Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The first invasive studies of cardiac electrophysiology (EP) in humans were performed epicardially. During its golden age (1970s to 1980s), electrical activation maps were acquired in the operating room by surgeons. These studies greatly advanced our understanding of the predominant mechanisms underlying common atrial and ventricular arrhythmias and paved the way for the first curative surgical procedures for electrical disorders. This approach would eventually be superseded by endocardial access because of its superior safety profile and the importance of the endocardial substrate in many arrhythmias. Advances in diagnostic and ablation catheter design as well as in energy sources (from fulguration to radiofrequency [RF]) have rendered surgical approaches rarely required.
For years, surgical epicardial access was largely restricted to cardiac defibrillation or treating refractory arrhythmias. In 1996 Sosa et al. developed a technique to percutaneously access the epicardium to treat recurrent electrical storms in a subpopulation of patients with Chagas disease. This approach created new opportunities in the treatment of arrhythmias, primarily from the ventricles, and has advanced our understanding of the arrhythmogenic substrates of different cardiomyopathies.
Nowadays, percutaneous epicardial access is commonly performed in tertiary EP centers for ventricular tachycardia (VT) ablation in patients with varied cardiac pathologies, including nonischemic dilated cardiomyopathies (DCMs) and arrhythmogenic right ventricular (RV) cardiomyopathy (ARVC).
The pericardial sac is situated posterior to the sternum and at the level of the second to sixth costal cartilages. The fibrous pericardium, the outermost layer of the sac, is attached to the sternum anteriorly and to the central tendon of the diaphragm inferiorly and fuses with the adventitia of the great vessels superiorly. The inner fibrous pericardium is lined by the parietal serous pericardium, which reflects on itself to form the visceral serous pericardium, which is adherent to the epicardium. The thickness of parietal pericardium ranges from 0.8 to 2.5 mm. The pericardial cavity is the potential space between the parietal and visceral layers of the serous pericardium and contains roughly 20 mL of physiologic fluid in normal conditions. In approximately 1 out of every 10,000 patients, the pericardium is congenitally absent. Features suggestive of this rare disorder include poor R wave progression on electrocardiogram (ECG), signs of cardiac displacement, and a sharp aortopulmonary window on the chest radiograph (although it can be more easily confirmed by computed tomography [CT] or magnetic resonance imaging [MRI]).
Under normal conditions, a catheter inserted within the pericardial space can move freely over most of the epicardial surface. It is bounded only by pericardial reflections, such as the oblique and transverse sinuses and the pulmonary vein recesses. The oblique sinus is a cul-de-sac along the posterior left atrial (LA) wall and is bounded by the pericardial reflections of the left and right pulmonary veins and superiorly by the transverse sinus/LA roof. Its inferior opening is bounded by the two inferior pulmonary veins. The ligament of Marshall is situated at the posterolateral aspect of the LA, and the fold of Marshall may be seen to the left of the oblique sinus. The transverse sinus is a tunnel-shaped anatomic space between the great vessels and the roof of the LA, connecting the left and right sides of the pericardial cavity. The right pulmonary artery lies superior to the transverse sinus, and the roof of the LA forms its floor. The phrenic nerves lie near the pericardium. The left phrenic nerve passes behind the left brachiocephalic vein, over the aortic arch and pulmonary trunk, and over the LA appendage. In the majority of patients, it goes laterally over the obtuse margin of the left ventricle (LV), but in a minority of patients, it goes anteriorly close to the left anterior descending artery. The right phrenic nerve descends along the brachiocephalic vein, follows the right anterolateral border of the superior vena cava, passes close to the anterior wall of the right superior pulmonary vein, and then travels along the lateral aspect of the right atrial wall to finally join the lateral inferior vena cava (IVC).
Epicardial ablation has the potential to cause significant morbidity and even mortality; therefore it is predominantly performed in high-volume expert centers with surgical back-up. The most common indication for an epicardial approach is VT. Selecting patients who most benefit from this strategy involves considering characteristics of the VT and the cardiac substrate, including the results of previous cardiac mapping and details of scar on imaging.
Percutaneous epicardial access was first described in Brazil, where the prevalence of Chagas-associated epicardial VT created a need for epicardial ablation. Nevertheless, epicardial ablation has proven useful in various other cardiomyopathies, including ischemic cardiomyopathy (ICM), , nonischemic DCM, ARVC, , sequelae from myocarditis, and Brugada syndrome. Furthermore, combined endocardial-epicardial ablation can improve the efficacy of ablation for scar-related VT. The 2019 expert consensus statement on catheter ablation of ventricular arrhythmias has outlined situations in which epicardial access is deemed useful or could be considered ( Table 134.1 ). This chapter outlines factors to consider when making this decision.
| Indication | Class |
|---|---|
| In patients with symptomatic ventricular arrhythmias from the epicardial outflow tract or LV summit in an otherwise normal heart for whom antiarrhythmic medications are ineffective, not tolerated, or not the patient’s preference, catheter ablation can be useful. | IIA |
| In patients with prior myocardial infarction and recurrent episodes of symptomatic sustained VT for whom prior endocardial catheter ablation has not been successful and who have ECG, endocardial mapping, or imaging evidence of a subepicardial VT substrate, epicardial ablation may be considered. | IIB |
| In patients with NICM, epicardial catheter ablation of VT can be useful after failure of endocardial ablation or as the initial ablation approach when there is a suspicion of an epicardial substrate or circuit. | IIA |
| In patients with ARVC who have failed one or more attempts of endocardial VT catheter ablation, an epicardial approach for VT ablation is recommended. | I |
| In patients with ARVC, a first-line combined endocardial/epicardial approach for VT ablation is reasonable. | IIA |
A previous unsuccessful endocardial VT ablation performed by an experienced electrophysiologist is possibly the clearest indication for considering an epicardial approach because an epicardial substrate is found in 73% to 80% of such cases, irrespective of the type of underlying cardiomyopathy.
Multiple algorithms have been proposed to predict an epicardial source of VT. These generally perform best in patients with nonischemic cardiomyopathy (NICM). According to Berruezo et al., the following ECG criteria are predictive of an epicardial origin for VT:
A pseudodelta wave greater than 34 ms (sensitivity, 83%; specificity, 95%)
An intrinsic deflection time greater than 85 ms (sensitivity, 87%; specificity, 90%)
An RS complex duration greater than 121 ms (sensitivity, 76%; specificity, 85%)
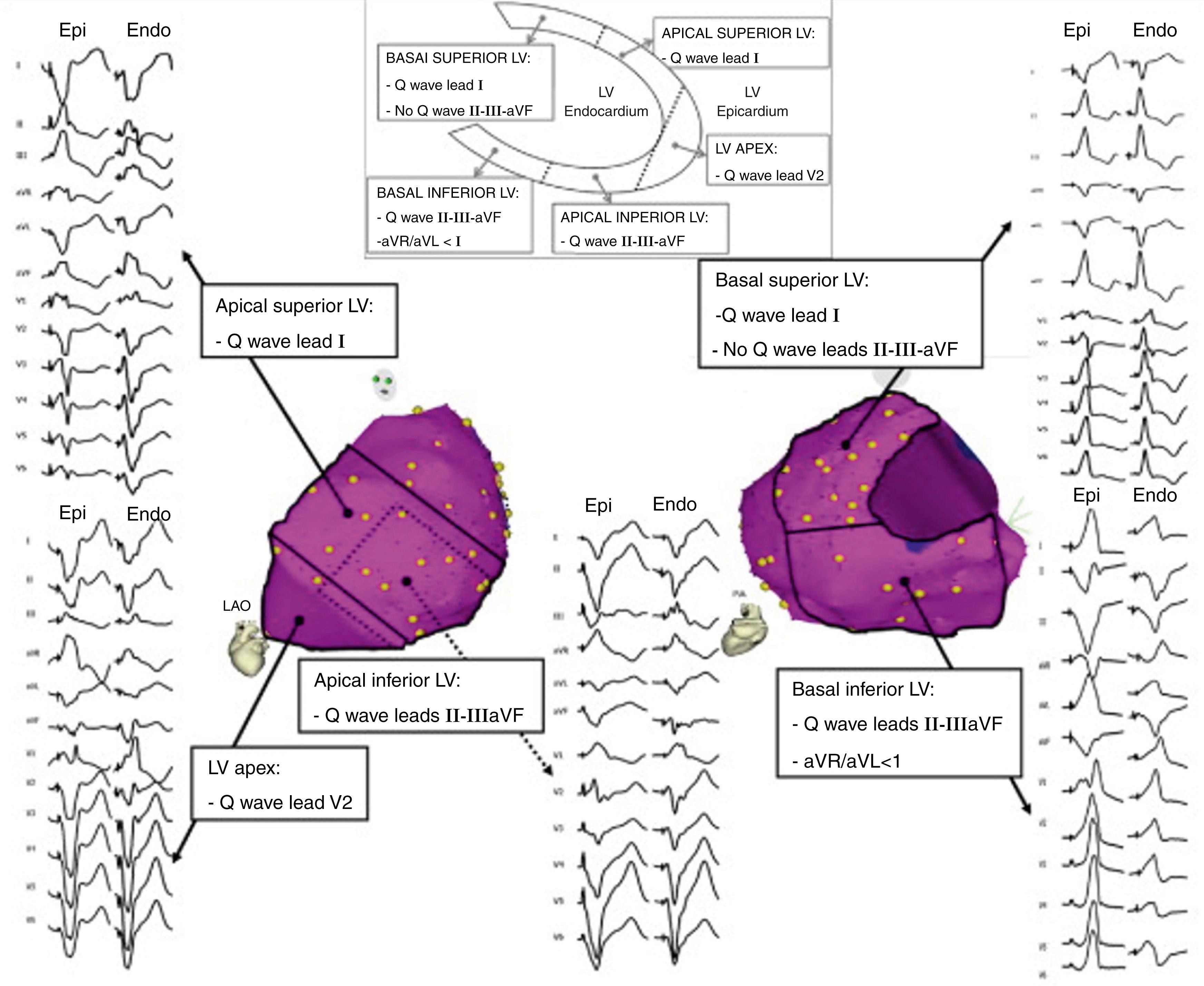
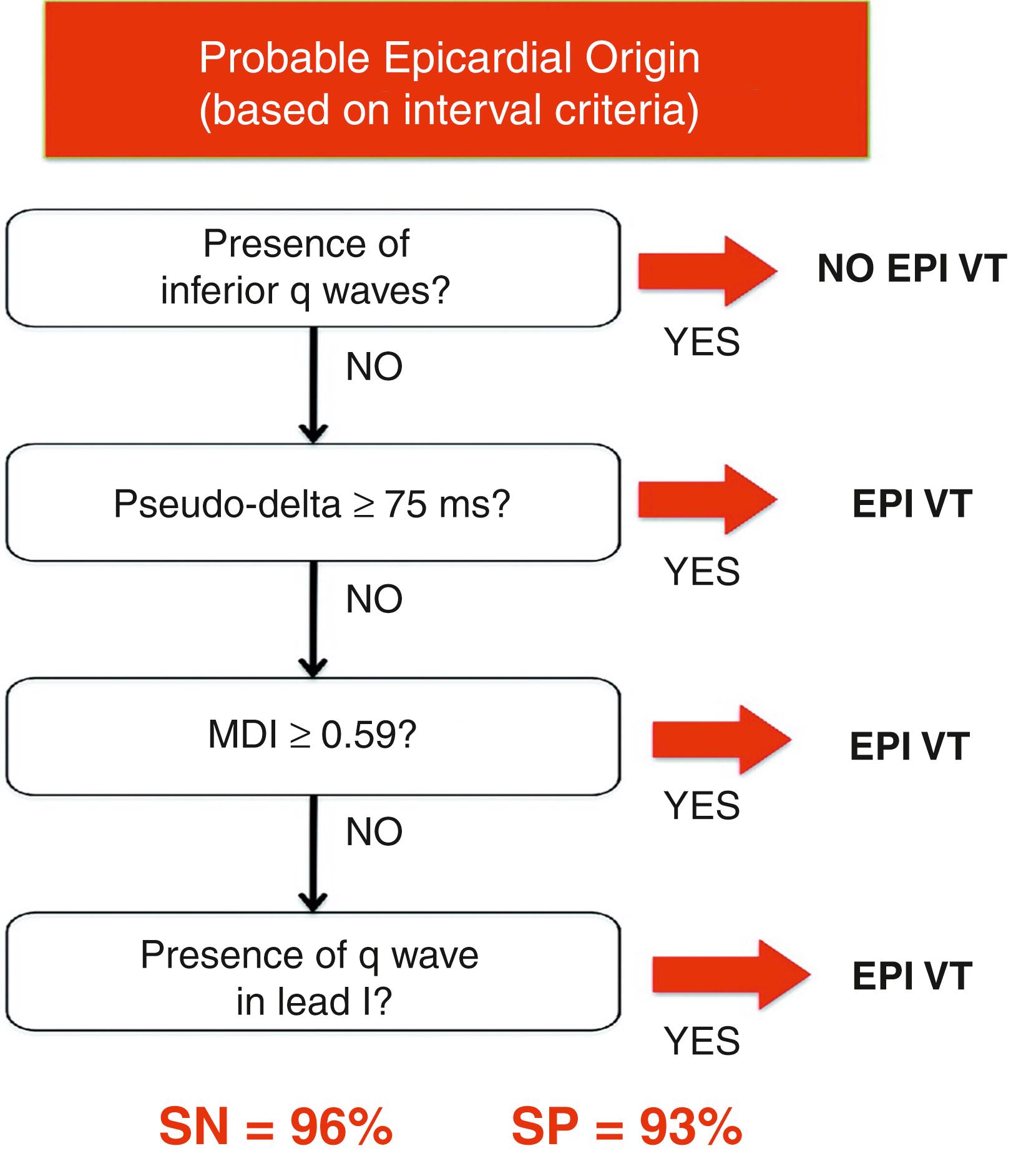
Complementary studies have improved the discriminative ability of such algorithms ( Fig. 134.1 ). For instance, the presence of a Q wave in the inferior leads or in lead I increases the diagnostic specificity for an epicardial source. Marchlinski et al. pooled such criteria and developed an algorithm for use in patients with NICM and VT with a reported 96% sensitivity rate and 93% specificity for an epicardial origin ( Fig. 134.2 ). Nevertheless, other groups have failed to identify reliable ECG criteria to predict an epicardial origin of VT in patients with ICM.
In patients with implantable cardioverter-defibrillators (ICDs), careful analyses of stored electrograms (EGMs) can suggest an epicardial source of VT. With epicardial VT, far-field ventricular EGMs are longer and have longer onset-to-peak durations relative to endocardial VT sources. In ARVC, the relative timing of the far-field (coil to pulse generator) and near-field (RV tip to ring electrode) VT EGMs can be helpful; a far-field QRS onset to near-field EGM interval of less than 30 ms was associated with an endocardial VT origin, whereas an interval of greater than 60 ms favored an epicardial origin.
With the use of gadolinium enhancement, MRI is able to indicate areas of fibrosis with a spatial resolution of approximately 2 mm with high-resolution sequences. Ventricular scar, which is often critical for VT maintenance, can therefore often be localized preoperatively to the epicardium or midmyocardium. These data can be critical when considering the risks and benefits of epicardial access. CT scan with acquisition performed late after contrast injection can also help identify scar.
The location of ventricular scar varies considerably between patients, but certain cardiomyopathies exhibit characteristic patterns. The likelihood of an epicardial substrate can therefore vary based on the underlying etiology, as summarized in Table 134.2 . , , ,
| Study | N | ICM (%) | DCM, Including Myocarditis Sequalae (%) | ARVC (%) | HCM (%) | Idiopathic VT (%) | Chagas Disease (%) |
|---|---|---|---|---|---|---|---|
| Schmidt et al. | 59 | NA | 80 | 83 | NA | 75 | NA |
| Sacher et al. | 156 | 82 | 92 | 100 | NA | 71 | NA |
| Henz et al. | 17 | NA | NA | NA | NA | NA | 82 |
| Sosa et al. | 10 | NA | NA | NA | NA | NA | 100 |
| Dello Russo et al. | 218 | NA | 30 | NA | NA | NA | NA |
| Ueda et al. | 5 | NA | NA | NA | 60 | NA | NA |
| Santangeli et al. | 22 | NA | NA | NA | 59.1 | NA | NA |
| Inada et al | 4 | NA | NA | NA | 75 | NA | NA |
| Dukkipati et al. | 10 | NA | NA | NA | 80 | NA | NA |
| Sarkozy et al. | 444 | 6 | NA | NA | NA | NA | NA |
| Fernandez Armenta et al. | 22 | NA | NA | 90 | NA | NA | NA |
| Haqqani et al. | 18 | NA | NA | 100 | NA | NA | NA |
| Nakahara et al. | 19 | 41 | 83 | NA | NA | NA | NA |
| Cano et al. | 22 | NA | 82 | NA | NA | NA | NA |
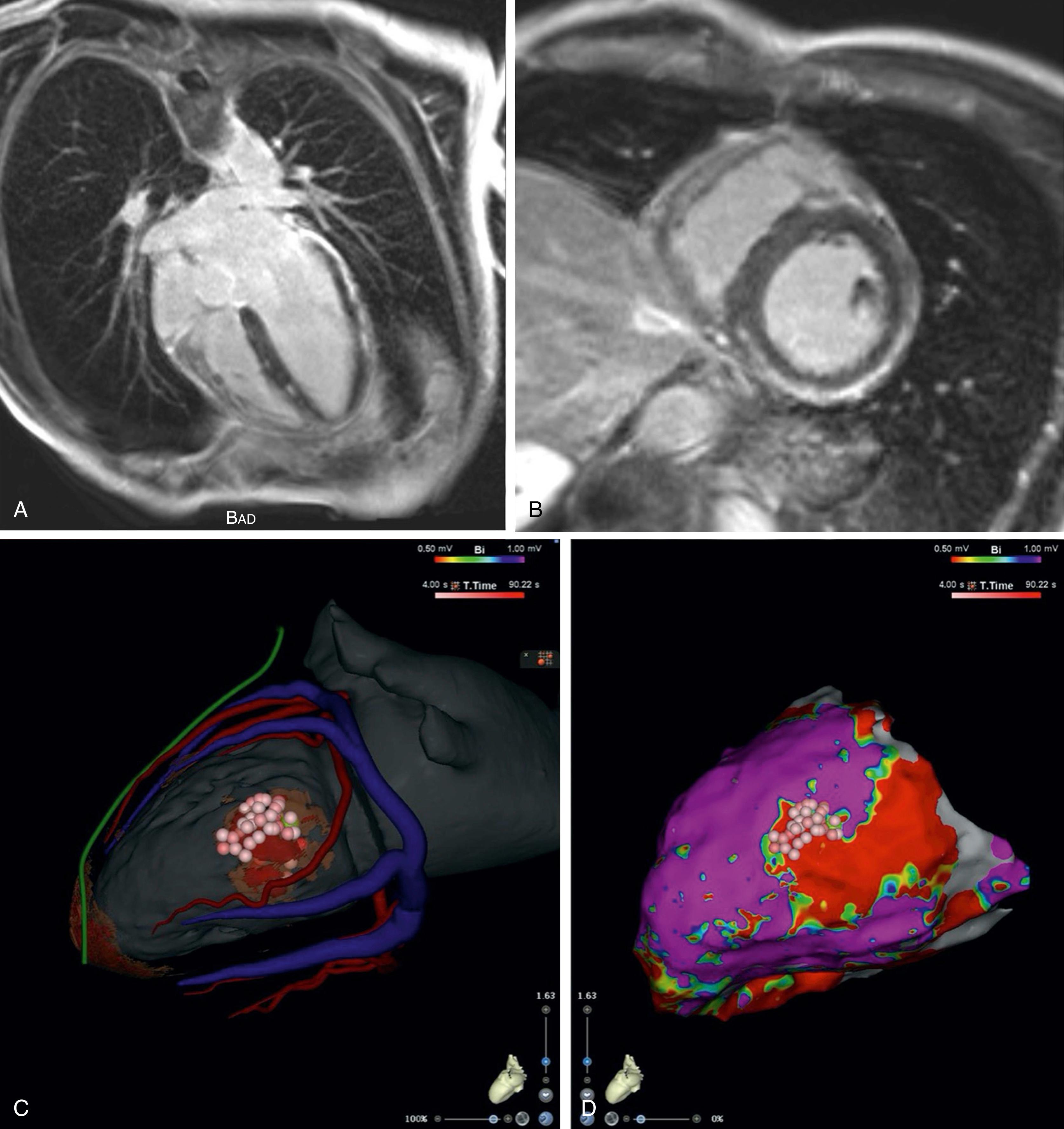
As previously mentioned, epicardial ablations were first described in patients with Chagas disease. In this condition, the myocardium and coronary arteries are affected by a chronic infectious and inflammatory process, which manifests as refractory VT in some cases. Critical VT circuit isthmuses are therefore often best reached epicardially. Accordingly, the addition of epicardial mapping and ablation to the standard endocardial approach in this patient population has increased procedural success. Similarly, in patients with VT attributed to sequelae from previous myocarditis, epicardial access is often required ( Fig. 134.3 ).
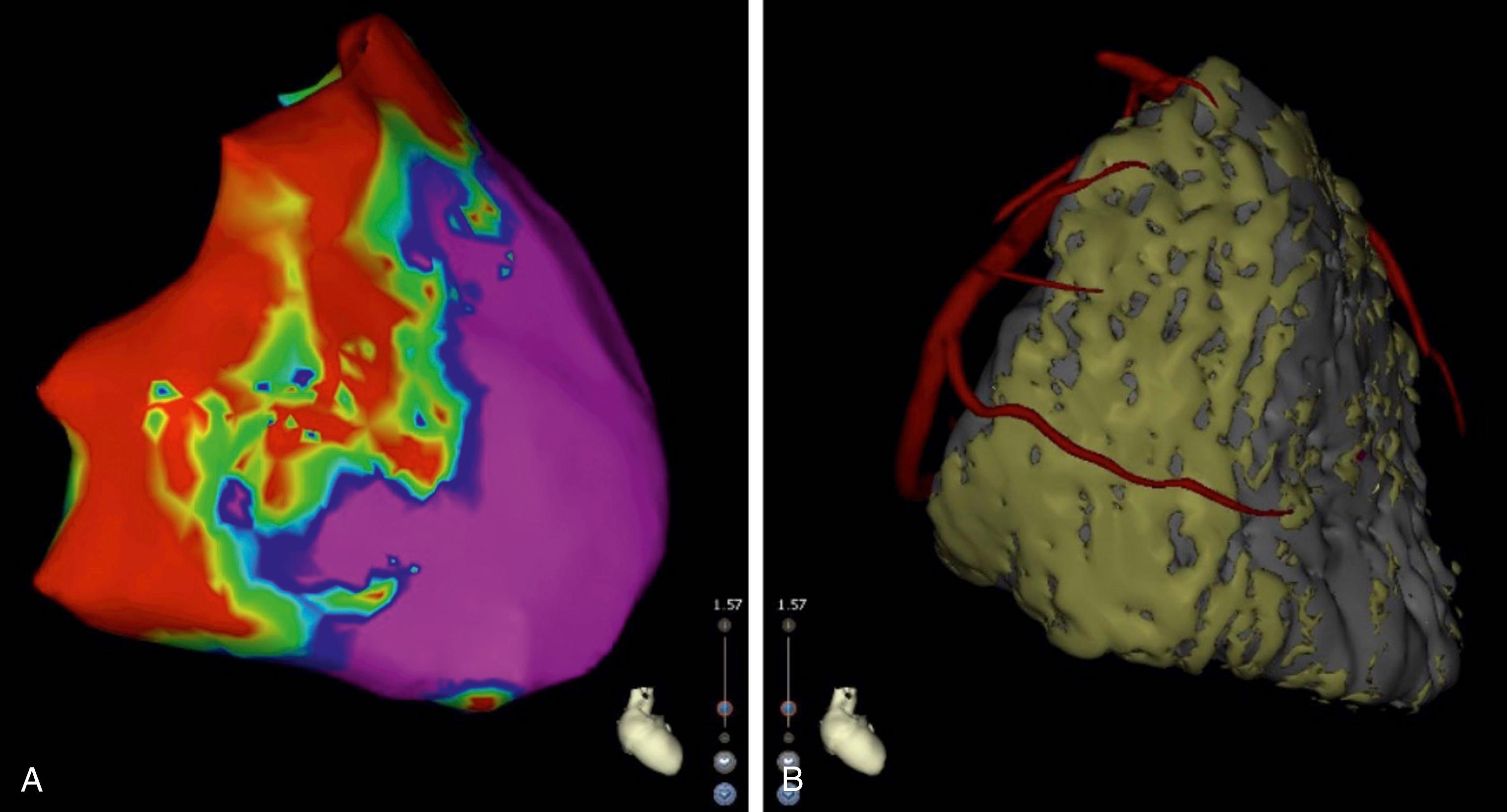
Fernandez-Armanta et al.’s series of 22 patients with ARVC and VT suggested that up to 90% of these patients had a critical epicardial isthmus. Haqqani et al. performed detailed endocardial and epicardial mapping in 18 patients with ARVC, finding evidence that VT circuits could be contained entirely within the epicardium. Consistent with these findings, combined endocardial and epicardial ablation in patients with ARVC has been associated with better outcomes than endocardial ablation alone. , The substrate of interest in this patient population tends to be located at the lateral tricuspid annulus, at the RV free wall, and at the outflow tract ( Fig. 134.4 ). CT imaging can characterize fibrofatty infiltration in this condition with a spatial resolution of 0.3 mm, which is superior to that of MRI.
In ICM, the critical isthmus of the VT circuit is most often found endocardially because the subendocardium is the most vulnerable to ischemia and thus is the first myocardial tissue to be injured during myocardial infarction. Routine epicardial mapping and ablation in ischemic VT is not recommended. Nevertheless, a combined endocardial-epicardial approach may be justified in selected patients when cardiac imaging suggests that the infarct is transmural.
In a series of nine patients with Brugada syndrome and recurrent ventricular fibrillation (VF), Nademanee et al. identified arrhythmia substrate on the epicardial aspect of the RV outflow tract in all cases. Ablation at these regions rendered ventricular arrhythmias noninducible in seven of nine patients and normalized the Brugada ECG pattern in eight. Outcomes at 20 months (±6 months) were excellent, with no recurrent arrhythmia in eight of nine patients off medication. A recent systematic review of ablative strategies for symptomatic Brugada syndrome suggested that epicardial substrate modification was more effective than endocardial-only approaches for the prevention of arrhythmia recurrence.
Among patients with NICM, Tzou et al. noted the following intracardiac EP criteria during VT as predictive of an epicardial origin:
Diffusely early activation (>2 cm 2 area with equally earliest activation [within 10 ms])
Sequence of a far-field EGM followed by a near-field EGM in the region of earliest endocardial activation
Inability to capture the far-field component of the earliest EGM (stim-QRS delay shorter than EGM-QRS delay) or inability to reproduce morphologic features of the VT complex with stimulation at the earliest endocardial site of activation
Moreover, unipolar endocardial voltage has been shown to be well correlated with epicardial bipolar voltage and scar. In the LV, a value less than 8.27 mV has been associated with epicardial scar in NICM. In cases of right-sided VT, a unipolar endocardial voltage of less than 1.66 mV predicts dense epicardial scar (<0.5 mV), although a unipolar endocardial voltage threshold of up to 5.5 mV for RV epicardial scar has been proposed in patients with ARVC.
Very rarely, arrhythmias other than VT or VF have been targeted using epicardial approaches, including accessory pathways (specifically between the right appendage and RV) and resistant arrhythmias, involving the pulmonary veins. ,
Epicardial access and ablation carries a nonnegligible risk for serious complications. Multicenter observational registries have provided clues as to potential relative contraindications, usually because these circumstances render the procedure more technically challenging.
Previous cardiac surgery is often associated with pericardial adhesions, which can hinder catheter maneuverability in the pericardial space and can cause significant bleeding if they are torn. They are more frequently found on the anterior aspect of the LV. Nevertheless, in selected cases, an epicardial approach can still be undertaken as reported by some expert centers. It is our opinion that such cases should only be performed at high-volume centers with expertise with this approach. Surgical access via subxiphoid or lateral thoracic windows to dissect through pericardial adhesions is an alternate option. In contrast, a history of previous percutaneous epicardial access has not been associated with increased risk.
Organomegaly, specifically hepatomegaly or splenomegaly, can increase the risk for inadvertent injuries to these organs during attempted epicardial access.
Morbid obesity can require deeper needle advancement relative to the skin and a modified angle of penetration with the subcostal approach.
Alternatives to consider when percutaneous access is not possible are surgical access via a mini subxiphoid approach when targeting the inferior LV wall, an intercostal lateral thoracic approach when targeting the lateral or anterior LV wall ( Fig. 134.5 ), or thoracoscopy. Furthermore, to target zones of interest that are not readily accessed with the standard epicardial approach, alternative techniques have been proposed, such as endocardial puncture through the right atrial appendage, direct intercostal access, , and puncturing through a distal branch of a lateral coronary vein to inflate the pericardium with CO 2 to facilitate access.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here