Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital and developmental disorders of the lung manifest almost exclusively in pediatric and neonatal patients and include a wide array of disorders resulting from abnormalities of lung architecture, cellular function, and metabolism. All of these disorders are uncommon, and most are rarely encountered in clinical practice; however, together, they account for significant morbidity and mortality in the pediatric population and can often be a diagnostic challenge for the pathologist.
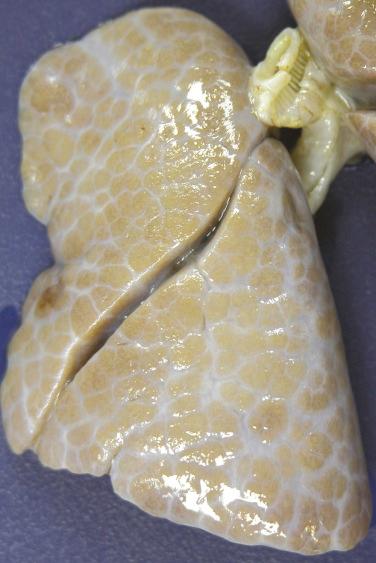
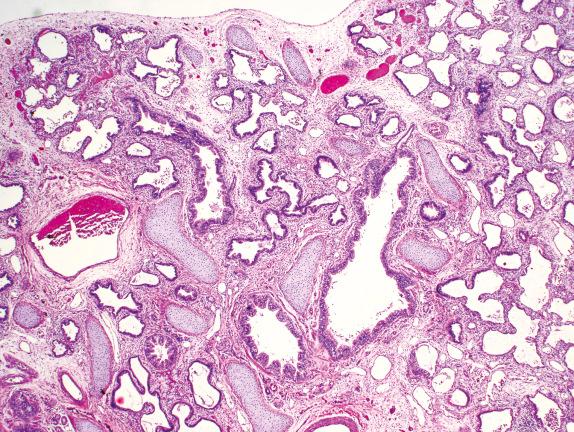
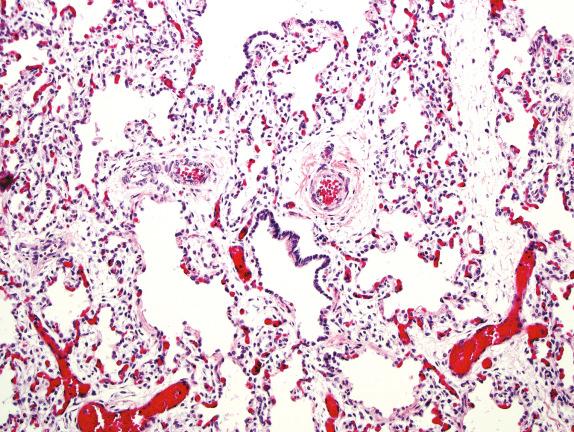
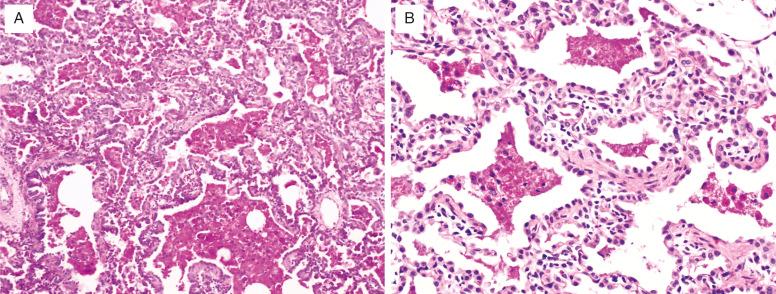
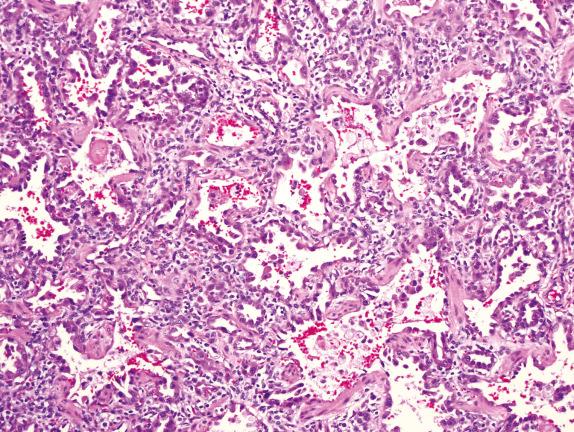
Diffuse developmental disorders of lung architecture are rare entities that typically present in the neonate. Babies with acinar dysgenesis (also called acinar dysplasia ) have an early arrest of lung development resulting in respiratory distress and death in the first few hours of life ( Figs. 4.1 and 4.2 ). Most reported cases have occurred in otherwise normal female infants (9:1 female:male ratio) at or near term. Although the pathogenesis is not known, an abnormality of epithelial-mesenchymal interaction during development has been proposed. Congenital alveolar dysplasia refers to a poorly characterized disorder with arrest in lung development at a later stage and thus with a somewhat less severe presentation ( Fig. 4.3 ). Similar to acinar dysplasia, affected infants have respiratory difficulty at birth but may be sustained for some time with ventilatory support. Alveolar capillary dysplasia (ACD) with misalignment of pulmonary veins typically causes respiratory symptoms and persistent pulmonary hypertension in term or near-term infants in the first days of life. Initially identified only at autopsy, increasing clinical suspicion has resulted in diagnostic lung biopsies to exclude the diagnosis in neonates and infants ( Fig. 4.4 ). Although there are exceptions, most affected infants die in the first month of life despite intensive supportive measures. ACD is associated with other malformations, particularly involving the cardiac, gastrointestinal, and genitourinary systems. Hypoplastic left heart syndrome is the most frequently associated cardiac malformation. Sibling pairs have been reported. In 2009 FOXF1 gene mutations and deletions were described as the cause of ACD.
A group of developmental disorders with abnormal parenchymal development and sometimes abnormal vasculature
Rare
ACD most common
Uniformly lethal
Term or near-term neonates
Marked female predominance in acinar dysgenesis
Acinar dysgenesis—immediate respiratory distress; cannot be supported
Congenital alveolar dysplasia—early respiratory distress; can be supported
ACD—respiratory symptoms and persistent pulmonary hypertension in a term infant in the first days to weeks of life; may be associated with other malformations
Acinar dysgenesis—small hazy lungs; often air leak with resuscitation
Congenital alveolar dysplasia and ACD—diffusely hazy lungs
Fatal despite maximal supportive measures
Survival is limited to hours in acinar dysgenesis and weeks in congenital alveolar dysplasia
Death usually in the first month in ACD, although longer survivals are reported
Lung transplantation may be an option for some
Small lungs with small lobules and thickened white interlobular septa
Normal to increased lung weight
Deeply congested
Normal to increased lung weight
Developmental arrest in pseudoglandular phase, with normal cytodifferentiation but nearly absent acinar development
Sometimes prominent airway smooth muscle and ectopic cartilage
Developmental arrest in late canalicular/early saccular phase
Reduced alveolar wall capillaries in some cases
May have arterial hypertensive changes
Malpositioned dilated veins accompany arterioles in the lobules and small pulmonary arteries adjacent to bronchioles
Striking muscularization of small pulmonary arteries and arterioles
Dilated and congested veins and venules
Dilated central channels in alveolar walls, but deficient alveolar capillaries
Normal position of larger veins adjacent to bronchi
Lobular architectural simplification (variable)
Lymphangiectasia (one-third of cases)
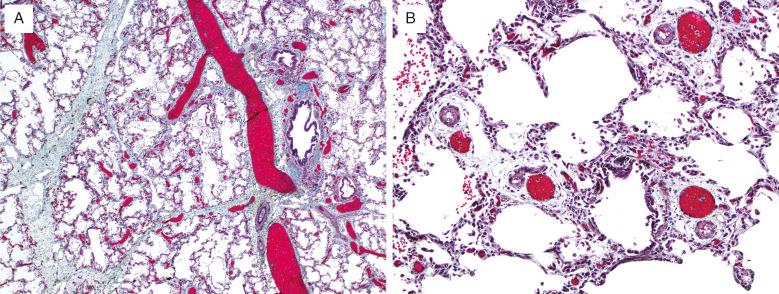
Acinar dysplasia should be distinguished from other, less severe forms of pulmonary hypoplasia. Congenital alveolar dysplasia may be difficult to separate from the changes seen in the lungs of infants with respiratory failure who have been intensively supported for long periods. The typical history of respiratory failure in a term infant and the uniformity of the histologic changes are key observations in this situation. Congenital alveolar dysplasia shows normal venous anatomy, whereas ACD is characterized by deficiency of capillaries within the alveolar walls, abnormal dilated veins within the bronchovascular bundles, and accompanying muscularized pulmonary arterioles within the lobules. These dilated veins are thought to represent dilated bronchial veins secondary to shunting due to high pulmonary arterial pressures.
These diffuse developmental disorders are universally fatal. Lung transplantation may be an option for babies with ACD if recognized early.
As a group, genetic mutations affecting surfactant metabolism are an important cause of respiratory distress in otherwise healthy term or near-term infants, as well as an emerging cause of chronic lung disease in older children and adults. Mutations or deletions in six different genes are now recognized to cause surfactant dysfunction disorders. Surfactant protein B (SFTPB gene) mutations were first recognized in 1993, followed by mutations in surfactant protein C (SFTPC gene). Since 2004, mutations in the gene encoding the adenosine triphosphate (ATP)–binding cassette transporter subfamily A3 (ABCA3) have emerged as the most common cause of genetic surfactant disorders presenting in infancy. Abnormalities in the NKX2-1 gene encoding thyroid transcription factor (TTF1) may cause impaired surfactant regulation. Mutations in the genes encoding the granulocyte macrophage colony-stimulating factor (GM-CSF) receptor (CSF2RA, CSF2RB) are another genetic cause of pulmonary alveolar proteinosis.
Heterogenous group of inherited disorders of surfactant metabolism
Rare
Death in infancy (SFTPB, SFTPC, ABCA3, NKX2-1)
Chronic disease in infants and older children (ABCA3, SFTPC, NKX2-1)
Chronic lung disease and fibrosis in adults (SFTPC)
Infancy—SFTPB
Infancy and older children—NKX2-1
Infants, older children, and adults—SFTPC, ABCA3, CSF2RA, CSF2RB
Term infants with respiratory difficulty in first weeks and months of life (SFTPB, SFTPC, ABCA3)
Chronic lung disease in older children (SFTPC, ABCA3)
SFTPB, ABCA3—autosomal recessive
SFTPC, NKX2-1—autosomal-dominant loss of function
May have family history; parents and grandparents sometimes affected with SFTPC
Clinical manifestations typically milder for SFTPC than for SFTPB
Recurrent pulmonary alveolar proteinosis (CSF2RA, CSF2RB)
“Crazy paving” pattern on chest computed tomogram (SFTPB)
Diffuse ground-glass opacity similar to hyaline membrane disease
Chronic interstitial disease (SFTPC)
Variable prognosis
Treatment with supportive measures, steroids, and hydroxychloroquine may delay the need for lung transplantation in SFTPC disease
Whole-lung lavage for CSF2RA, CSF2RB
In infancy, heavily consolidated-appearing lungs. Some with pulmonary alveolar proteinosis pattern may have yellow material visibly distending airspaces.
In infancy, all show diffuse prominent alveolar epithelial hyperplasia with at least focal periodic acid–Schiff–positive globular or granular proteinaceous material in alveoli
Classic pulmonary alveolar proteinosis pattern—associated most commonly with SFTPB mutations, as well as CSF2RA and CSF2RB
Variant pulmonary alveolar proteinosis pattern with some lobular remodeling, interstitial widening, foamy macrophages, and granular proteinosis—more commonly associated with ABCA3 mutations
Chronic pneumonitis of infancy pattern with lobular remodeling, interstitial widening, abundant foamy macrophages, few cholesterol clefts, mild interstitial inflammation, and sparse proteinosis—usually associated with SFTPC mutations
Desquamative interstitial pneumonia pattern—usually associated with SFTPC mutations
Nonspecific interstitial pneumonia—associated with SFTPC and ABCA3 mutations in infants and older children
Idiopathic pulmonary fibrosis—associated with SFTPC mutations in older children and adults
Electron microscopy
SFTPB —absence of normal lamellar bodies with hybrid lamellar/multivesicular bodies and absent tubular myelin
ABCA3 —distinctive tiny lamellar bodylike structures with electron-dense inclusion “fried egg”; sometimes absence of lamellar bodies
SFTPC, NKX2-1, CSF2RA, CSF2RB —nonspecific ultrastructural abnormalities
Surfactant gene mutation studies on blood or frozen lung tissue
Pulmonary alveolar proteinosis pattern—Acquired proteinosis in immunodeficient patients or those with antibodies to GM-CSF. These patients are typically older and do not have the diffuse marked alveolar epithelial hyperplasia seen in the setting of congenital alveolar proteinosis.
Chronic pneumonitis of infancy—Other forms of chronic interstitial lung disease with endogenous lipoid pneumonia.
In infants, mutations of SFTPB and ABCA3 typically manifest as pulmonary alveolar proteinosis ( Fig. 4.5 ), whereas SFTPC mutations typically result in more lobular remodeling and less alveolar proteinosis, yielding a pattern of chronic pneumonitis of infancy ( Fig. 4.6 ). SFTPC mutations are also recognized as a cause of idiopathic and familial pulmonary fibrosis in older children and adults, and ABCA3 mutations have also been identified in a few older children and young adults with chronic fibrosing lung disease. NKX2-1 disorders have variable pathologic features, including impaired alveolarization, accumulation of foamy macrophages, and chronic pneumonitis of infancy pattern. GM-CSF disorders result in accumulation of abundant pulmonary alveolar proteinosis material, with little to no lobular remodeling. It should be noted that there are cases with morphology consistent with surfactant dysfunction in which no mutation has been identified in the known genes. Other genes and clinical patterns of disease will almost certainly be identified as more is learned about this complex set of metabolic disorders. Mutations in surfactant proteins and related proteins may also be genetic modifiers affecting the natural history and prognosis of various forms of acquired lung disease.
Congenital pulmonary airway malformations (CPAMs) have been classified by Stocker according to cyst size and histomorphologic resemblance to segments of the developing bronchial tree and airspaces, from proximal (CPAM, type 0) to distal (CPAM, type 4). The two most common of these are more simply described as the large cyst type (Stocker type 1) and the small cyst type (Stocker type 2) CPAMs. The other three morphologic types are all thought to represent different pathogenetic processes: acinar dysplasia (type 0), pulmonary hyperplasia (type 3), and low-grade cystic pleuropulmonary blastoma (PPB) (type 4).
Focal developmental malformation of the lung, usually forming a single large, often multiloculated, cystic structure
Uncommon, but commonest of the cystic maldevelopments
Any lobe may be involved, lower lobe somewhat more commonly, and the left lung slightly more than the right
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here