Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Please access Videos to view the corresponding videos for this chapter.
Please access Videos to view the corresponding videos for this chapter.
The approaches to the skull base via the paranasal sinuses were introduced in the late 19th and early 20th centuries. Tumors of the sellar region were first approached through various incisions in the forehead to gain access to the sphenoid sinus via the ethmoid sinuses. In 1910, Cushing introduced a sublabial incision combined with a transseptal, transsphenoidal approach that eventually became the gold standard for decades. Coincidentally, on the same day that Cushing implemented his sublabial approach, June 4, 1910, Oscar Hirsch gained access to the sphenoid sinus and sella using an endonasal approach. Eventually, many adopted the endonasal approach, but Cushing’s influence delayed its acceptance for decades. In fact, Cushing’s transsphenoidal approach to the sella turcica was conceptually unchanged for approximately 70 years. However, its logistics operation and outcomes were changed greatly by technological advances, which provided improved visualization, intraoperative image guidance, and instrumentation. Dott, Guiot, and Hardy, respectively, added pneumocisternography and encephalocisternography, radiofluoroscopic guidance, and the operative microscope. All of these revolutionized the surgery, driving its standardization and improving outcomes. A similar technology-driven transformation began in the 1980s with the development of early intraoperative computed tomography (CT) and magnetic resonance (MR) image guidance systems and the introduction of the endoscopic transethmoidal approach by Jankowski, the endoscopic transseptal approach by Sethi, and the endonasal endoscopic approach by Carrau and Jho. , At the same time, otolaryngologists were developing and refining functional endoscopic sinus surgery (FESS). It was the collaboration between otolaryngologists and neurosurgeons that led to the expansion of the endoscopic endonasal transsphenoidal approach to the other areas of the skull base.
The application of rod-lens endoscopy ( Box 50.1 ) to the endonasal transsphenoidal approach has allowed its expansion while improving its visualization. This is due to a basic difference in the optical properties of the endoscope when compared to those of the microscope, namely its ability to deliver light to a surgical target. A microscope visualizes from a distance and delivers a cone of light with its apex at the surgical target. As light travels in a straight line, any structure between the target and the lens of the microscope will obstruct its passage, thus requiring a superficial exposure that is wider than the deep exposure (“ice cream cone” effect) or modifying the angle of visualization to favor one aspect of the field over others. Conversely, an endoscope delivers a cone of light with its apex at the tip of the lens, and can be advanced close to the target, bypassing obstacles that could obstruct the passage of light. This allows a smaller superficial exposure while allowing a much wider working field in the depth (“flashlight” effect). This is a distinct advantage when approaching deep lesions with complex surroundings, such as those involving the skull base and paranasal sinuses. However, the adoption of smaller corridors may impede the introduction and movement of the dissection instruments. Hence, endoscopic visualization can be achieved through minuscule corridors; however, efficient instrumentation requires the amplification of the initial visualization corridor. In addition, modifications of existing and development of new instrumentation have facilitated this process.
Another significant difference between endoscopic and microscopic optics is the loss of three-dimensional visualization. However, this problem is easily overcome using an active, handheld endoscope that continuously moves following the dissecting instruments and providing visual cues such as shadows and perspective. At the same time, the surgeons gain proprioceptive cues by keeping one instrument on or near the object of focus. Three-dimensional awareness is recreated via proprioception and triangulation of the instruments. This is a critical component of the learning curve, but is relatively easy to overcome.
Despite these differences, endonasal extirpative techniques do not differ from the standard microsurgical techniques. Two-handed dissection by the operating surgeon is required; therefore, two surgeons are needed. Identification of critical structures, central debulking of intradural lesions, followed by extracapsular dissection and fine, sharp dissection are all critical components of microsurgery and should be directly translated to endoscopic skull base surgery.
One of the main reasons these approaches have gained acceptance is that many skull base lesions are medial and anterior to the surrounding neurovascular structures. A direct anterior, median approach provides a distinct advantage, as these structures do not have to be displaced or transgressed to access them (i.e., the critical vessels and cranial nerves are at the periphery of the surgical target). This principle guides the selection of an endoscopic endonasal approach (EEA) for the surgical treatment of tumors of the skull base. Vascularity, tumor consistency or fibrosity, and size represent important surgical considerations, but do not constitute contraindications to an EEA.
The key to the development of the endonasal approaches to skull base pathology was the collaboration between otolaryngologists and neurosurgeons. An understanding of the anatomy and surgical intricacies of the sinonasal tract acquired by otolaryngologists melded with the experience and adeptness of neurosurgeons in the surgical treatment of pathologies of the sella and parasellar areas. This was supplemented even further by the proficiency regarding skull base anatomy and techniques learned through the open and microscopic approaches.
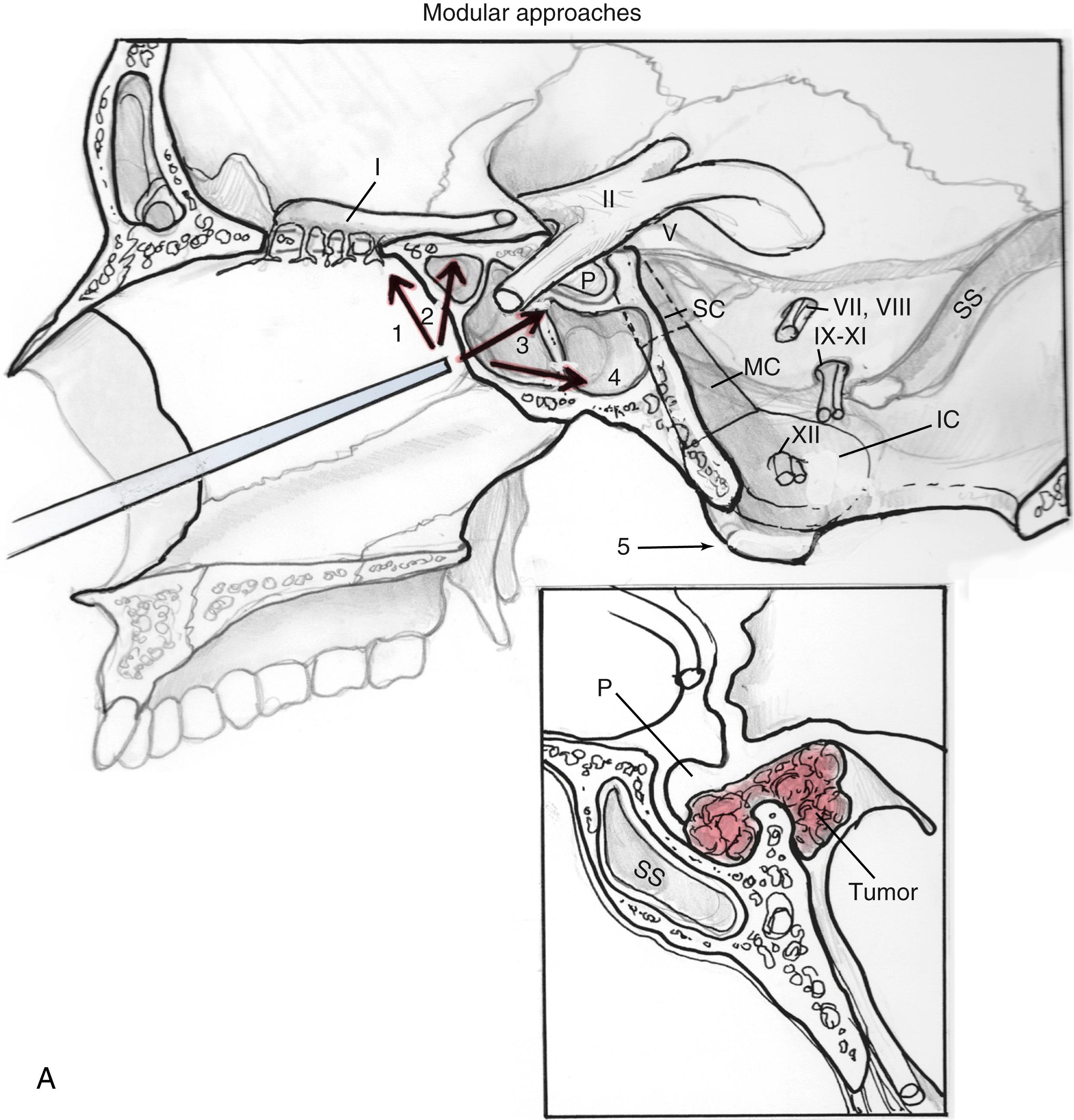
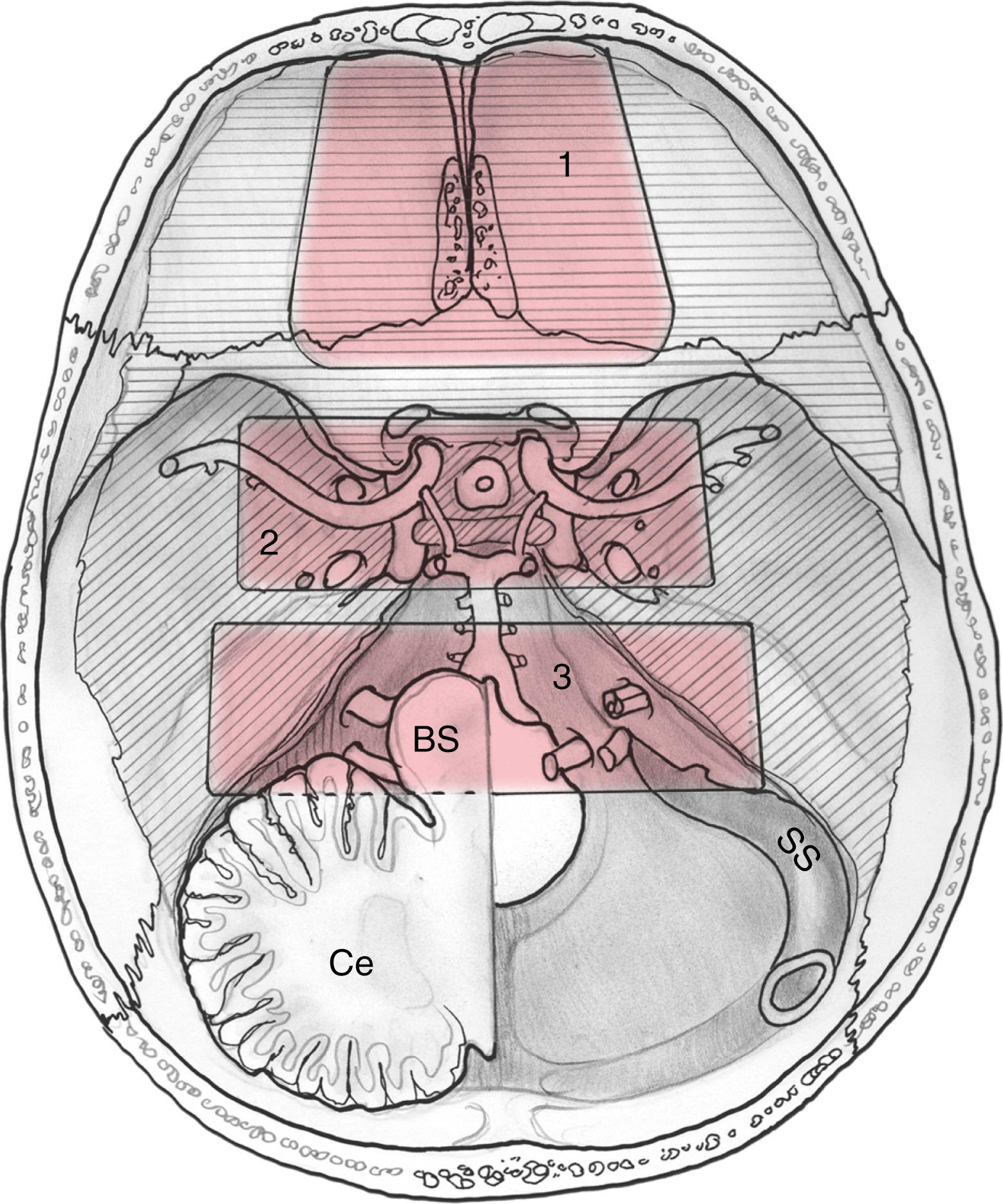
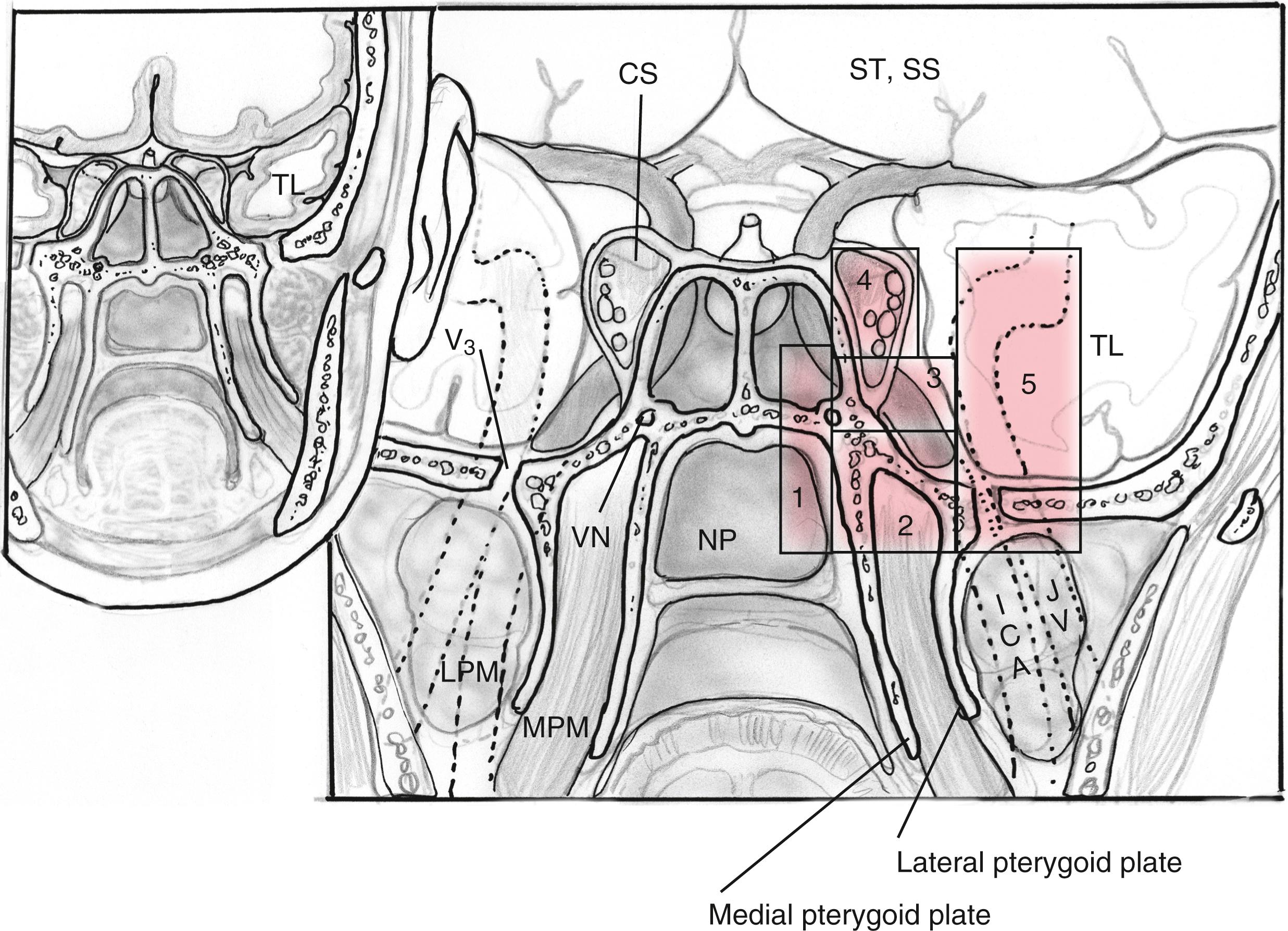
EEAs can be divided according to the geographic location of the surgical target into those that are centered in the sagittal or median planes (between the internal carotid arteries [ICAs]) and those in the coronal or paramedian planes (lateral to the ICAs) ( Box 50.2 ). The sagittal plane can be divided from rostral to caudal (i.e., anterior to posterior) according to the area that comprises the corridor into transfrontal, transcribriform, transplanum or transtuberculum, transsellar, transclival, and transodontoid approaches ( Fig. 50.1 ). The coronal plane is somewhat more complex in that the lateral expanded approaches also vary based upon which fossa is involved ( Fig. 50.2 ). When dealing with lesions in the anterior fossa, the coronal approaches consist of the supra- and transorbital approaches. The middle fossa is the most complex due to the presence of the ICA and is broken, according to the anatomical relationship of the lesion to this critical vessel, into five “transpterygoid” anatomical zones of approach: the petrous apex (posterior to the paraclival ICA), petroclival junction (inferior to the petrous ICA), quadrangular space or Meckel cave (superior to the medial petrous ICA), superior cavernous sinus (lateral to the parasellar ICA), and the infratemporal fossa (inferior to the lateral petrous ICA and anterior and medial to the parapharyngeal ICA) ( Fig. 50.3 ). Finally, the posterior fossa or inferior expanded approaches consist of the transcondylar and parapharyngeal space approaches.
The sagittal plane, also referred to as the rostral-caudal plane, represents the region between the ICA as it rises and courses along the ventral skull base from a caudal to rostral direction as well as the rostral extension of this median region.
The transfrontal approach is the most anterior expanded endonasal approach. A transfrontal approach starts with identification of the nasofrontal recesses and continues with a Draf 3 or endoscopic Lothrop procedure (wide frontal sinusotomies from orbit to orbit with a superior nasoseptal defect and removal of the floor of the frontal sinuses). The anterior attachment of the middle turbinate provides a posterior landmark to preserve olfaction (when a unilateral resection is possible) and avoids the violation of the cribriform plate with resultant cerebrospinal fluid (CSF) leak. A Draf 3 approach can be used in isolation for rare lesions such as nasal dermoid cysts or fibro-osseous tumors and more commonly for frontal sinusitis or mucoceles. In pediatric patients with dermoid cysts, the cyst is drained endonasally, but the septum should be resected up to the skull base. The transfrontal approach is also used in conjunction with the transcribriform approach for accessing the anterior extent of lesions such as olfactory groove meningiomas. A key anatomic landmark for this region is the frontoethmoidal recess.
The transcribriform approach is a commonly used approach for anterior skull base (ASB) tumors, such as olfactory groove meningiomas and esthesioneuroblastomas. In addition, it is frequently used for the repair of posttraumatic and iatrogenic CSF leak repairs.
A complete sphenoethmoidectomy is performed bilaterally and the nasofrontal recesses are exposed. A large exophytic tumor within the nasal cavity can be debulked to improve the instrumentation space and to better ascertain the origin and extension of the tumor. The superior nasal septum is transected, extending from the crista galli to the sphenoid rostrum, as needed. As mentioned earlier, a transfrontal approach can be performed to establish the anterior margin. The transcribriform approach provides direct access to the vascular supply of tumors such as olfactory groove meningiomas, allowing early devascularization. The anterior and posterior ethmoidal arteries should be identified early and cauterized or clipped. The lateral bony margins are drilled to form “gutters” or osteotomies spanning the length of the tumor or planned resection. If necessary, the lamina papyracea can be removed as well to allow the lateral displacement of the periorbital and orbital contents to enhance the exposure of the orbital roof. This can extend all the way to the mid-orbital line at the level of the superior rectus muscle. After the lateral margins are drilled, the planum or tuberculum can be drilled as needed to establish the posterior margin. To avoid accidental trauma, it is safest to preserve the bone of the optic canals intact as long as possible during the resection. Finally, the dura is dissected from the crista galli to allow its removal. The remaining bone of the cribriform plate can now be dissected free ( Fig. 50.4 ). For benign extradural lesions, the dura may be left intact. This may not be possible over the olfactory filaments, which often have an accompanying sleeve of meninges. The olfactory filaments can be cauterized to minimize CSF leakage, but should nonetheless undergo formal repair (see later). Obviously, olfaction (when present) is sacrificed if a bilateral approach is undertaken.
The dura is opened at the lateral extent of desired resection using a fine blade and scissors extending the durotomies back bilaterally. This is done to minimize bleeding from branches of the anterior falcine artery and the inferior sagittal sinus and for better control of the frontoorbital and frontopolar arteries. Horizontal durotomies at the most posterior and anterior end of the resection are extended to the midline (i.e., falx cerebri). The falx and inferior sagittal sinus must be systematically addressed next. Pistol-grip, endonasal, and bipolar electrocautery are used with one blade on either side of the falx and sinus to coagulate it before transecting it to allow the release of the tumor and dura anteriorly. The anterior falcine branches may be encountered during tumor resection for access to the falx; their cauterization provides additional tumor devascularization. Dura can be resected en bloc as needed for tumors such as esthesioneuroblastoma. Histopathological analysis of the surgical margins is requested both intra- and postoperatively.
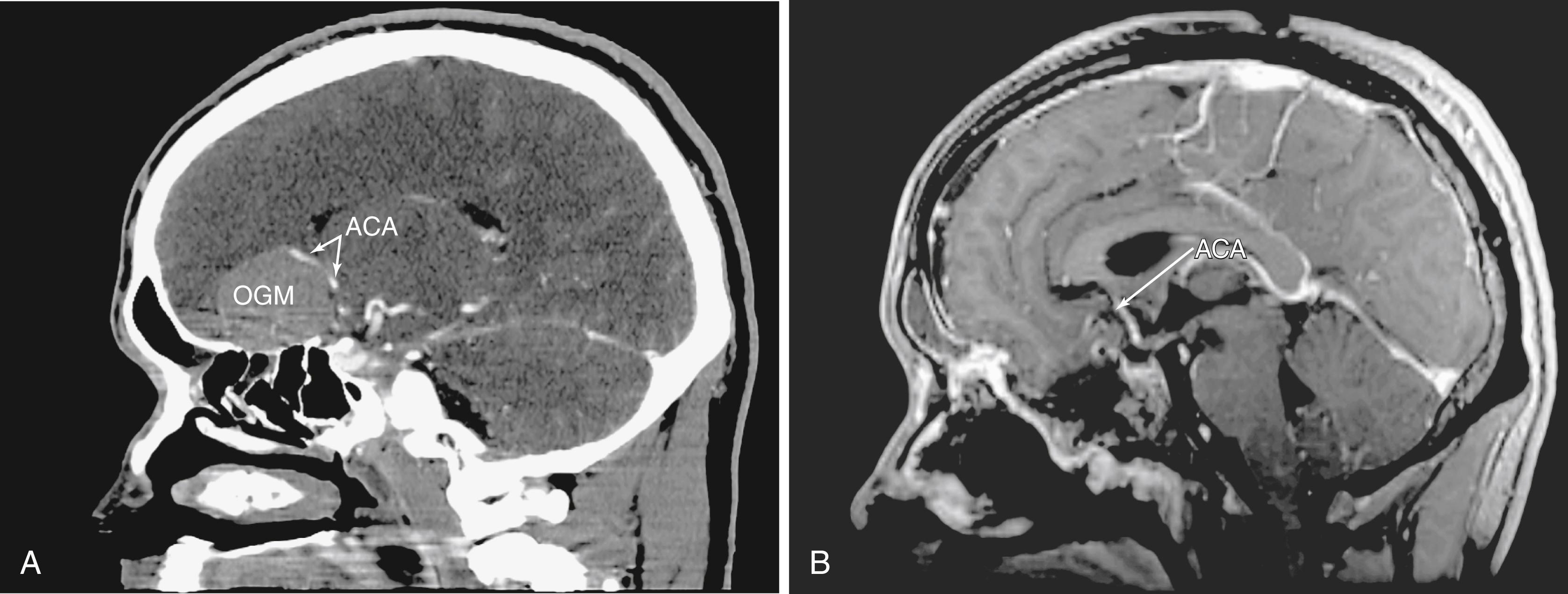
Intradural tumor resection must be performed with caution, especially when approaching the interhemispheric fissure, as one will frequently encounter the branches of the anterior cerebral artery or even the anterior communicating artery on the surface of the tumor ( Fig. 50.5 ). An endonasal ultrasonic aspirator or two suctions can be used depending upon the tumor consistency and proximity of involved structures. Microsurgical principles, including the preservation of the arachnoid planes (when possible) and the sharp dissection of critical structures, are followed throughout the surgery. Visualization may require the use of angled endoscopes (45 or 70 degrees).
Following a logical progression, the transplanum approach was the first expansion of a traditional transsphenoidal approach. This approach provides a natural corridor for suprasellar tumors such as craniopharyngiomas, tuberculum sella meningiomas, and large pituitary adenomas extending to the anterior cranial fossa. It may also be used to biopsy or resect infundibular lesions such as metastases or hypophysitis. This approach is often an integral addition to the transsellar and transcribriform approaches.
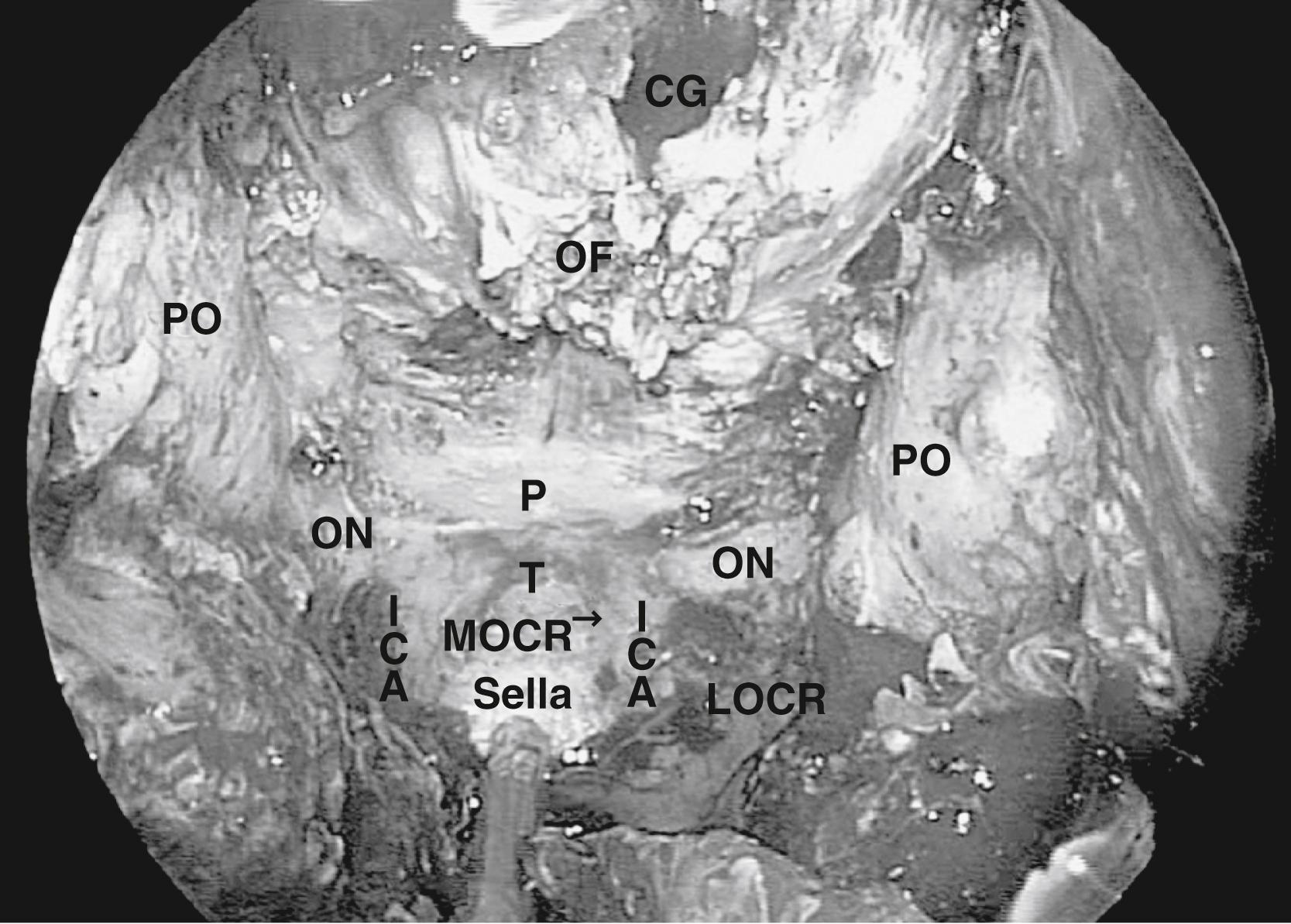
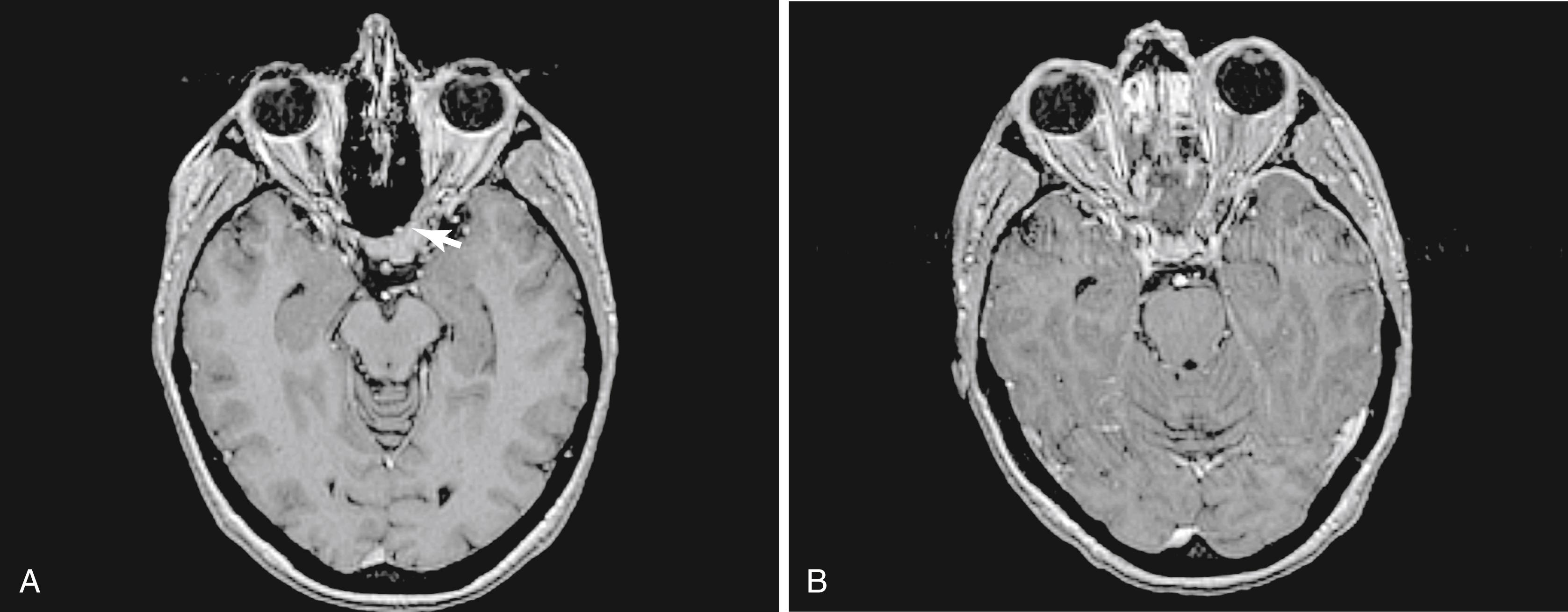
A transsphenoidal exposure (see “ Transsellar Approach ”) is augmented by a posterior ethmoidectomy. The posterior ethmoidal arteries, located just in front of the sphenoid sinus rostrum, are a good landmark to indicate the anterior extent of the transplanum approach. Respect of this boundary helps to preserve olfaction (i.e., the cribriform plate is anterior to the posterior ethmoidal arteries) while providing adequate access. The lateral margins are the optic nerves that form the sides of a trapezoid, which include the anterior planum and tuberculum sellae. The optic nerve canals must be identified to avoid damage to the nerves, which do not need to be exposed in the majority of patients. Whenever drilling over or near the optic canals, it is important to irrigate constantly to avoid heat transmission from the drill to the nerves. After the sella is exposed, the planum can be drilled until it is thin and somewhat mobile (it can be displaced with a gentle push). This can require an angled endoscope for adequate visualization depending upon the slope of the ASB. Although somewhat counterintuitive, the planum is easiest to remove in an anterior to posterior direction after the bone has been adequately thinned, exposing the dura at the anterior extent and laterally. Exposure of the optic nerves is not needed in the great majority of patients. However, in many tuberculum meningiomas, there is an extension of the disease into the medial optic canals ( Fig. 50.6 ). In these patients, the bone overlying the tumor in the optic canal should be thinned (blue-lined) with a drill and carefully removed with a dissector. This extension of the disease is not easily accessed transcranially, which would require the release and retraction of an often already compromised optic nerve.
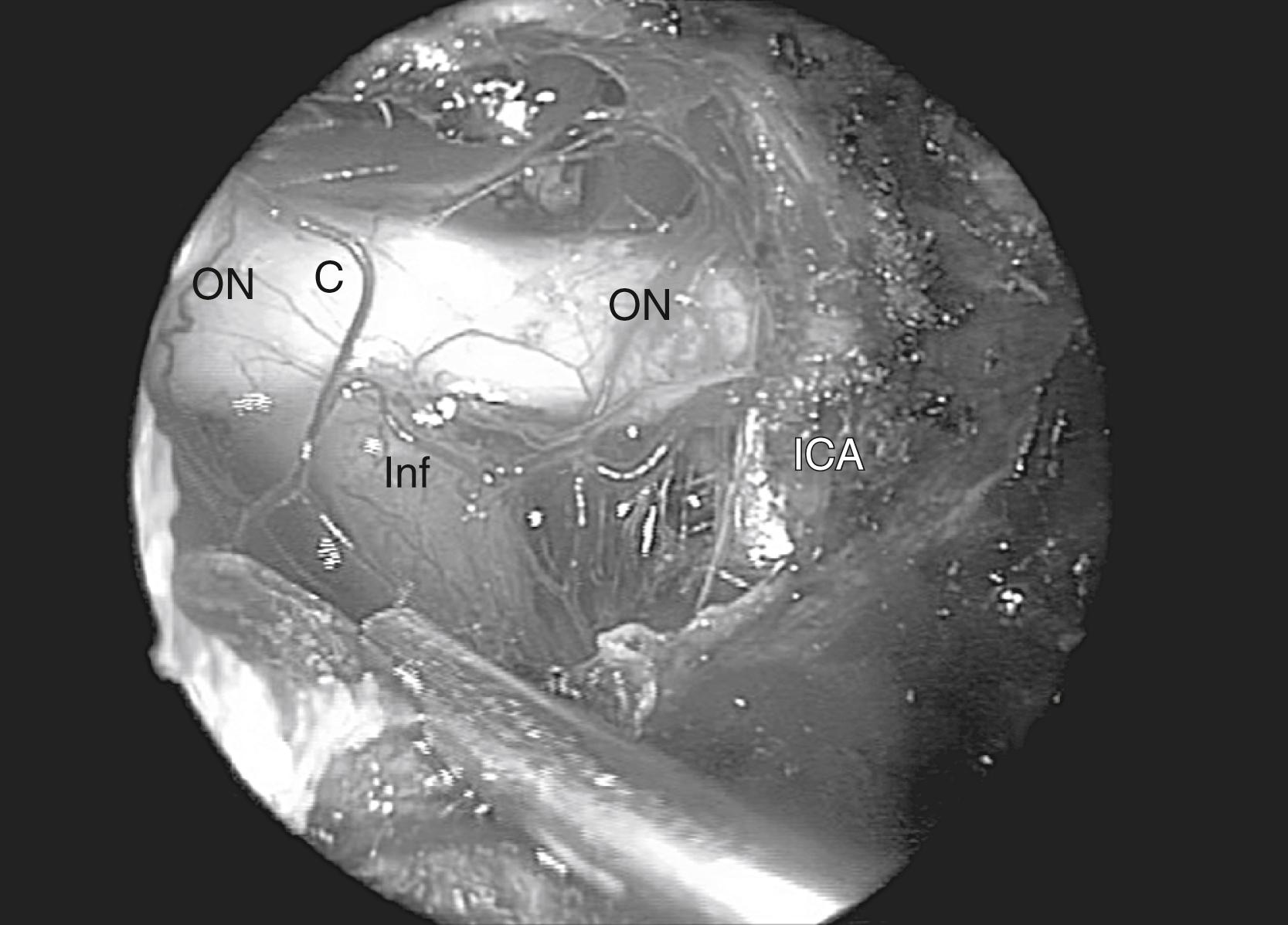
When approaching suprasellar lesions, the superior intercavernous sinus (SIS) can often merely be retracted inferiorly rather than being transected. If, however, it must be traversed, it is useful to open the dura of the sella below the SIS in the midline as well as the dura of the planum above the SIS. This provides access on both sides of the sinus to perform a controlled ligation, with either a bipolar or clips. Alternatively, its bleeding can be controlled using hemostatic paste. The dura can be reduced bilaterally with bipolar coagulation, taking precautions not to damage the optic nerves. In addition, one must not coagulate the superior hypophyseal artery (SHA), because this can lead to hypopituitarism. Once the dura is opened, the tumor resection proceeds carefully, systematically identifying critical structures. First, the supraclinoid ICA is identified and the resection proceeds until the ipsilateral optic nerve is identified. This is followed by the identification of the chiasm, then of the contralateral optic nerve and supraclinoid ICA ( Fig. 50.7 ). In order to gain direct and unencumbered access to the paraclinoid ICA, it is imperative to remove the medial opticocarotid recess (mOCR); this represents the lateral extension of the tuberculum. The mOCR is the key anatomical landmark for this module.
The transsellar approaches are well known for enabling access to pituitary adenomas as well as the myriad of sellar pathologies, such as Rathke cleft cysts (RCCs), arachnoid cysts, and rare craniopharyngiomas. In addition, by using a pituitary transposition, retroinfundibular lesions, such as granular cell tumors and some craniopharyngiomas, can be easily accessed while potentially preserving the pituitary function. The endoscopic approaches to the sella are different from standard microscopic approaches in that, although they require more exposure, they provide more lateral access. Tumor that extends into the medial cavernous sinus can be accessed effectively using an EEA ( Fig. 50.8 ). Most often, this is achieved by following the path of the tumor where it has transgressed the medial wall of the cavernous sinus. For extensive and anterior extension, the bone overlying the cavernous ICA, which guards the medial cavernous sinus, must be carefully thinned and removed to allow some displacement of the ICA.
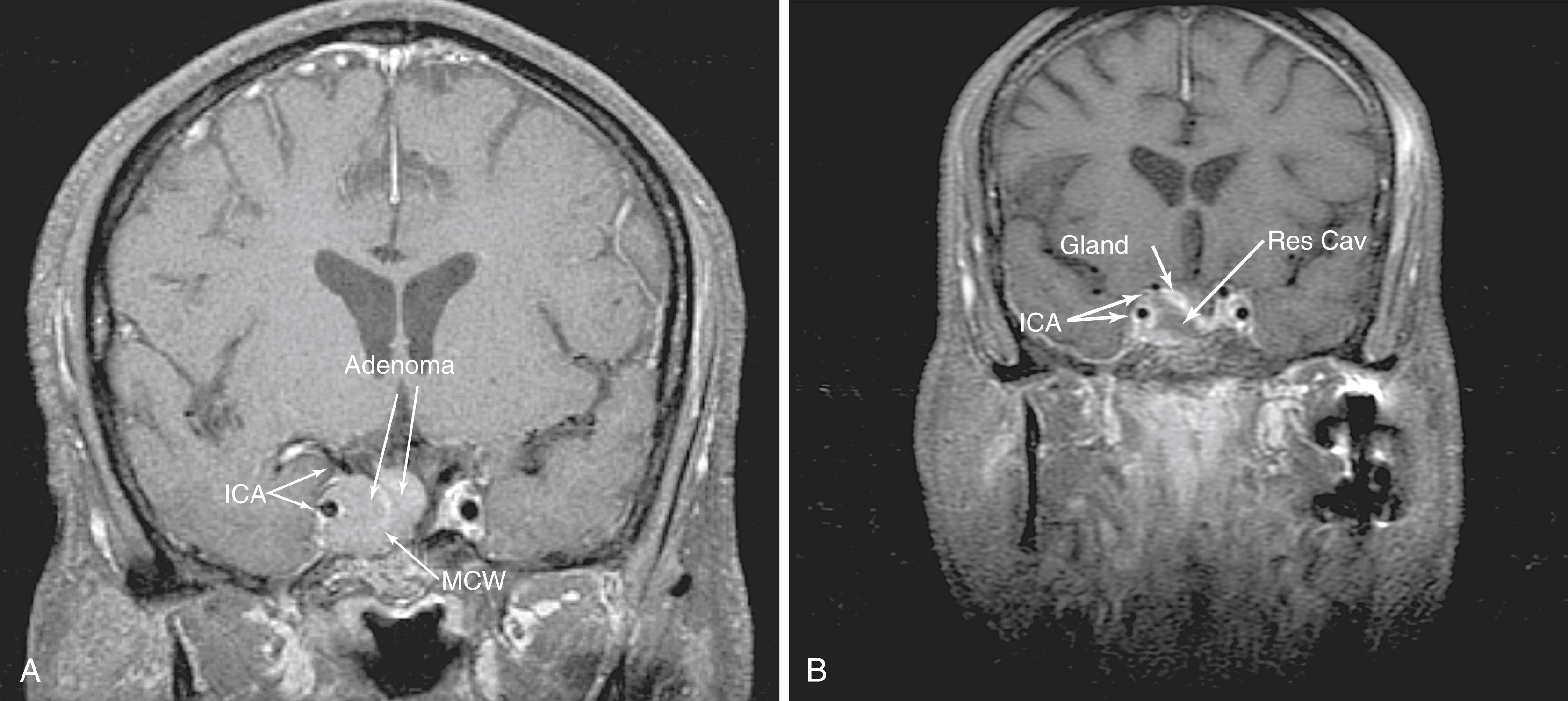
Greater exposure is required because room must be made for instruments to work around the endoscope while maintaining visualization. As a result, the sphenoid must be opened completely to work unencumbered in the sella. For example, lesions such as RCCs, which are commonly in the pars intermedia, can be approached from below, under the anterior gland (subsellar approach) rather than through it. This requires drilling the floor of the sphenoid sinus flush with the clival recess to allow this inferior access and visualization that the endoscope provides. The guiding anatomic principle for this module is complete bony removal and exposure of the sella from “blue to blue”; that is, laterally from cavernous sinus to cavernous sinus and from the SIS superiorly to the inferior intercavernous sinus (IIS) inferiorly.
The transclival approaches may be easier to understand if divided into the superior, middle, and inferior clival approaches. The superior clivus extends from the posterior clinoid superiorly to the Dorello canal inferiorly. The middle clival segment continues from the Dorello canal down to the jugular foramen and the lower third extends from the jugular fossa to the basion. The superior clival approach is usually combined with a transsellar approach and often involves a pituitary transposition (see later) and posterior clinoidectomy. The middle clival approach essentially removes the bulk of the clivus and exposes the midbrain with its own potential pitfalls and nuances. The inferior clivectomy provides access to the foramen magnum.
The pituitary transposition serves to access lesions directly posterior to the pituitary gland or infundibulum, such as retroinfundibular craniopharyngiomas, granular cell tumors, and chordomas. In addition, clival lesions, such as chondroid tumors (e.g., chordomas and chondrosarcomas), often involve this portion of the clivus ( Fig. 50.9 ). Although conceptually simple, this technique is technically demanding. The pituitary gland is dissected free from the sella anteriorly and laterally and lifted with the posterior dura to allow access to the space just behind it. The bands of fibrous connective tissue that anchor the gland to the cavernous sinus along the lateral walls must be sharply dissected to free and preserve the gland. There is often some venous bleeding that can be controlled with oxidized methylcellulose, gelatin sponges, or hemostatic paste. The inferior hypophyseal arteries may need to be sacrificed. In our experience, as long as the SHAs are preserved, gland function will be preserved. After all the lateral connective bands are released, the aperture in the sellar dura through which the stalk enters should be opened to release the stalk for transposition. This should be done carefully with a fine scissors with visualization of the SHAs. At this point, the gland can be finally raised from the sella and held superiorly with fibrin glue. This gives direct access to the dorsum sellae and posterior clinoids, which can then be drilled and removed for access to the interpeduncular and basilar cisterns. There is more brisk venous bleeding during this drilling, as the clival venous plexus extends into the clinoids and dorsum sellae. This tight space, surrounded by critical structures with often brisk bleeding, requires an experienced team for a safe and controlled approach. In our opinion, this may be the most complex EEA procedure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here