Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work is supported in part by grants from the US Public Health Service (HL133127, HL108037, and HL081707) and the American Heart Association (18SFRN34230125).
A major challenge in prescribing antiarrhythmic drugs is the occurrence of drug-induced ventricular arrhythmias that can lead to syncope and sudden cardiac death. In recent years, an increasing number of cardiac and noncardiac drugs have been identified that cause proarrhythmia, defined as the generation of new or worsened arrhythmias during drug therapy. It is critical to recognize proarrhythmia, given that it is a reversible cause of life-threatening ventricular arrhythmias. The epidemiology of this adverse effect has been poorly understood given a reliance on the reporting of anecdotal cases, but an increased risk of sudden cardiac death has also been identified as a first clue for harm during therapy for specific drugs. From a historical standpoint, proarrhythmia has had a major effect on the pharmaceutical industry, with the development of a drug class halted in some cases. Moreover, drug-induced ventricular arrhythmias have been a major reason for drug withdrawal from the US market in recent decades.
The concept of proarrhythmia represents an extreme example of interindividual variability in drug response. There is an improved recognition of pharmacokinetic and pharmacodynamic mechanisms that lead to its occurrence, encompassing drug interactions, genetic variants, and clinical risk factors. Specific proarrhythmic syndromes have been identified, each with a distinct underlying mechanism. Typically, proarrhythmic drugs modify the same ionic currents that are implicated in congenital causes of a specific arrhythmia. The optimal approach to therapy is to recognize risk factors a priori to avoid treating high-risk patients, to recognize proarrhythmia when it occurs for prompt discontinuation of the offending agent, and to use therapy that is selective for the specific proarrhythmic syndrome.
Both pharmacokinetic and pharmacodynamic risk factors contribute to the development of proarrhythmia ( Table 102.1 ). Pharmacokinetics describes the absorption, distribution, metabolism, and excretion of drugs in vivo, processes that are highly regulated by tissue-specific expression of specific drug-metabolizing and drug transporter molecules. Elevated plasma concentrations can promote exaggerated electrophysiologic effects that increase the likelihood of proarrhythmia, and this scenario can occur through several mechanisms. , First and most obviously, drug overdose can lead to toxic concentrations. Second, dysfunction of the organ responsible for drug elimination can lead to supratherapeutic levels. Thus drugs such as sotalol and dofetilide that are renally excreted cannot be used safely in the setting of significant renal disease. Third, drug interactions can alter pharmacokinetic properties to cause elevated plasma concentrations, particularly in patients exposed to polypharmacy. A high-risk scenario occurs with an electrophysiologically active drug that has a single route of elimination, such as a specific CYP enzyme, drug transporter, or the kidneys, can be inhibited by other medications or disease.
| Pharmacokinetic | Pharmacodynamic | |||
|---|---|---|---|---|
| Gene | Potential Effect | Gene | Potential Effect | |
| Digitalis toxicity | MDR1 | Polymorphisms linked to increased blood level | RYR2, CASQ2, ANK2 | Loss-of-function variants may predispose to digitalis-mediated arrhythmias |
| Torsades de pointes | CYP2D6 | Poor metabolizer at increased risk for thioridazine-related torsades de pointes | KCNQ1, KCNH2, KCNE1, SCN5A | Subclinical congenital long QT syndrome mutations predisposed to torsades de pointes |
| SCNA S11037 | Y allele confers increased risk in African Americans | |||
| SCN5A R1193Q | Q allele confers increased risk in Asians | |||
| Sodium channel blocker toxicity | Poor metabolizer at increased risk for flecainide-related adverse effects; ultrarapid metabolizer at increased risk for encainide-related toxicity | SCN5A | Ventricular fibrillation during drug challenge in patients with Brugada syndrome | |
A notable example of this is the antihistamine terfenadine. Drug-induced torsades de pointes (TdP) was considered an unusual curiosity, but it gained major attention when reported in a patient taking terfenadine and the CYP3A4 inhibitor ketoconazole. At the time, terfenadine was widely used with an excellent safety profile, and it was being considered for over-the-counter marketing. Terfenadine is a potent QT-prolonging agent that is normally extensively metabolized by CYP3A4 to fexofenadine, which possesses antihistaminic and no electrophysiologic effects. However, CYP3A is subject to inhibition by a wide variety of drugs, including macrolide antibiotics, azole antifungals, antiretrovirals, Ca 2+ channel blockers, and grapefruit juice. As additional cases were reported, and the indication for terfenadine was not life-threatening, the drug was ultimately withdrawn from the market and replaced by fexofenadine (Allegra). This scenario was repeated with the antihistamine astemizole, the gastroprokinetic agent cisapride, and multiple antibiotics, and drug-induced long QT syndrome (DiLQTS) became a leading cause for withdrawal or relabeling of drugs in the United States over the ensuing decade. This recognition prompted regulatory changes in the drug development process within the United States and Europe, mandating studies to determine whether a new investigational drug blocked a specific K + current known as I Kr , which is targeted by numerous drugs, or prolonged the QT interval prior to human studies.
Another example is digoxin, which undergoes renal and biliary excretion by P-glycoprotein. This drug efflux pump is subject to inhibition by multiple agents that also inhibit CYP3A, including quinidine, amiodarone, and dronedarone, and cotherapy with these agents can lead to elevated plasma concentrations and digoxin toxicity. As a third example, propafenone is eliminated by CYP2D6, which has genetically determined activity. In approximately 7% of whites and African Americans homozygous for loss-of-function DNA variants, termed “poor metabolizers,” the parent drug accumulates while side effects, including bradycardia, are increased compared with “extensive metabolizers.” Multiple antipsychotics (notably thioridazine) are also eliminated by CYP2D6, and poor metabolizers are at increased risk of drug-induced proarrhythmia (see Table 102.1 ). CYP2D6 activity can also be inhibited by drugs such as quinidine and antidepressants (e.g., fluoxetine, paroxetine, and bupropion), and cotherapy with these agents essentially renders the patient a poor metabolizer with increased proarrhythmic risk. Flecainide undergoes dual elimination by CYP2D6 and renal excretion. In patients who are poor metabolizers, the onset of renal failure can lead to excessive plasma concentrations and flecainide toxicity.
For a given drug concentration, the clinical effects that are observed can vary widely among individuals, and characterization of this variability is termed pharmacodynamics. Variability can occur in the amount or function of the drug target, or there can be variability in the biologic milieu in which drug target interactions take place. It is well recognized that patients with DiLQTS can harbor mutations in the genes responsible for the congenital form of the disease, including KCNQ1, KCNH2, SCN5A, and KCNE1 (see later). SCN5A variants that are more common in specific populations (S1103Y in African Americans and R1193Q in Asians) are linked to DiLQTS as well. Similarly, a number of drugs that block cardiac Na + channels can cause the transient appearance of Brugada-pattern ST elevation on the electrocardiogram (ECG) that is otherwise absent. Approximately one-third of Brugada patients have mutations in SCN5A, but in the remainder, mutations in rhythm-related genes cannot be detected. Although other genes have rarely been implicated in this syndrome, the clinical relevance of these mutations has not been clearly validated.
In addition to genetics, other risk factors for drug-induced ventricular arrhythmias are pharmacodynamic in nature. For TdP, these include female sex, bradycardia, heart failure and left ventricular (LV) hypertrophy, electrolyte abnormalities, and others as described in the following sections. Each of these patient-specific factors increase risk for QT prolongation. Similarly, factors that promote reentry increase risk of Na + channel blocker toxicity, including conduction slowing, myocardial scar, and ischemia.
It was recognized in the 1920s that the initiation of quinidine therapy could occasionally lead to syncope. However, it was not until the advent of continuous electrocardiographic monitoring in the 1960s that Selzer and Wray identified polymorphic ventricular tachycardia (VT) as the etiology of this event. In 1966, the French cardiologist Dessertenne described a case of polymorphic VT in the setting of complete heart block, which he termed torsades de pointes, or “twisting of points,” referring to the pattern of a military braid ( Fig. 102.1 ). After subsequent descriptions of the congenital long QT syndrome, it was recognized that this drug-induced arrhythmia was also linked to QT prolongation.
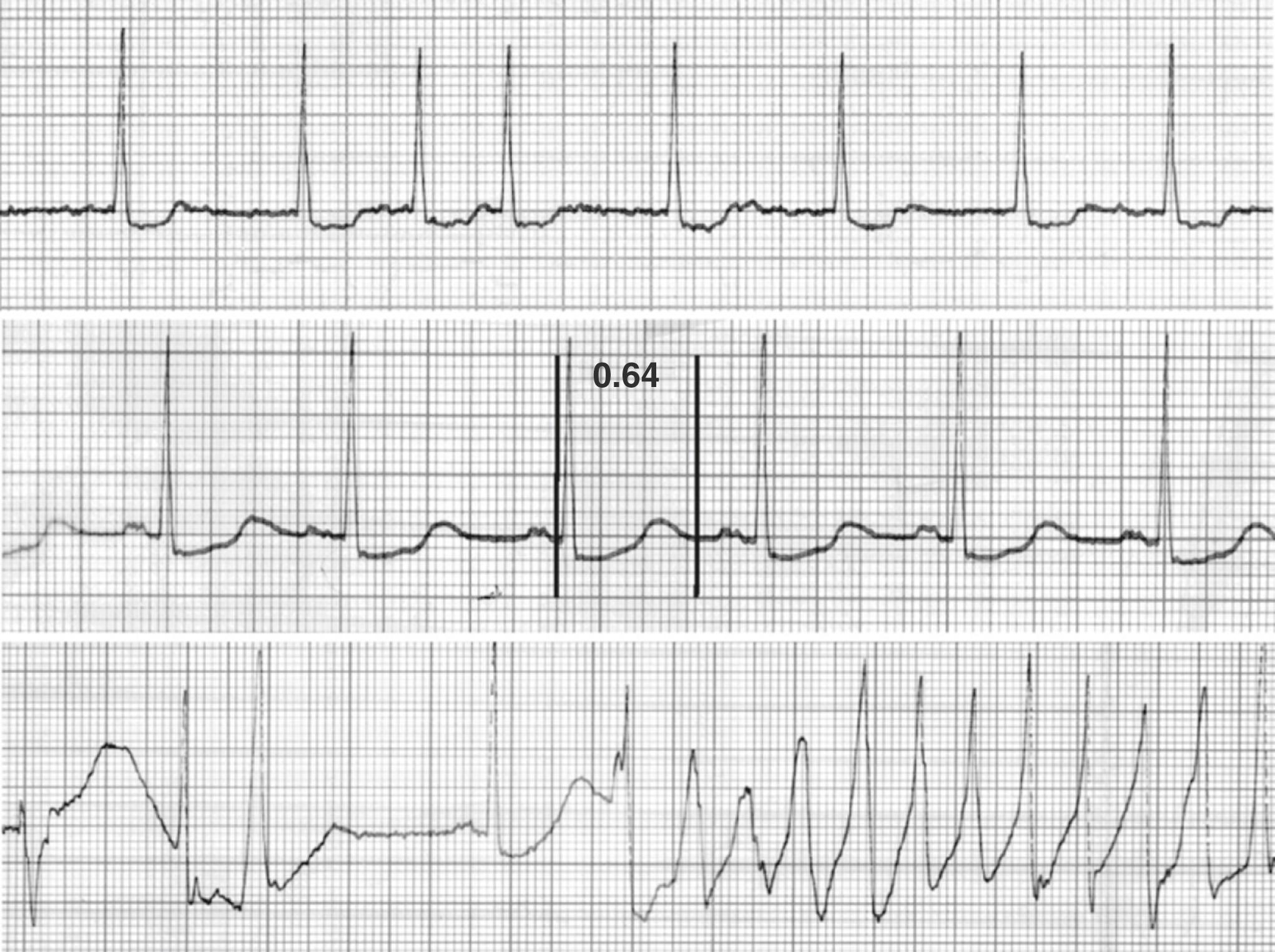
Although patients may be asymptomatic on presentation, development of TdP can lead to palpitations, presyncope or syncope, seizure-like activity, or a cardiac arrest. After puberty, the 99th percentile QTc values are 470 ms in males and 480 ms in females on the 12-lead ECG. Values greater than this are consider prolonged, and a QTc interval greater than 500 ms is distinctly abnormal. In general, the longer the QT interval is, the greater the risk of TdP. T wave flattening with prominent U waves is often seen, whereas T wave alternans is more unusual. Significant dispersion of repolarization, or the difference between the longest and shortness QT intervals on the 12-lead ECG, is often present. However, the spatial difference between the T wave peak to end interval divided by the QT interval (Tpe) appears to be superior in predicting TdP. Physiologic repolarization is greatest at long R-R intervals. In addition, QT-prolonging antiarrhythmic drugs exert their greatest effects at slow rates (or after a pause), termed reverse-use dependence. The typical initiation sequence for TdP is a premature beat, followed by a pause, with the subsequent beat having a markedly prolonged QT interval that is interrupted by a premature ventricular contraction (PVC) triggering the arrhythmia (i.e., a short-long-short pattern [see Fig. 102.1 ]). Bursts of polymorphic VT are usually self-terminating, but occasionally they can degenerate to sustained VT or ventricular fibrillation (VF). During prolonged episodes, the arrhythmia can intermittently appear monomorphic.
The onset of DiLQTS typically occurs early after the initiation of drug therapy, but it can also occur during long-term administration (i.e., after a dose increase or development of additional risk factors outlined in the following).
Prolongation of action potential duration (APD) can occur with either a reduction in outward or an increase in inward currents. , Not surprisingly, congenital long QT syndrome mutations in KCNQ1, KCNH2, and SCN5A encoding the α-subunits of the slow and rapid delayed rectifier K + currents I Ks and I Kr , and the cardiac Na + current, respectively, reduce repolarizing K + currents or increase inward Na + current.
Virtually all drugs causing DiLQTS are high potency blockers of I Kr . The resultant increase in APD is most prominent during phase 3, leading to a triangular-type action potential morphology. Prolonged depolarization enables reactivation of inward current through L-type Ca 2+ or Na + channels, or the Na + -Ca 2+ exchanger, to generate early afterdepolarizations (EADs) and triggered beats ( Fig. 102.2 ). EADs are most easily elicited from Purkinje cells in the conduction system that have particularly long action potentials. For some drugs, including arsenic trioxide and pentamidine, the reduction in I Kr is caused by defective channel trafficking to the cell surface, whereas fluoxetine appears to cause both I Kr block and defective trafficking. Not all drugs that block I Kr cause TdP. For example, verapamil is a potent I Kr blocker, but its Ca 2+ channel blocking properties appear to counterbalance this effect, and DiLQTS does not occur. It is likely that a similar effect is operative for amiodarone, which blocks both Ca 2+ and Na + channels in addition to I Kr . Thus the incidence of TdP with amiodarone is less than 1% despite dramatic QT prolongation during long-term therapy. Although EAD-mediated triggered activity likely initiates TdP, existing data support both focal activity from multiple sources and reentry created by dispersion of repolarization for its maintenance. The K V 11.1 channel, also known as hERG, which generates I Kr , is subject to block by many different drugs, and it appears that its unique structure is responsible. The channel has a wide inner vestibule with a pore that contains multiple aromatic groups that provide high-affinity binding sites for different compounds.
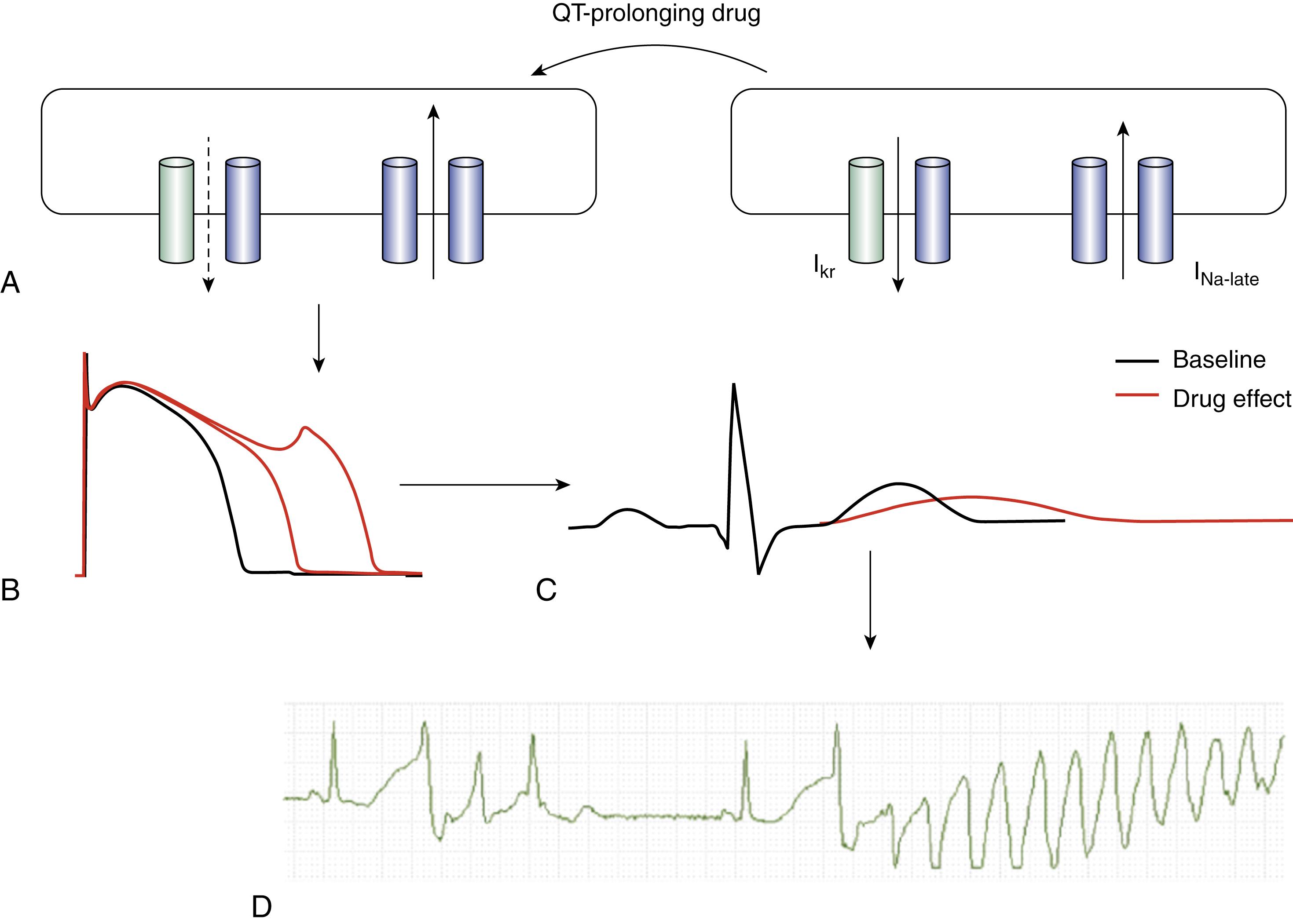
More recently, another drug-induced mechanism has been identified, in which persistent or late Na + current that occurs during the plateau phases of the action potential is increased (see Fig. 102.2 ). , In effect, these drugs phenocopy SCN5A mutations occurring with the congenital LQTS that cause defective fast inactivation. This mechanism is at least partially responsible for QT prolongation caused by the tyrosine kinase inhibitor nilotinib. In cardiomyocytes exposed to nilotinib, action potential prolongation did not occur acutely; rather it occurred after a period of hours. This was reversed by intracellular administration of phosphatidylinositol 3,4,5-trisphosphate (IP 3 ), a downstream effector of phosphoinositide 3-kinase (PI3K). Although APD increase was associated with depression of multiple ionic currents (I Kr , I Ks , I Ca,L , and peak I Na ), increased late Na + current was identified as the principal mechanism of APD prolongation. Additional studies revealed that some but not all drugs that block I Kr also increase late Na + current, and this additive effect likely accounts for the high QT-prolonging potency of drugs such as dofetilide.
A unifying hypothesis to understand the role of risk factors in TdP is the concept of repolarization reserve. This posits that in normal hearts, multiple ionic currents mediating action potential repolarization serve as redundant protective mechanisms to prevent extreme deviations. In this situation, an I Kr blocker will not cause DiLQTS. However, when acquired conditions impair one or more redundant mechanisms, the same drug can cause marked QT prolongation and TdP because of “reduced repolarization reserve.”
Antiarrhythmic drugs are responsible for the overwhelming preponderance of drug-induced TdP; thus therapy with these agents constitutes the strongest risk factor. , , , , The overall incidence ranges from 1% to 8% with quinidine, sotalol, and dofetilide. DiLQTS also occurs with noncardiovascular drugs, but the incidence appears to be much lower. This was first reported for antipsychotic drugs, and multiple drugs in other therapeutic categories have also been implicated, most notably antidepressants, antibiotics, antiretroviral agents, and anticancer drugs ( www.crediblemeds.org ). In some cases, QT-prolonging drugs were identified to increase the risk of sudden cardiac death in large epidemiology studies, including antidepressants, typical and atypical antipsychotics, and erythromycin, an effect likely caused by QT-related proarrhythmia. For erythromycin, risk was increased severalfold during cotherapy with drugs that inhibit its elimination. For the vast majority of drugs, TdP risk increases as a function of dose/plasma concentration. A notable exception is quinidine, which can cause marked QT prolongation at low concentrations because of its high-potency block of I Kr .
The unpredictability of DiLQTS and its similarity to the congenital LQTS led to a search for genetic causes (see Table 102.1 ). As noted previously, mutations in congenital LQTS genes have been identified in patients with drug-induced TdP: These patients have a normal QT interval at baseline but develop excessive QT prolongation during drug challenge. Such incomplete penetrance has been well documented in families with the congenital form, indicating latent or subclinical phenotypes. The incidence of subclinical congenital LQTS mutations was estimated to be approximately 10% to 20%. However, a more recent study of DiLQTS patients using next-generation sequencing of targeted arrhythmia-related genes found that 37% of patients harbored such mutations. In addition, common DNA variants that occur in a larger percent of the population have also been implicated. The variant D85N in the KCNE1 gene, which encodes a K + channel-modulating subunit, increases risk of DiLQTS. The S1103Y and R1193Q variants in SCN5A causing the congenital LQTS are enriched in African Americans (13%) and Asians (6%), respectively. ,
Early series documented a 2- to 3-fold female predominance in DiLQTS. After puberty, the QT interval shortens in men but not women, an effect caused by remodeling of repolarizing K + currents caused by testosterone. Androgens also appear to be protective against DiLQTS, and as discussed later, suppression of these hormones during anticancer therapy increases risk. As noted previously, bradycardia results in longer action potentials at baseline, whereas I Kr blockers demonstrate reverse-use dependence, or potency that is enhanced at slow rates. Often, DiLQT develops soon after the onset of complete heart block because of ventricular slowing. Hypokalemia can prolong cardiac repolarization under otherwise normal circumstances. This is caused by a reduction in I Kr that is counterintuitive to electromechanical considerations and is mediated by enhanced fast inactivation. In addition, low extracellular potassium potentiates drug block of I Kr . Hypomagnesemia also increases risk of DiLQTS, likely through modulation of L-type Ca 2+ current to promote EADs.
Interestingly, TdP often develops after conversion of atrial fibrillation (AF) to sinus rhythm, whereas AF appears to be protective (see Fig. 102.1 ). This effect appears to be related to heart rate slowing, but a component of QT prolongation after conversion is irrespective of rate. Thus it is critical to repeat an ECG after conversion of AF to sinus rhythm to reassess the degree of QT prolongation. The presence of structural heart disease also increases the risk of DiLQTS. Both LV hypertrophy and heart failure promote electrical remodeling that prolongs repolarization. As indicated earlier, factors that impair drug elimination causing excessive plasma concentrations of QT-prolonging drugs can be a critical precipitating factor for TdP, including organ insufficiency and coadministration with drugs that inhibit enzymes (e.g., CYP3A4 and CYP2D6) and transporters (e.g., P-glycoprotein) responsible for excretion. Additional risk factors that have been identified include older age, inflammation, and hypogonadism with testosterone deficiency.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here