Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In contrast to set-piece wars of the past, modern warfare is characterized by a “nonlinear” battlefield with intermittent engagements of an elusive enemy. Soldiers wear advanced protective gear including helmets, ballistic eyewear, ceramic torso plates, and Kevlar neck, arm, and groin pieces. Even transport vehicles are surrounded in ballistic armor. In addition to typical weapons such as high-velocity automatic rifles, mortars, and grenades, high-energy improvised explosive devices (IEDs) are used. The protective gear of modern warfare has significantly reduced injury rates among deployed soldiers; however, direct strikes that find gaps in the body armor or indirect fire attacks on bases, where soldiers without protective gear are targeted, can result in highly destructive injuries.
Combat medical care has also evolved significantly in recent times. There is a renewed emphasis on combat medic training to provide lifesaving care under fire; in-theater transport care is now more commonly provided by highly trained medics and even midlevel practitioners and physicians; and advanced surgical care has been pushed closer to combat activities as part of the in-theater trauma system. Although hemorrhage remains the most common cause of prehospital death, soldiers with historically nonsurvivable injuries are now arriving alive at forward facilities. As a result of these many advances, and despite the injury severity of our combat wounded reaching historically high levels, the case fatality rate is at an all-time low of less than 10%.
Thoracic injuries still represent a significant proportion of combat wounds, including both blunt and penetrating injuries. Ballistic vests afford significant protection to our soldiers, converting potentially lethal injuries into minor contusions in many cases ( Fig. 61-1 ). However, significant chest injuries still occur in 5% to 10% of casualties ( Fig. 61-2 ). These patients require advanced management by multiple teams of providers in several medical facilities over many thousands of miles. This chapter describes the historical context of this management strategy and the current approach to complex thoracic injuries in multiply injured combat casualties.
Prior to World War I, surgical intervention on a combat chest wound was rare, and mortality from these injuries was over 60% ( Fig. 61-3 ). The concept of negative intrathoracic pressure was first demonstrated by Ludwig in 1847 and then by Aron in 1900. However, in the early 1900s, the nuances of thoracic physiology and its clinical implications remained poorly understood. Indeed, the significance of negative intrathoracic pressure was not fully appreciated until 1923.
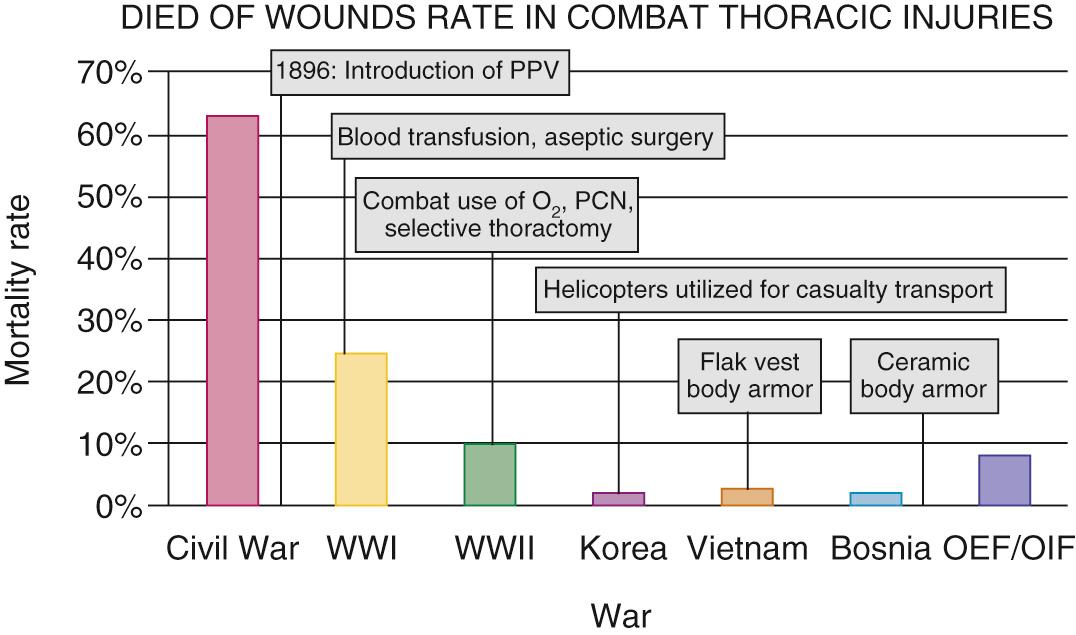
It was in this era that Dr. Evarts Graham was appointed to the Surgeon General's Empyema Commission. This famous commission was tasked with determining the optimal treatment for complex parapneumonic effusion and empyema resulting from streptococcal pneumonia, which had spread among enlisted troops afflicted by the influenza pandemics of 1917 and 1918 and was associated with a mortality of up to 90% in some of the camps. The commission determined that, in contrast to pneumococcal empyema, which responded well to open drainage, the preferred treatment in patients with streptococcal infections was repeated thoracentesis followed by delayed open drainage. This new approach reduced the mortality from streptococcal empyema to less than 5%. Graham was inspired to further explore respiratory mechanics and to confirm his clinical observations in the laboratory by developing an animal model of streptococcal empyema. Through this work, he determined conclusively that the mediastinum was, in fact, not a fixed structure as it had been viewed previous to his work and that patients with streptococcal empyema subjected to early thoracotomy very likely died of the open pneumothorax in the setting of minimal respiratory reserve.
Dr. Graham continued to exert his influence in World War II by ensuring that thoracic-trained surgeons were appointed to the Second Auxiliary Surgical Group, which then formed the first Thoracic Surgery Center in Tunisia in 1943. Among these surgeons was Dr. Lyman Brewer who, along with Dr. Edward Churchill, shaped the approach to managing combat thoracic injuries and postinjury empyema. World War II saw a significant increase in the use of formal thoracotomy for the management of combat wounds; in fact, in many cases, thoracotomy was being overused. Brewer, with Churchill's support, called for a more measured approach and outlined the indications for thoracotomy :
Continued bleeding (severe)
“Traumatic” thoracotomy (large sucking wound)
Thoracoabdominal wound
Miscellaneous
Heart or esophagus repair
Trachea or bronchus repair
Foreign body larger than 2 cm
Interestingly, these indications for thoracotomy largely apply even in modern combat casualty care.
Routine use of tube thoracostomy was not widely accepted during World War II. Although the thoracic drainage system developed by Lilienthal in 1926 was available in WWII, intermittent thoracentesis was favored for the management of pleural effusions and hemothorax.
The modern three-chamber drainage system was described in 1952 by Howe, and the technique of tube thoracostomy was being widely used in civilian trauma. However, repeated thoracentesis remained the primary management strategy for traumatic pneumothorax and hemothorax along with instillation of antibiotics into the thoracic cavity even through the Korean War. It was not until multiple civilian centers reported the usefulness of tube thoracostomy in thoracic trauma in the 1960s that this approach became the mainstay for both primary management of simple intrathoracic problems like pneumothorax and postoperative management following major thoracotomy for trauma.
Although positive pressure ventilation with a facemask was first used by Brewer as early as 1944 for the management of posttraumatic pulmonary edema (i.e., modern-day acute respiratory distress syndrome), it was not until the development of plastic endotracheal tubes, rapid blood gas analysis, and the polio epidemic of 1952 (negative pressure ventilation) that mechanical ventilation became a routine part of critical care management of patients with severe thoracic trauma. All of these capabilities are now readily available, even in the most austere combat casualty care environments. In addition to these routine management tools, modern combat casualty care has also advanced the concepts of damage control surgery, damage control resuscitation, and critical care air transport to maximize survival of combat casualties as summarized in the remainder of this chapter.
One of the greatest advances in modern combat casualty care has been the establishment of a formal trauma system for the care of our wounded soldiers. In November 2004, the Joint Theater Trauma System (JTTS) was created to apply lessons learned from civilian trauma systems across the United States to the combat theaters in Iraq and Afghanistan. The fundamental aspects of this system included the continual presence of a senior trauma surgeon—the theater trauma director—and a cadre of nurse specialists trained in performance improvement and data collection.
Now in its 10th year of operation, this team is responsible for educating deployed medical units on the most current clinical practice guidelines (CPGs) (available at http://www.usaisr.amedd.army.mil/clinical_practice_guidelines.html ). This team also conducts a weekly performance-improvement case-based teleconference that spans the globe. Finally, this team and its parent organization, the Joint Trauma System (JTS), track outcomes over time and advocate for policy and practice changes in order to continually improve the care that is delivered. This systematic approach stands in stark contrast to the first Gulf War, during which the lack of an organized trauma system resulted in numerous mistakes in combat casualty care. As the result of almost a decade of work, the value of this is evidenced by the lowest case fatality rate among combat casualties in all of recorded history.
It is interesting to note the current deployment patterns for surgeons by specialty and military branch. Currently, all three branches have cardiothoracic surgeons on active duty and in the reserves. These surgeons function primarily as general surgeons when deployed. In one report, 79% of cases performed by cardiothoracic surgeons were general surgical cases, whereas 21% were index thoracic or major vascular procedures ( Table 61-1 ). The U.S. Army does not differentiate surgical subspecialists for deployment purposes. Cardiothoracic surgeons, trauma surgeons, vascular surgeons, colorectal surgeons, surgical oncologists, pediatric surgeons, and laparoscopic surgeons all deploy as general surgeons. This is true of both active duty and reserve surgeons in the army. During the height of the operational tempo, the U.S. Air Force deployed both cardiothoracic and vascular surgeons specifically to the aeromedical evacuation hubs in Iraq and Afghanistan to ensure definitive vascular reconstruction was performed prior to long-range transport in patients with major vascular injuries in the torso or extremities. In at least one case, the deployed cardiothoracic surgeon also functioned as the “trauma czar,” who was responsible for ensuring the stability and readiness of each casualty prior to evacuation. Similarly, the U.S. Navy has deployed at least one cardiothoracic surgeon as the theater trauma director, who is tasked with supervising the research team while also ensuring uniformity across the various treatment facilities in theater and adherence to the CPGs as much as possible. Consequently, maintaining a broad skill set is vitally important for all military surgeons regardless of their specialty. Cardiothoracic surgeons, as a result of comprehensive training in general surgery, thoracic surgery, vascular surgery, and critical care, are uniquely prepared to provide care to the multiply injured combat casualty.
| Total Cases | Thoracotomy | Sternotomy | Major Vascular | Cardiac/Thoracic Aorta | Total General Surgery N (%) | Total Cardiothoracic or Vascular N (%) | |
|---|---|---|---|---|---|---|---|
| Surgeon 1 | 104 | 8 | 2 | 19 | 0 | 75 (72) | 29 (28) |
| Surgeon 2 | 151 | 10 | 5 | 15 | 3 | 121 (80) | 30 (20) |
| Surgeon 3 | 204 | 12 | 2 | 21 | 2 | 169 (83) | 35 (17) |
| Surgeon 4 | 186 | 14 | 4 | 17 | 0 | 151 (81) | 35 (19) |
| Mean N (%) | 161 (100) | 11 (7) | 3 (2) | 18 (11) | 1 (0.6) | 127 (79) | 34 (21) |
By far, most potentially preventable combat deaths result from hemorrhage. This finding is true of deaths prehospital (killed in action [KIA]) and those patients who arrive at a medical facility with detectable vital signs but then go on to expire (died of wounds [DOW]). Consequently, great emphasis has been placed on identifying new means of controlling both compressible (i.e., extremity) and noncompressible hemorrhage using hemostatic dressings, inflatable polymers, and endoaortic balloon placement.
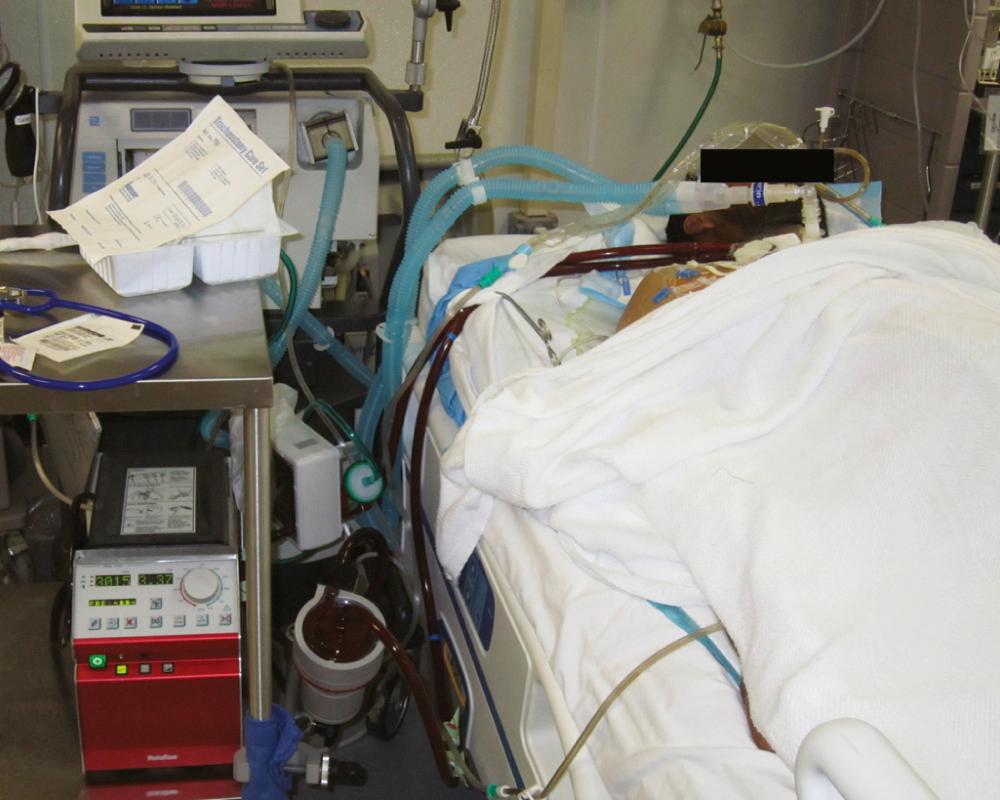
Other potentially preventable causes of death include airway obstruction, tension pneumothorax, and multiorgan failure. Airway management has been addressed by teaching combat medics placement of a cricothyroidotomy in tactical situations. For tension pneumothorax, the traditional anterior needle decompression approach has received scrutiny because postmortem studies have shown that often the needle does not enter the pleural space. This finding has generated interest in identifying alternative approaches such as the lateral placement technique and modification of existing devices such as a Veress needle, which can be used if tension pneumothorax is suspected. Finally, multisystem organ failure deaths are being addressed by introducing advanced organ support modalities like renal replacement therapy (RRT) and extracorporeal membrane oxygenation (ECMO) further forward in the combat theater ( Fig. 61-4 ).
Increased attention has also been paid to the care delivered en route from point of injury to a military treatment facility (MTF). For the most critically injured soldiers, dispatching transport teams with flight paramedics who have a higher level of training has been shown to improve survival. The United Kingdom has taken this one step further and has created a team with flight nurses and physicians, who recover casualties from point of injury. This so-called medical emergency response team (MERT) has also proven beneficial for the most severely injured casualties.
In a mature combat theater, combat casualties typically pass through multiple levels of care prior to evacuation. Within the combat theater, there are three levels of MTFs, ranging from an aid station with no surgical capability (level I) to a site with far forward general and orthopedic surgeons (level II) and finally an austere hospital-type facility that has more advanced imaging capabilities and more surgical subspecialties (level III). However, movement through this system is not always a strictly linear process. The JTS has been instrumental in advocating that a balance be struck between expedient transport and transport to a location where the patient's problem can be managed. For example, a patient with an open skull fracture would typically be moved to a level III facility with a neurosurgeon, if at all possible, and a patient with an open chest wound and shock would be taken to either a level II or level III facility—whichever is closer—rather than a level I facility.
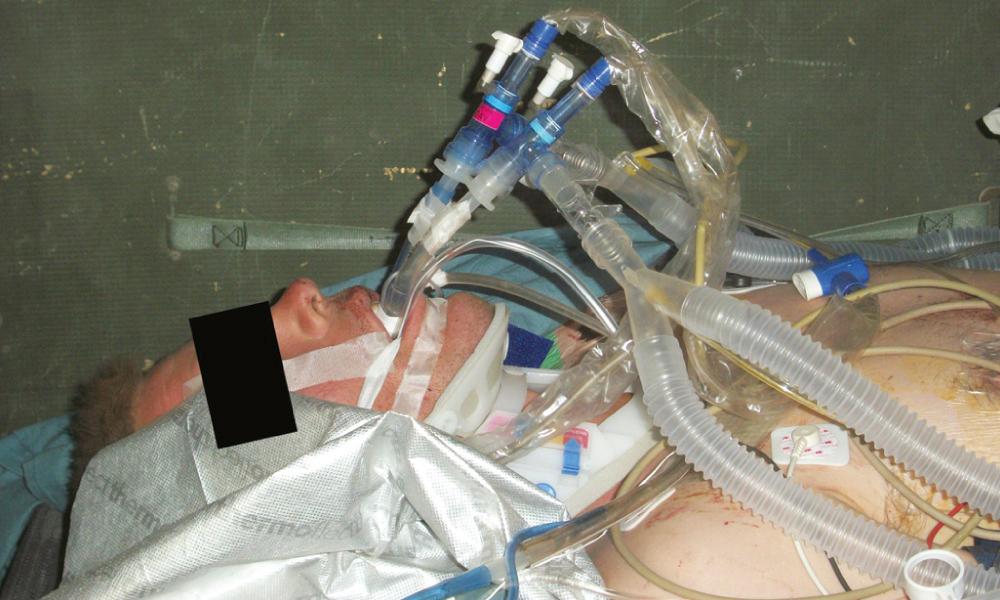
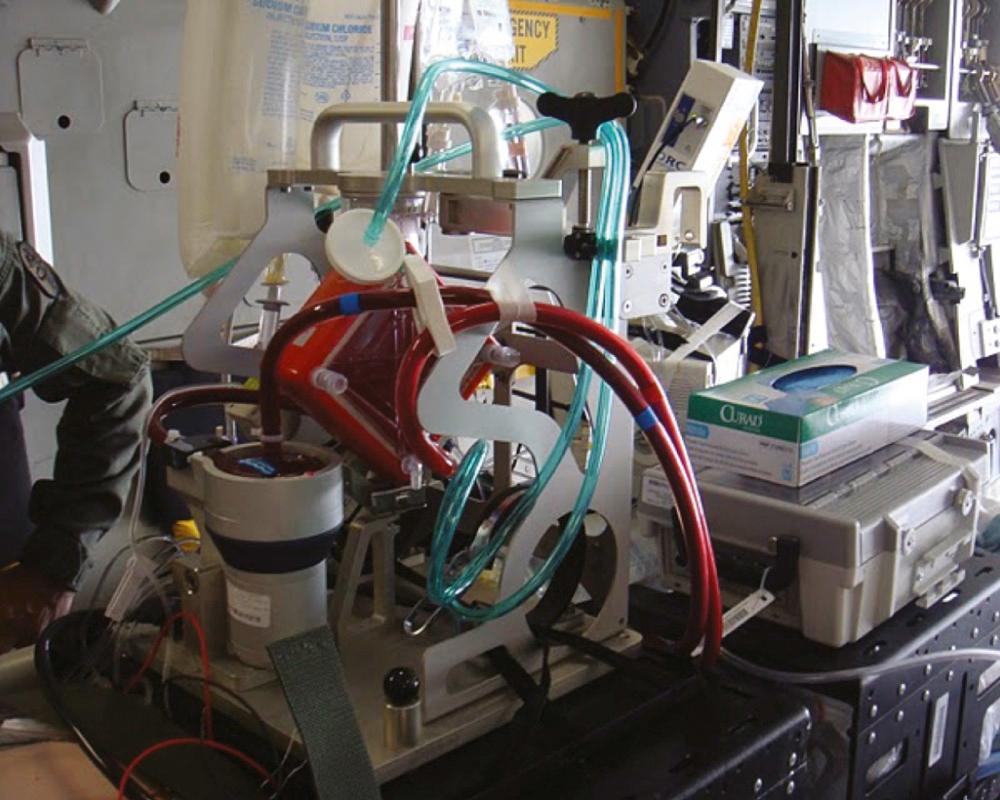
All combat casualties within the theater who cannot return to duty eventually filter into the level III facility designated as the aeromedical hub for long-range evacuation to a level IV facility (Landstuhl Regional Medical Center in Germany in this case) and then on to a level V facility in the United States where they receive definitive care. Modern ICU-level care can be provided in transport by a Critical Care Air Transport Team (CCATT); however, the patient should be stabilized or at least “stabilizing” prior to transport. For patients with thoracic trauma, this typically means that any intrathoracic (i.e., noncompressible) hemorrhage has been controlled, any air leaks are managed with tube thoracostomy that is well secured to the patient, any coagulopathy has been reversed, and the patient is on stable and moderate to low ventilator settings or even extubated. In the vast majority of cases, these requirements can be met during a short stay in the level III facility. However, in some cases, advanced maneuvers such as independent lung ventilation ( Fig. 61-5 ), inhaled pulmonary vasodilators, rescue ventilator modalities, or even ECMO are required to ensure safe transport. The need for such interventions, which are beyond the standard CCATT capabilities, was first recognized in 2005 and resulted in the establishment of the Acute Lung Rescue Team (ALRT) based at the level IV facility. As of 2010, this team had performed 24 complex patient transports, and as of 2011, 10 U.S. patients had been transported with either pumpless lung support or full ECMO with a 90% survival ( Fig. 61-6 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here