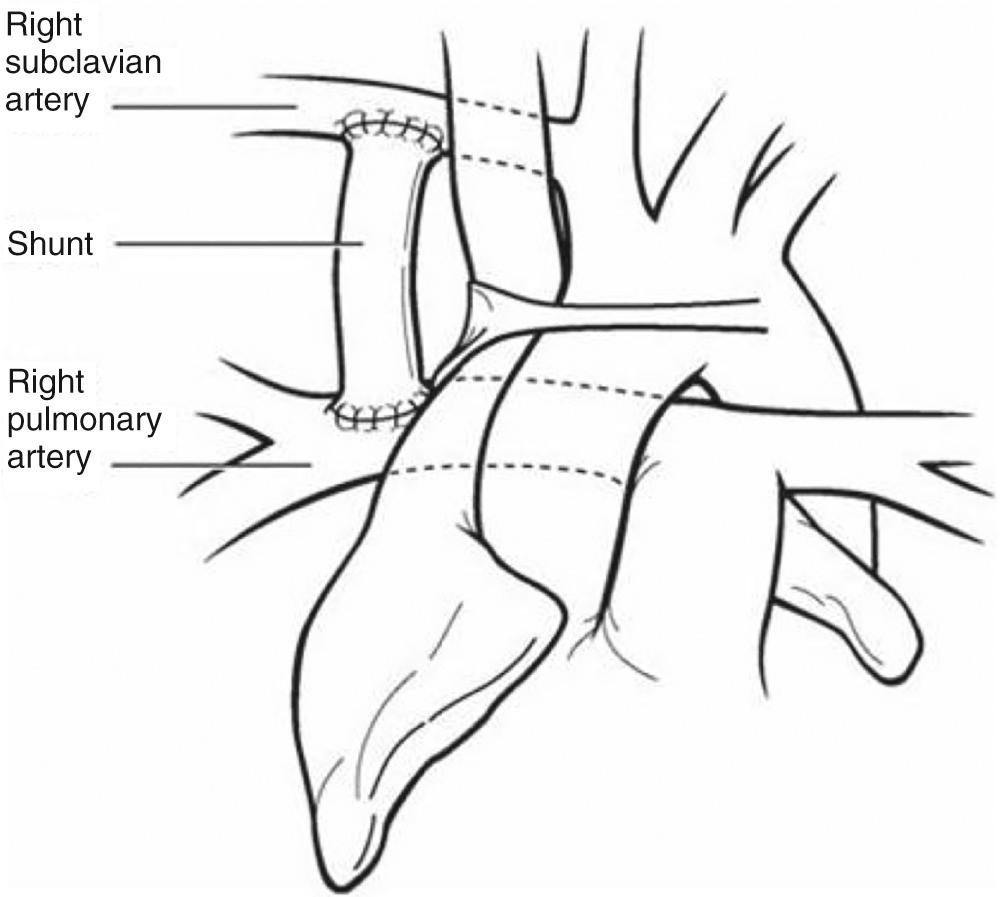
Stenting of the arterial duct to provide a stable source of pulmonary blood flow was first reported in 1992. A recent retrospective multicenter analysis suggests that duct stenting may provide equivalent results to a surgical shunt in patients with suitable anatomy. Unlike the patent arterial duct as an isolated lesion, the duct in cyanotic heart disease has wide morphologic variability. It may be long and curled, rendering stent implantation more challenging. The newer coronary stents have better flexibility and trackability, making ductal stenting safer. Decisions regarding shunt type or the use of duct stenting frequently vary by institution, being based on anatomy and perceived local expertise; as yet no universally accepted criteria have been developed. However, as techniques improve, duct stenting will play a bigger role in initial palliation.
In the patient with duct-dependent pulmonary blood flow, creation of a reliable source of flow is generally performed within the first week of life. Classically a thoracotomy is performed on the side opposite arch dominance and an ePTFE graft is used to create a shunt between the innominate artery and the ipsilateral proximal pulmonary artery. This approach results in a primary median sternotomy incision for the next-stage operation, but the disadvantage is that the duct cannot be controlled or ligated at the completion of the procedure. Having both a patent arterial duct and a systemic-to-pulmonary artery shunt results in hypotension and low-velocity flow through the synthetic shunt, which predisposes to shunt thrombosis; however, this does not become apparent until the duct begins to close. In the current era a median sternotomy incision is commonly used for shunt construction. There are several advantages to a median sternotomy. The duct can be ligated after construction of the shunt and the adequacy of the shunt can therefore be determined. The innominate artery and the proximal branch pulmonary artery are more easily accessed. If necessary, cardiopulmonary bypass support can be used for construction of the shunt if the patient does not tolerate branch pulmonary artery occlusion. Patch arterioplasty is necessary for proximal branch pulmonary artery stenosis in the region of duct insertion.
Thoracotomy: The patient is placed in the lateral decubitus position and a posterolateral thoracotomy incision is made on the side opposite the aortic arch through the fourth interspace. An incision is made in the mediastinal pleura posterior to the superior caval vein, and the pulmonary and innominate arteries are identified. Lymph nodes along the side of the trachea are resected and the pulmonary artery is dissected as far medially as possible. The innominate artery is fully mobilized. Heparin is administered, typically, 100 units/kg, and the innominate artery is isolated. A longitudinal incision is made in the inferior surface of the innominate artery. A 3.5- or 4.0-mm graft is commonly used. The graft is cut in a beveled fashion to accommodate the angle of the innominate artery and an anastomosis is constructed with fine monofilament suture. An alternative approach is to perform the proximal anastomosis to the subclavian artery. The use of the smaller subclavian artery as the origin of the shunt will add resistance to shunt and allows for larger-caliber graft to be used; it may also be useful in the smaller patient to accommodate a normal-caliber graft. Once the proximal anastomosis is complete, the graft is cut to the appropriate length to reach the pulmonary artery. The innominate artery is de-aired, flow is restored, and the graft is controlled with a fine vascular clamp. After isolating the pulmonary artery, a longitudinal arteriotomy is made in the pulmonary artery and the distal anastomosis is performed. Clamps and snares are removed from the pulmonary artery and the shunt is opened. There should be a drop in blood pressure corresponding to flow in the shunt with an increase in pulmonary artery saturations. Prostaglandin infusion is halted.
Sternotomy: The patient is placed in the supine position and a median sternotomy incision is performed. After exposing the heart, the innominate artery is mobilized. The proximal ipsilateral pulmonary artery is likewise exposed. It may be possible to encircle the duct at this point, but care should be taken, as this may not be tolerated because it can result in excessive hypoxemia; in addition, the duct can be bruised or injured, and this would also compromise pulmonary blood flow. The shunt is constructed the same as for a thoracotomy approach. Following completion of the shunt and after it has been opened, the duct can be encircled and snared to make certain that there will be adequate pulmonary blood flow with the newly constructed shunt. If the saturations remain satisfactory, the duct is ligated. If the patient develops excessive hypoxemia with snaring of the duct, a stepwise approach to determine the problem should be undertaken, including making certain that ventilation is adequate. The shunt should finally be inspected for technical issues. Revision of the shunt or replacement with larger graft should be considered if occlusion of the duct is not tolerated.
With initiation of spontaneous respiration after birth, PVR begins to drop. For patients with a fUVH and without anatomic limitation to pulmonary or systemic blood flow, heart failure will predictably develop with the drop in PVR.
Pulmonary artery banding is the first stage of palliation in neonates with unrestrictive pulmonary blood flow and no systemic outflow tract obstruction ( Fig. 71.3 ). Pulmonary artery banding relieves the volume load on the heart; otherwise heart failure may ensue. Pulmonary artery banding reduces pulmonary artery pressure, allowing for continued remodeling of the pulmonary vascular bed for subsequent second stage of palliation. Neonatal PVR reaches a nadir around the third or fourth week of life, and traditionally pulmonary artery band placement has been performed after 2 or 3 weeks of age. It has been thought that placing a pulmonary artery band in the early neonatal period may lead to the need to reoperate for band readjustment once the PVR falls. Recent data, however, suggest that delay is not necessary, and that pulmonary artery banding can be performed during the first week or two of life.
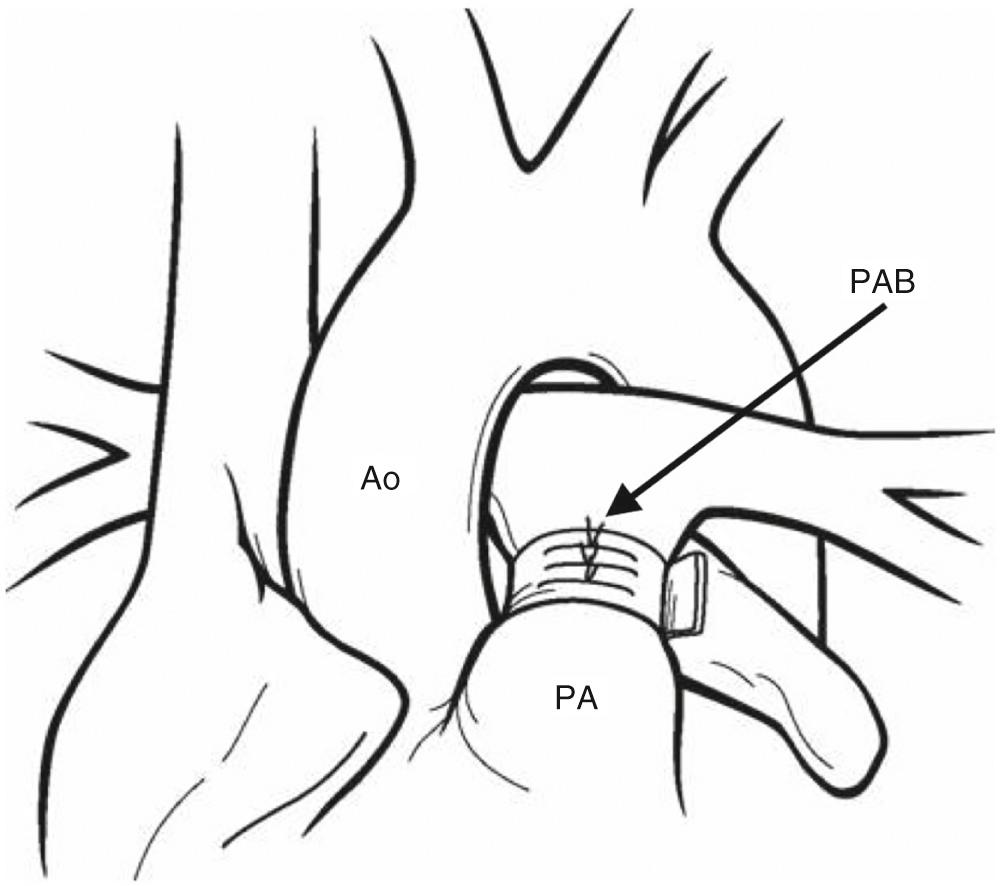
Ligation of the main pulmonary artery and placement of a systemic-to-pulmonary artery shunt has been advocated by some as a strategy to manage the patient with unrestricted pulmonary blood flow. The theoretical advantage is the ability to more precisely control pulmonary blood flow. This is uncommonly used in practice due the increased risk of systemic-to-pulmonary artery shunt compared with pulmonary artery banding.
Pulmonary artery banding can be performed through a thoracotomy or median sternotomy incision. If the arterial duct is patent or there is any question of patency, the duct is ligated. The main pulmonary artery can be banded with a variety of material including ePTFE, polyester tape, or silk. The aorta is separated from the pulmonary artery and the tape passed around the main pulmonary artery. The length of band can be estimated according to the Trusler rule. The length of the band should be 20 mm plus the number of millimeters corresponding to the child's weight in kilograms. Neonates with transposition physiology will be more cyanotic due to unfavorable streaming. In this case the length should be 24 mm plus the child's weight to prevent excessive hypoxemia. Arterial saturation and pressure in the distal pulmonary artery should be monitored during banding. The patient should be placed on an FiO 2 of 50% to decrease the amount of dissolved oxygen. The band should be adjusted to achieve a distal pulmonary artery pressure of 15 to 20 mm Hg or one-fourth of the systolic blood pressure. The saturation should be 80% to 85%. A common technique is to use hemoclips to make the final adjustments. This allows the band to be tightened and loosened in 1-mm increments. The final adjustment is commonly a compromise between achieving a satisfactory pressure and avoiding excessive hypoxemia. Once the proper tightness is achieved, the band is secured with multiple sutures between the band and the pulmonary artery. Ideally the band should be above the sinotubular junction of the pulmonary valve and not impinging on the origin of either pulmonary artery. If it is not properly secured, the band can slip and compromise the origin of a pulmonary artery, typically the right pulmonary artery, and result in excessive hypoxemia. If the band is positioned too far proximally, it can encroach on the pulmonary valve and result in leaflet damage, which can cause regurgitation.
Obstruction to systemic flow can occur due to arch hypoplasia with coarctation, aortic valve stenosis, subaortic stenosis, or a combination of these lesions. These newborns will be maintained on prostaglandin to maintain systemic output. Creation of unobstructed systemic outflow is important to maintain cardiac output and prevent hypertrophy of the ventricle, which would compromise future palliation. Patients with fUVH and obstructed systemic blood flow will also have unobstructed pulmonary blood flow, and relief of systemic outflow obstruction must be combined with restriction of pulmonary blood flow.
For isolated coarctation with or without arch hypoplasia and without additional left ventricular outflow tract obstruction (aortic valvar or subaortic stenosis), standard coarctation repair can be performed (see also Chapter 45 ). If the arch hypoplasia is distal to the origin of the left carotid artery, the arch can be approached via a left thoracotomy. If there is proximal arch hypoplasia, an approach via a median sternotomy using cardiopulmonary bypass will be necessary. The procedure can be combined with pulmonary artery banding.
In certain groups of fUVHs with a dominant left ventricle and discordant ventriculoarterial connections, the aorta can arise from a rudimentary outflow chamber or right ventricle and connect to the left ventricle via an interventricular communication. A restrictive interventricular communication will result in systemic outflow tract obstruction. When the interventricular communication is small at birth, aortic hypoplasia—frequently with coarctation and occasionally with an interrupted aortic arch—may be present. Even when the interventricular communication is nonrestrictive at birth, it can become narrow over time, resulting in obstruction, and the tendency for systemic outflow obstruction to progress after pulmonary artery banding has been well recognized. Strategies to prevent development of outflow tract obstruction or manage its presence include an anastomosis between the pulmonary root and the ascending aorta—the Damus-Kaye-Stansel (DKS) procedure simultaneously described by Paul Damus, Michael Kaye, and Horace Stansel in the 1970s ( Fig. 71.4 ). The anastomosis of the pulmonary root and aorta bypasses the restrictive interventricular communication. A direct approach at enlargement of the interventricular communication is challenging in the neonate and risks injury to the conduction system with resultant complete heart block.
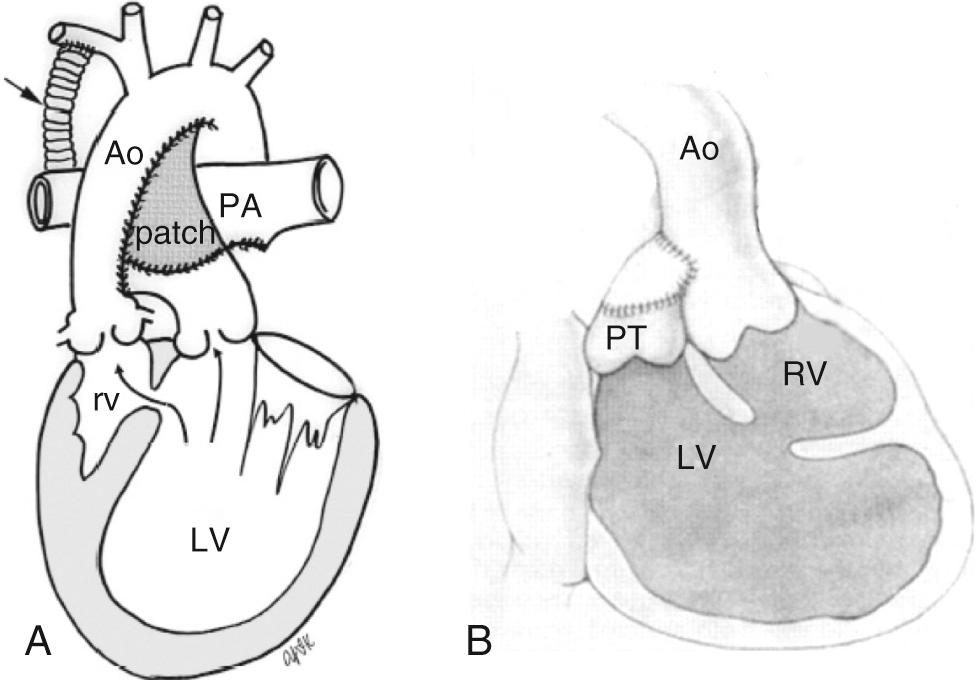
A DKS procedure requires cardiopulmonary bypass; therefore coarctation repair with pulmonary artery banding offers the newborn a less morbid procedure. In the current era, where a bidirectional superior cavopulmonary anastomosis is generally performed during infancy, an interventricular communication area greater than 1 to 2 cm 2 /m 2 (corresponding to a interventricular communication diameter of 7 mm or greater) is a reasonable cutoff identifying those patients who are unlikely to develop obstruction during infancy prior to a superior cavopulmonary anastomosis. In these patients, arch repair and pulmonary artery banding can be considered for neonatal palliation and a more definitive procedure to prevent systemic ventricular outflow obstruction can be part of the second-stage palliation.
More severe forms of systemic ventricular outflow tract obstruction are frequently associated with coarctation and arch hypoplasia. This includes more severe forms of fUVH with a dominant left ventricle and discordant ventriculoarterial connection (tricuspid atresia with transposed great vessels), and those with dominant right ventricle and concordant ventriculoarterial concordant connections (e.g., hypoplastic left heart syndrome). For these neonates there are two surgical options, Norwood palliation and so called “hybrid palliation.”
The Norwood procedure includes arch reconstruction, a DKS type root amalgamation, creation of a nonrestrictive atrial septal defect, and placement of an appropriately restrictive source of pulmonary blood flow—either a modified Blalock-Taussig shunt or a shunt from the right ventricle to the pulmonary artery ( Fig. 71.5 ). The Norwood procedure requires cardiopulmonary bypass with a period of aortic cross-clamping and perfusion techniques that allow for aortic arch reconstruction. This remains one of the higher-risk procedures commonly performed in the newborn period.
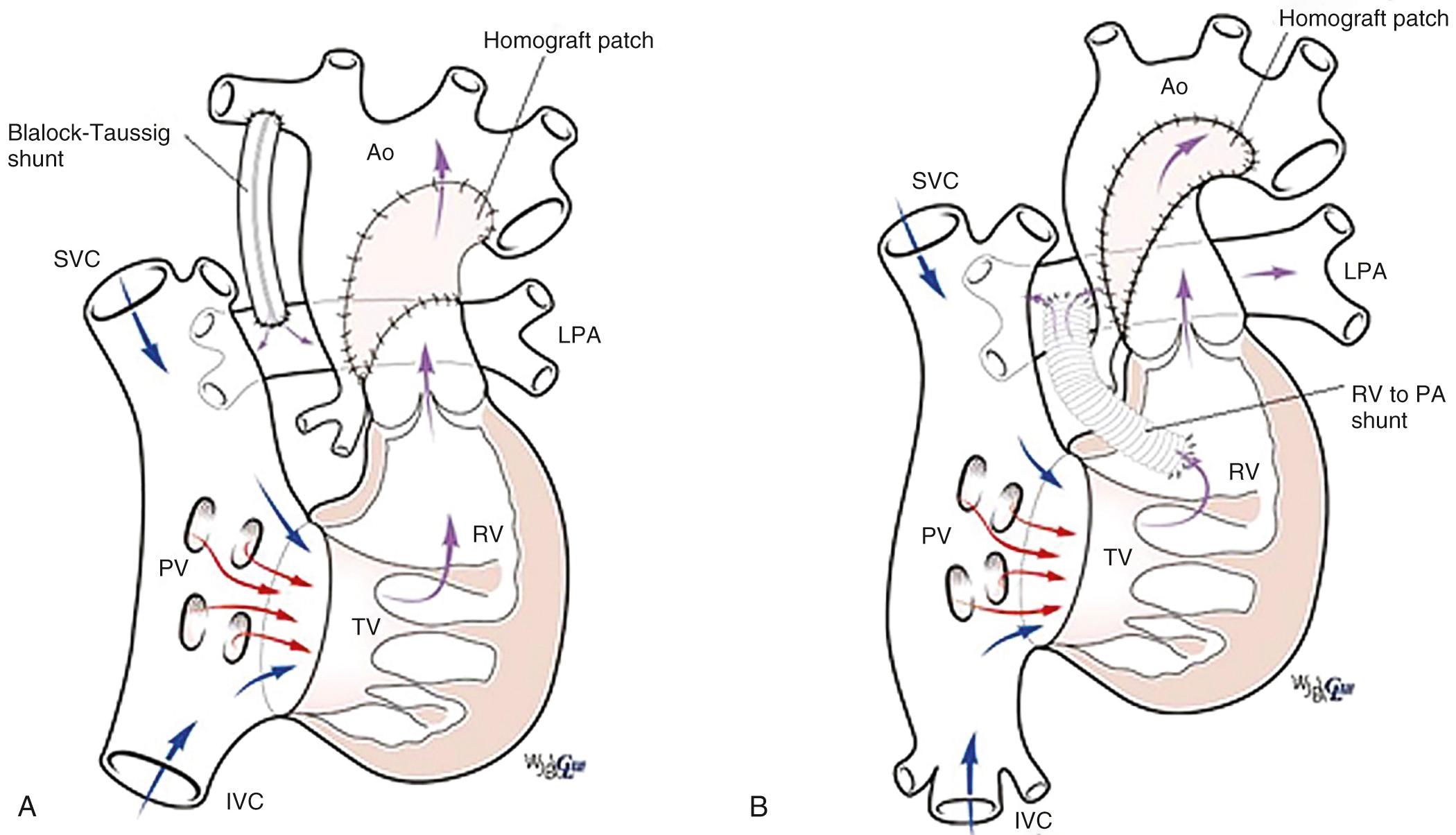
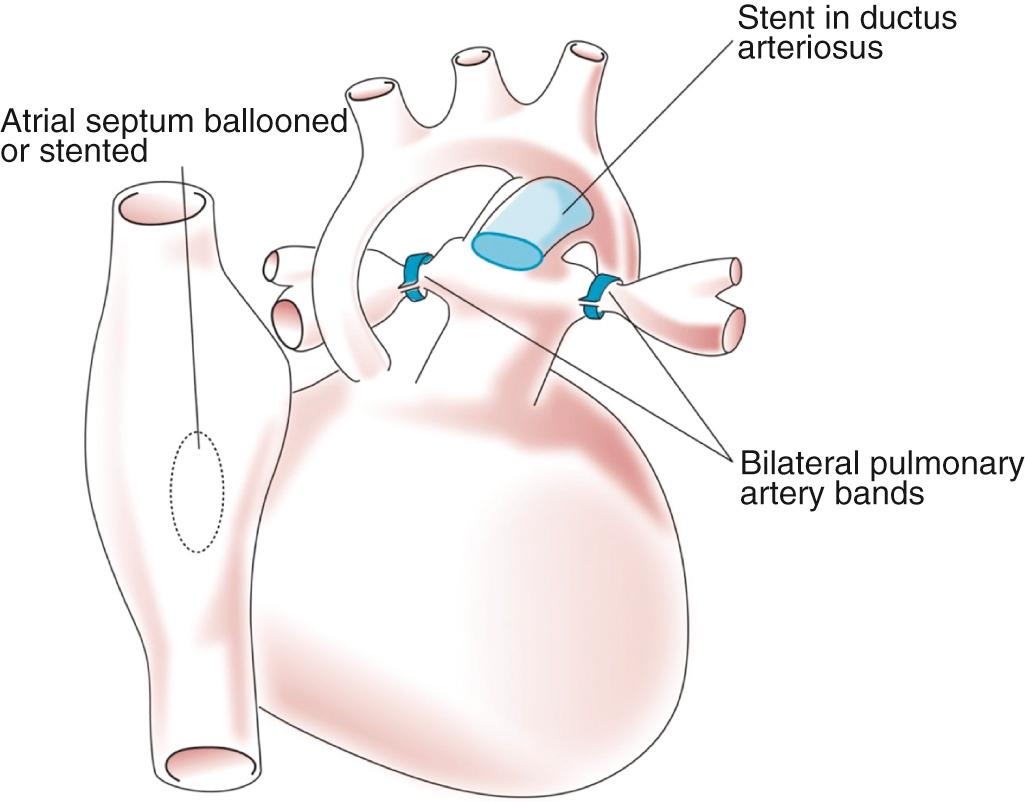
The hybrid procedure is an alternative that provides for neonatal palliation including banding of the branch pulmonary arteries and maintenance of the arterial duct with either a stent or a prostaglandin infusion ( Fig. 71.6 ). If not present, a nonrestrictive atrial septal defect is created using interventional catheter-based techniques. The advantage of the hybrid procedure is that is does not use cardiopulmonary bypass. Definitive management of left ventricular outflow tract obstruction and arch hypoplasia involves a Norwood-style reconstruction combined with the second-stage procedure. Some programs use the hybrid procedure for all neonates with fUVH and severe arch hypoplasia. This avoids a long period of cardiopulmonary bypass in the vulnerable neonate. Other programs reserve this alternate strategy for patients with risk factors for cardiopulmonary bypass including; prematurity, low birth weight, infection, necrotizing enterocolitis, and intracranial hemorrhage. The disadvantage of the hybrid procedure is a more challenging period between neonatal palliation and the comprehensive second-stage procedure, including the predictable need for catheter-based reintervention directed at the atrial septum or narrowing between the stented arterial duct and the more proximal aortic arch, referred to as retrograde arch obstruction. In addition, the second-stage procedure is more complicated and requires a long period of cardiopulmonary bypass for arch reconstruction and reconstruction of the banded pulmonary arteries. At present the decision between the Norwood procedure and the hybrid approach remains largely program-specific without an obvious advantage to either procedure in the average-risk neonate.
The DKS is performed through a median sternotomy incision using cardiopulmonary bypass. Patients suitable for an isolated DKS do not have arch obstruction, and profound hypothermia is not necessary. After cross-clamping and cardioplegia, an anastomosis—generally with patch augmentation—is made between the great vessels above the sinotubular junction of the semilunar valves. There are two general techniques. In the first the pulmonary root is transected above the sinotubular junction and a vertical incision is made in the ascending aorta adjacent to the pulmonary root. The adjacent proximal edges of the aorta and pulmonary root are joined, and the remainder of the connection is accomplished with a patch. The second technique, sometimes called the “double-barreled” technique, involves complete transection of both great vessels above the sinotubular junction. The great vessels are joined at their “kissing” point and the ascending aorta is then anastomosed to this double-barreled root. Patch augmentation is frequently required to make for the size discrepancy between the combined root and the ascending aorta. A source of pulmonary blood flow is necessary. In the neonate with a single left ventricle, this will be a systemic-to-pulmonary artery shunt. In an infant that was suitably palliated with a pulmonary artery band, a bidirectional superior cavopulmonary anastomosis can be performed.
The Norwood procedure ( ) is performed through a median sternotomy with cardiopulmonary bypass. After initial dissection, the patient is cannulated for cardiopulmonary bypass. Arterial cannulation is achieved by cannulating either the pulmonary artery, arterial duct, or using an ePTFE graft anastomosed to the innominate artery. Single venous or bicaval cannulation can be used for venous return. Once cardiopulmonary bypass has been established, flow to the pulmonary arteries is prevented by snaring either the branch pulmonary arteries or the ductus arteriosus. Three perfusion strategies have been described for the period of arch reconstruction; deep hypothermic circulatory arrest at 18°C, antegrade cerebral perfusion at deep hypothermia at 18°C to 22°C, or continuous perfusion at moderate hypothermia. The last approach requires placement of an additional arterial cannula to supply flow to the descending thoracic and abdominal aorta during arch reconstruction. The site of distal cannulation can include cannulation of the arterial duct, descending thoracic aorta at the level of the diaphragm, or femoral artery.
During a period of aortic cross-clamping and altered perfusion, arch reconstruction including amalgamation of the aortic and pulmonary roots is accomplished ( Fig. 71.7 ). A nonrestrictive atrial septal defect is created. The cross clamp is released and rewarming completed. Finally a source of pulmonary blood flow is established—either a systemic-to-pulmonary artery shunt or a right ventricle-to-pulmonary artery conduit. Once rewarming has been completed and cardiac function has returned, vasoactive support is initiated. Commonly milrinone and catecholamines are used. Prior to weaning from bypass, the patient should be in an AV sequential rhythm, hematocrit should be at least 35%, ionized calcium should be within the normal range, and the systemic vascular resistance should be about 12 Woods units. The SVR can be estimated by dividing the mean arterial pressure by the cardiac index. Further adjustments of vasoactive agents are made to achieve the target SVR. For the patient with a systemic-to-pulmonary artery shunt, the shunt is opened as the cardiopulmonary bypass flow is reduced. After successful weaning from bypass, modified ultrafiltration can be performed. Evaluation of the procedure is then undertaken. This can include echocardiography, either epicardial or transesophageal, to evaluate function, the adequacy of the atrial septal defect, and the degree of tricuspid valve regurgitation. Regional perfusion as assessed by NIRS should be used to assess DO 2 . Residual systemic outflow obstruction can be ruled out by comparing the systemic ventricular systolic pressure with a pressure measured from the femoral or umbilical artery catheter. Once the clinician is satisfied with the repair, the venous cannula is removed and protamine is administered to reverse anticoagulation. The arterial cannula can be left in place until after protamine has been administered. It is essential to achieve complete hemostasis. Transfusion of platelets and fibrinogen—either fresh frozen plasma or cryoprecipitate—is common. Careful inspection of the surgical sites should be undertaken and additional sutures placed as necessary. For continued bleeding that does not seem to be surgical and has not responded to component transfusion, additional agents such recombinant factor VIIa or prothrombin complex concentrates can be considered.
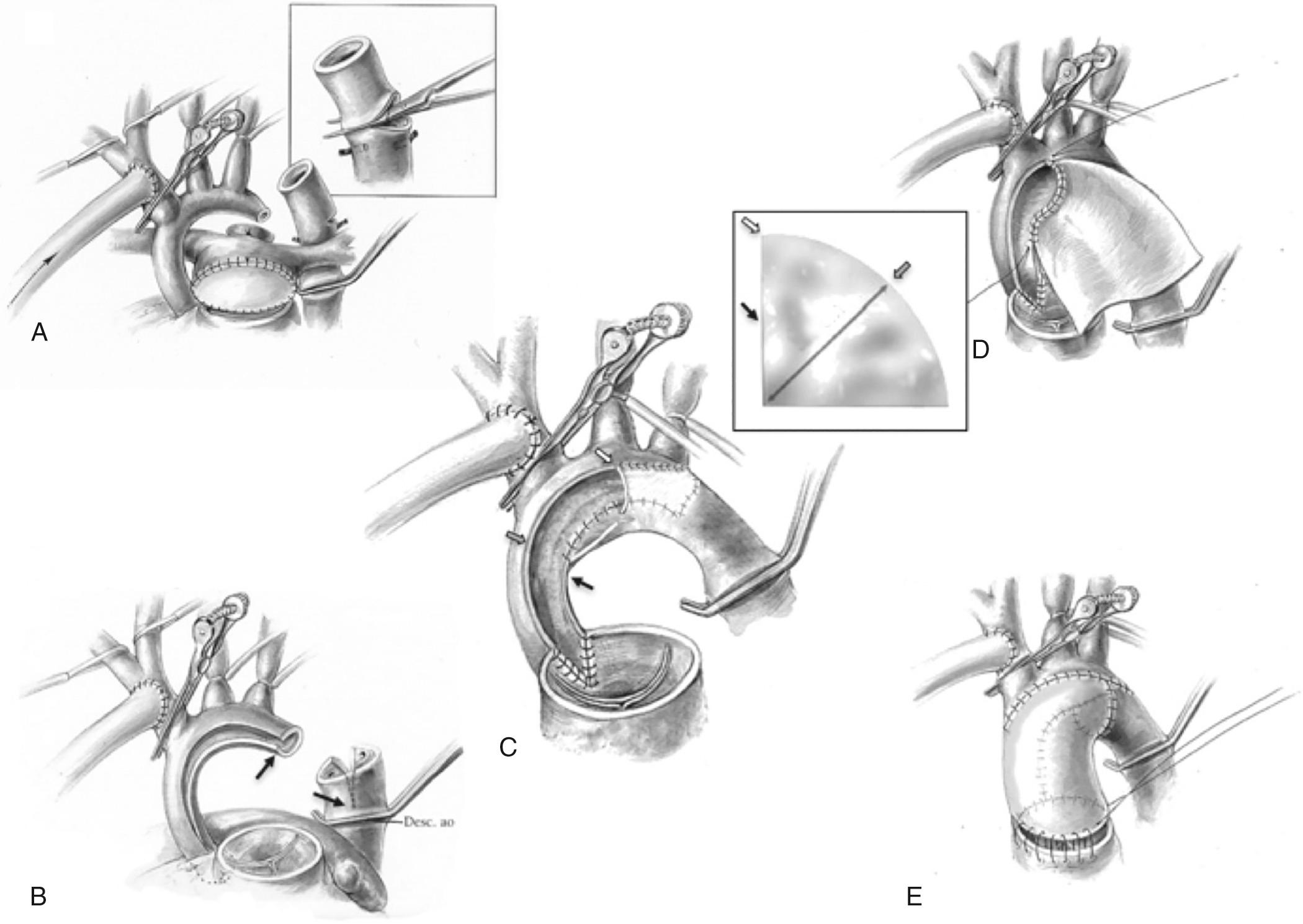
Once hemostasis has been achieved, the decision to close versus leaving the sternum open is made. For those patients who have had a long bypass run and are on more than the usual inotropic support, delayed sternal closure may be necessary. Although some programs routinely leave the sternum open, there is little question that this prolongs the length of time to extubation and, as a consequence, also CICU length of stay and hospitalization. Therefore it seems reasonable to determine whether the sternum can be closed primarily. Sternal closure sutures are placed and the sternum reapproximated. The hemodynamics are evaluated and if there is no perturbation, sternal closure can be completed and the remainder of the incision closed in the usual manner. If sternal approximation results in important hypotension and/or desaturation or if there is evidence of decreased systemic DO 2 as assessed by NIRS or venous saturation, the sternum should be left open. Once the patient begins to diurese and vasoactive support can be weaned, chest closure can be performed. If the patient fails to progress, residual lesions should be ruled out.
The hybrid approach is defined by placement of individual branch pulmonary artery bands. Ductal patency is maintained with either a stent or prostaglandin. In addition, catheter-based creation of a nonrestrictive atrial septal defect is necessary if this is not already present. Typically the procedure is performed in a hybrid catheterization laboratory. A median sternotomy is performed. Branch pulmonary artery banding is performed first. Rings 2 to 3 mm in diameter are cut from an ePTFE tube graft that is 3.0 or 3.5 mm in diameter. With the typical hypoplastic left heart syndrome anatomy, the left pulmonary artery is banded first, as it is the most difficult to access. The right pulmonary artery is banded next. The adequacy of the banding can be directly measured by catheter and additional fine adjustments can be made. If a stent is to be placed to maintain duct patency, this is performed last. The atrial septal defect can then be enlarged using catheter-based techniques.
Finally, some neonates with a fUVH are born with without the need for neonatal surgical intervention. In general these are children with unobstructed systemic blood flow and restricted pulmonary blood flow; in them the physiology results in adequate systemic DO 2 , a “protected” pulmonary vascular bed with normal pulmonary artery pressures, and no significant obstruction to systemic or pulmonary venous return. This is most commonly seen in patients with tricuspid atresia (with atrioventricular and ventriculoarterial concordance) and a restrictive ventricular septal defect and in patients with isomerism and native obstruction to pulmonary blood flow (see Chapter 69 ). These patients require frequent monitoring in early infancy for adequate oxygenation as well as to confirm that pulmonary artery pressures are low; however, a small number of babies born with a fUVH may not require surgery until a planned superior cavopulmonary connection (SCPC) (see later).
Residual lesions, inadequately palliated anatomy, is associated with increased mortality and morbidity in patients with a fUVH. Palliative procedures for patients with fUVH are among the most challenging procedures. The patient will leave the operating room with a multidistribution rather than normal in-series circulation. It is essential that the palliative procedure achieve the anatomic goal and assessment of the success of the procedure is essential prior to leaving the operating room. Tools to assess the outcome of palliation will include evaluation of hemodynamics, filling pressure, blood pressure and pulse oximetry, and additional lesion-specific measures such as measurement of blood pressure in the upper and lower extremities after coarctation repair. For the patients undergoing main pulmonary artery banding, in addition to assessment of hemodynamics, measurement of distal pulmonary artery pressure, echocardiography and arterial and venous oxygen saturation and pO 2 may be needed.
Despite the relative simplicity of the procedure the systemic-to-pulmonary artery shunt is one of the higher-risk palliative procedures. If a shunt is too large, the patient can have excessive pulmonary blood flow at the expense of systemic blood flow. In addition, a shunt that is too large can result in diastolic hypotension, thus compromising coronary blood flow. If the shunt is too small, the patient will continue to have excessive hypoxemia. Intraoperative assessment of the adequacy of a shunt is challenging. The shunt is constructed in an anesthetized patient; as a consequence, VO 2 is markedly reduced. Saturations will be higher in the anesthetized state than when the patient is awake. Assessment of the shunt is easier if the ductus is closed at the completion of the procedure. For the patient undergoing placement of a systemic-to-pulmonary artery shunt, the goal is sustained relief of hypoxemia, and evaluation will include both the hemodynamics with special attention to the diastolic blood pressure as well as arterial and venous oxygen saturation and pO 2 .
Closed palliative procedures (performed without the use of cardiopulmonary bypass)—such as coarctation repair, pulmonary artery banding, and construction of a systemic-to-pulmonary artery shunt—may be complicated by the development of a restrictive atrial septal defect. The adequacy of the atrial septal defect should be evaluated preoperatively and plans for definitive management made prior to other palliative procedures.
The goal of Norwood palliation includes relief of systemic outflow tract obstruction, creation of a widely patent atrial septal communication, and creation of a reliable source of pulmonary blood flow that relieves hypoxemia, permits growth, but does result in heart failure. Intraoperative evaluation of the patient following the Norwood procedure is challenging. In addition to assessment of hemodynamics—especially blood pressure, central venous pressure and pulse oximetry—evaluation may include simultaneous measurement of ascending and descending aortic blood pressure, echocardiographic assessment of systolic function, AV valve regurgitation, size of atrial septal defect, and relief of arch hypoplasia. Measurement of superior caval vein saturation, a surrogate of mixed venous saturation and NIRS, can provide information on global DO 2 . Identification of residual lesions such as important residual arch obstruction or a restrictive atrial septal defect should prompt the clinician to consider immediate correction. The adequacy of the source of pulmonary blood flow can be more challenging. Excessive hypoxemia should prompt a stepwise evaluation as outlined earlier. Excessive pulmonary blood flow can be identified by higher arterial saturation and pO 2 , along with evidence of reduced systemic blood flow such as reduced superior caval vein saturation, reduced somatic NIRS values, and hypotension. Coronary insufficiency is a major cause of mortality after the Norwood procedure. Although severe coronary insufficiency is obvious—dusky, cyanotic myocardium and profoundly decreased function with inability to wean from bypass—the diagnosis of subtler coronary insufficiency is more challenging. Decreased function, new AV valve regurgitation, and ECG changes are common following the Norwood procedure and can occur even in the otherwise uncomplicated patient. Nevertheless, these findings should prompt evaluation of patency of the connection of the native aortic and pulmonary roots with either echocardiography or angiography.
Neonatal surgical palliation of the patient with fUVH must provide both a systemic and pulmonary output sufficient to provide DO 2 for the patient's metabolic demands and to promote healing. Optimally, systemic outflow obstruction will be completely relieved and a restricted source of pulmonary blood flow will be created. The source of pulmonary blood flow, although fixed to some degree, must be sufficiently large to support the infant to the second-stage palliation. Even in the optimally palliated child, the early postoperative period is commonly marked by periods of decreased total output from the single ventricle; this can make achieving satisfactory DO 2 challenging. In addition, there is a potential for residual lesions or additional complications that affect the goal of providing adequate systemic DO 2 . Within this group of diagnoses and surgical reconstructions, there is proportional higher mortality than other congenital heart surgery patients. The challenges of managing this group of patients has spawned the multidisciplinary CICU, which is emblematic of modern congenital heart programs. These challenges are summarized in Box 71.3 .
Bedside preparedness
Invasive monitoring
Bleeding
Delayed sternal closure
Assessment of adequate systemic oxygen delivery
Mechanical ventilation and considerations for tracheal extubation
Evaluation of the central nervous system
Feeding and nutrition
Deviations from the expected postoperative course
Evaluation and management of acute decompensation
Evaluation and management of failure to progress
Family support and discharge planning
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here