Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We appreciate the skilled efforts of the talented medical illustrators Courtney Fleming and Anthony Baker, whose original works are depicted in this chapter.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Cerebral edema is the result of excess fluid within cells and/or extracellular fluid accumulation in brain parenchyma.
Cerebral edema is classified as vasogenic, cytotoxic, interstitial, or osmotic.
Depending on the inciting event, cerebral edema can be the result of blood-brain barrier disruption, alterations of cellular metabolism, hydrocephalus-associated transependymal cerebrospinal fluid flow, osmotic dysregulation, and/or glymphatic system disruption.
MRI can be useful in distinguishing between vasogenic, cytotoxic, interstitial, and osmotic cerebral edema.
Management of cerebral edema can be medical and/or surgical. Treatment is specific to the underlying cause of edema and reducing potential for secondary injury.
Cerebral edema is the pathologic accumulation of fluid within cells and/or extracellular compartment of the brain. It can result from a variety of pathologic processes. Cerebral edema can cause significant neurological morbidity and mortality. Based on the underlying pathophysiology, cerebral edema is classified into four types: vasogenic, cytotoxic, interstitial, and osmotic. Because each form of edema has a different inciting event that is associated with a defined underlying pathophysiology, clinical impact and management strategies are different. The distinctive forms of cerebral edema (singular or in combination) can be associated with a number of neurological pathologies, including (but not exclusive to) brain tumors, infection (cerebritis or abscess), liver failure, poisoning, hydrocephalus, traumatic brain injury, hypertension, intraparenchymal hemorrhage, altitude changes, metabolic disorders, and cerebral infarction.
Here we define the mechanisms of cerebral fluid homeostasis, the pathophysiologic changes that disrupt fluid homeostasis and underlie the various forms of cerebral edema, and the management of the different types of cerebral edema and the strategies to mitigate their potential permanent effects.
The Monroe-Kellie doctrine is critical to understanding cerebral fluid homeostasis, pathophysiology, and patient management in edematous states. This doctrine defines the cranial vault (calvaria) as a fixed-volume structure. Within the fixed volume of the skull, the sum of the volume is derived from brain parenchyma (1250–1500 mL volume), cerebrospinal fluid (125–150 mL), and blood (125–150 mL). Per the Monroe-Kellie doctrine, increases in any of the three components results in a corresponding decrease in the other components. Under edematous conditions, increases of intraparenchymal fluid (edema) expand the brain tissue volume that necessarily leads to the reduction in cerebral blood and cerebrospinal fluid volume. Consequently, relative increases in brain volume due to cerebral edema can cause reduced blood volume (flow) and significant secondary injury from ischemia or brain herniation.
Although the brain represents a small portion of total body mass (approximately 2%–3% of total mass), its significant energy needs require approximately 20% of the total circulating blood volume to function. To accommodate the large circulating blood volume, the brain is supplied by an extensive capillary network (total area, 12–20 m 2 ). This vascular network is dense, and the mean distance between adjacent cerebral capillaries is approximate 40 microns. The tight proximity between capillaries and nervous system tissues (including neurons and astrocytes) permits the near immediate equilibration of needed nutrients and substrates to occur between the system circulation and neural tissues ( Fig. 70.1 ). Although the relationship between systemic circulation and visceral tissues allows the free exchange of plasma solutes, the exchange of solutes between the systemic circulation and brain is highly discriminatory. This CNS-specific selectivity is governed by the blood-brain barrier.
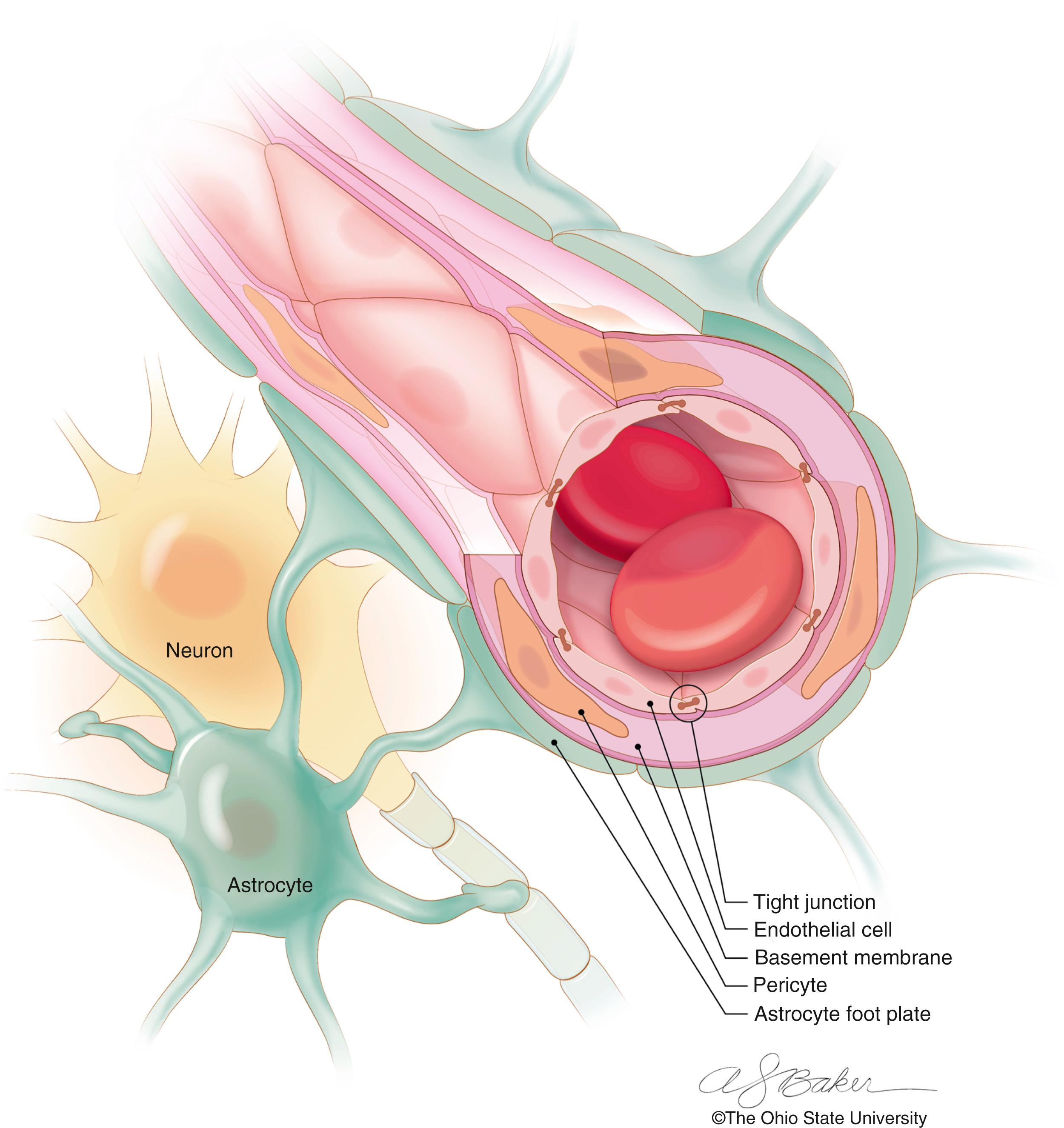
The blood-brain barrier allows the brain to regulate its physiologic microenvironment separately from the systemic environment/circulation. Dysfunction of the blood-brain barrier is an essential component in the understanding of the pathophysiology-specific forms of cerebral edema. Ehrlich (1885) first described the potential presence of the blood-brain barrier after his studies demonstrated that intravenously injected dye stained visceral structures but did not stain normal brain tissue. , Goldmann (1913) later demonstrated that injection of intraventricular dye stained brain tissue but did not stain systemic (nonneural) tissues. Taken together, these insights indicated the presence of a barrier between the systemic circulation and brain (blood-brain barrier). Since the discoveries of Ehrlich and Goldmann, the cellular and physiologic components of the blood-brain barrier have been better defined in normal and disease states.
The foundation of the blood-brain barrier is the tight junctions between the cerebral vascular endothelial cells (see Fig. 70.1 ). The blood-brain barrier is composed of a basement membrane, closely apposed astrocytic foot processes, pericytes, and vascular endothelial cells. Under normal conditions the tight junctions between endothelial cells restrict the passage of most large (greater than 180 Da), hydrophilic, and/or charged molecules by restricting paracellular movement (dominant mode of solute movement in the systemic circulation). Transport across the blood-brain barrier occurs via selective transport proteins, lipophilic diffusion, adsorptive transcytosis, receptor-mediated transcytosis, active adenosine triphosphate-driven transcellular transport, or efflux pumps. Notable molecules and compounds that can move across the blood-brain barrier include gases (e.g., oxygen, carbon dioxide), small lipophilic structures (e.g., methanol, ethanol), and nutrients (e.g., glucose, ascorbic acid).
Systemically, nonneural tissue interstitial fluid is derived from plasma ultrafiltrate that freely passes into peripheral tissues across the endothelial cells of systemic vasculature. Excess interstitial fluid and associated metabolic by-products are removed from the extracellular fluid compartment via lymphatic vessels and returned to the blood circulation. Historically, the nervous system was considered to lack lymphoid tissue and the cerebrospinal fluid was believed to function as a sink to resorb brain interstitial fluid and metabolic by-products. Studies have clearly defined an intracranial glial-lymphatic system (glymphatic system) that rapidly drains interstitial fluid via bulk (or convective) flow into perivenous channels within the brain. Whereas the blood-brain barrier represents a fluid input pathway to brain interstitial tissue, the glymphatic system represents a fluid pathway for eliminating excess brain fluid and solutes.
Within the glymphatic system, cerebrospinal fluid is driven along the periarterial spaces (Virchow-Robin spaces) by arterial pulsation, a pressure gradient, respirations, and vasomotor motility. The movement of cerebrospinal fluid continues in the loosely approximated perivascular spaces to the capillary complexes. There cerebrospinal fluid movement into the brain interstitial space is facilitated by the aquaporin-4 water channels expressed on astrocyte foot processes that envelop the cerebral microvasculature. Specifically, cerebrospinal fluid flows through the aquaporin-4 water channels into the brain parenchyma. Once in extracellular spaces of the brain, the fluid moves rapidly by convection to the perivenous spaces of the deep cerebral veins. This interstitial fluid is collected in the perivenous spaces and then drains out the cervical lymphatics, arachnoid granulations, and perineural space of cranial nerves. , Glymphatics may play a critical role in neuroimmunologic function, , and dysregulation has been associated with a variety of neurological disorders.
Based on landmark work by Klatzo (1967), cerebral edema was initially classified into two types (vasogenic and cytotoxic) edema. His studies demonstrated that vascular fluid leakage into the extracellular spaces of the brain through a disrupted blood-brain barrier led to vasogenic edema. Alternatively, he found that cytotoxic edema was due to intracellular swelling from cell injury or death. Later, interstitial and osmotic cerebral edema were identified. Interstitial cerebral edema results from pressure-driven transependymal bulk flow of ventricular cerebrospinal fluid under pressure (hydrocephalus) into brain parenchyma. Osmotic cerebral edema occurs as a result of passive water flow driven by an osmolarity gradient (hyperosmotic brain relative to hypo-osmotic plasma) across a normal blood-brain barrier. The four types of cerebral edema ( Table 70.1 ) are at times not distinct. One form of edema can lead to another, and/or multiple forms of edema may exist simultaneously. For example, ischemia can lead to different types of edema (cytotoxic and vasogenic).
| Feature | Vasogenic Edema | Cytotoxic Edema | Interstitial Edema | Osmotic Edema |
|---|---|---|---|---|
| Pathophysiology | Increased vascular permeability at the blood-brain barrier, with extracellular fluid accumulation | Metabolic failure of the Na + /K + -ATPase pump and other energy-expensive ionic balance metabolism with cellular swelling (neurons, glia, and endothelial cells) | Transependymal flow of cerebrospinal fluid into the periventricular extracellular space that is caused by a pressure-driven bulk flow | The brain is hyperosmolar with respect to plasma Water moves along the osmotic gradient into the brain |
| Composition of edema fluid | Plasma ultrafiltrate | Early: perivascular cerebrospinal fluid Late: plasma | Cerebrospinal fluid | Relatively hypo-osmotic plasma |
| Location of edema | Preferentially white matter | Gray and white matter | Periventricular white matter | Gray and white matter |
| Extracellular fluid volume | Increased | Decreased | Increased | Increased |
| Typical etiology | Brain tumors (primary and metastatic), lead toxicity, radiation necrosis, trauma, abscess and encephalitis, as well as later stages of infarction | Anoxia, diabetic ketoacidosis, hepatic encephalopathy, hypothermia, infarction/ischemia, trauma, infection, meningitis, Reye syndrome, water intoxication, and radiation necrosis | Hydrocephalus (most pronounced in acute obstructive edema) | Hemodialysis, too-rapid correction of hypernatremia, hypertensive crisis, syndrome of inappropriate antidiuretic hormone secretion |
| Response to therapy | Yes | Dependent on etiology | Yes | Dependent on etiology |
| Corticosteroids | Effective | Not effective | Not effective | Not effective |
| Diuretics | Minimally or not effective | Transiently effective | Transiently effective | Not effective |
| Hyper-osmolar therapy | Transiently effective | Transiently effective | Minimally or not effective | Not effective |
| Other treatment | Reversal of primary insult (e.g., removal of tumor), VEGF inhibition, other targeted disease-specific treatment | Reversal of primary insult Decompressive craniectomy for extensive and progressive medically refractory edema | Reversal of primary insult (e.g., removal of cerebrospinal fluid obstruction, ventricular shunt or third ventriculostomy) | Reversal of primary insult, restoration of normal osmotic gradient |
Vasogenic edema results from an abnormally permeable blood-brain barrier ( Fig. 70.2 ). Increased blood-brain barrier permeability can result from neoplastic processes (primary and metastatic), brain injury, infection, and/or inflammation. These pathologic processes result in disruption of the endothelial tight junctions and alterations in the astrocytic relationship with vascular endothelial cells. The increased permeability of the blood-brain barrier results in fluid and solute extravasation from the system circulation (plasma ultrafiltrate). The plasma ultrafiltrate includes fluid, serum proteins, ionic solute, and other plasma products. The fluid and serum accumulate in the extracellular (interstitial) spaces of the brain parenchyma, forming edema.
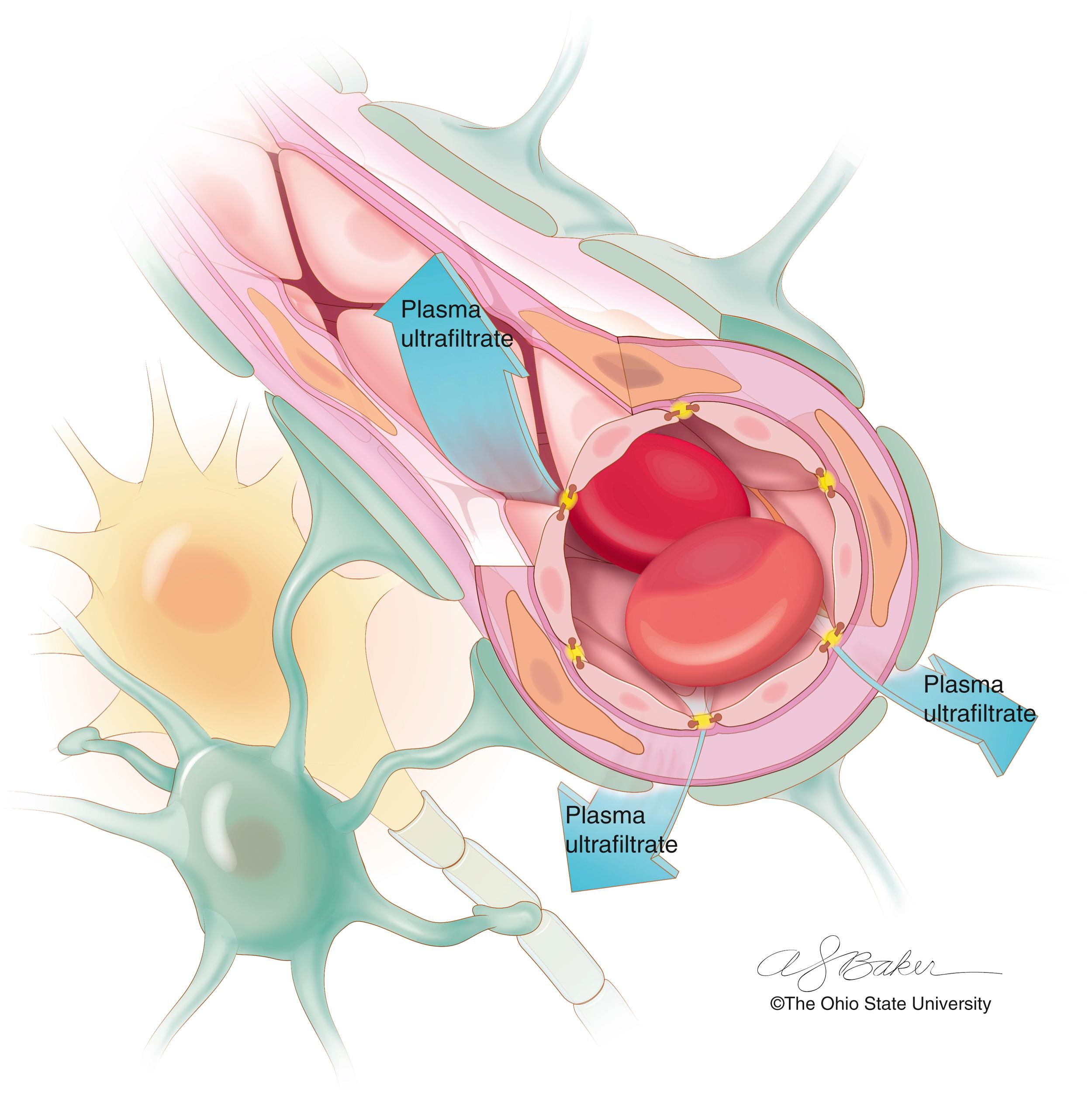
Vasogenic cerebral edema occurs frequently with brain tumors. Brain tumor vasculature often has a dysfunctional blood-brain barrier that permits the leakage of gadolinium on MRI into the peritumoral nervous tissue ( Fig. 70.3 ). Many tumors actively secrete angiogenic factors to amplify the proliferation of tumor-associated vessels. Vascular endothelial growth factor (VEGF) is an angiogenic protein secreted by numerous tumors, including those arising from the CNS. VEGF has a variety of actions including proliferation of endothelial cells, increasing capillary permeability in normal vessels, and the development of vessels with dysfunctional or absent tight junctions. Secretion of VEGF leads to substantially increased vascular permeability and fluid leakage at the blood-brain barrier from the intravascular compartment into the interstitial compartment.
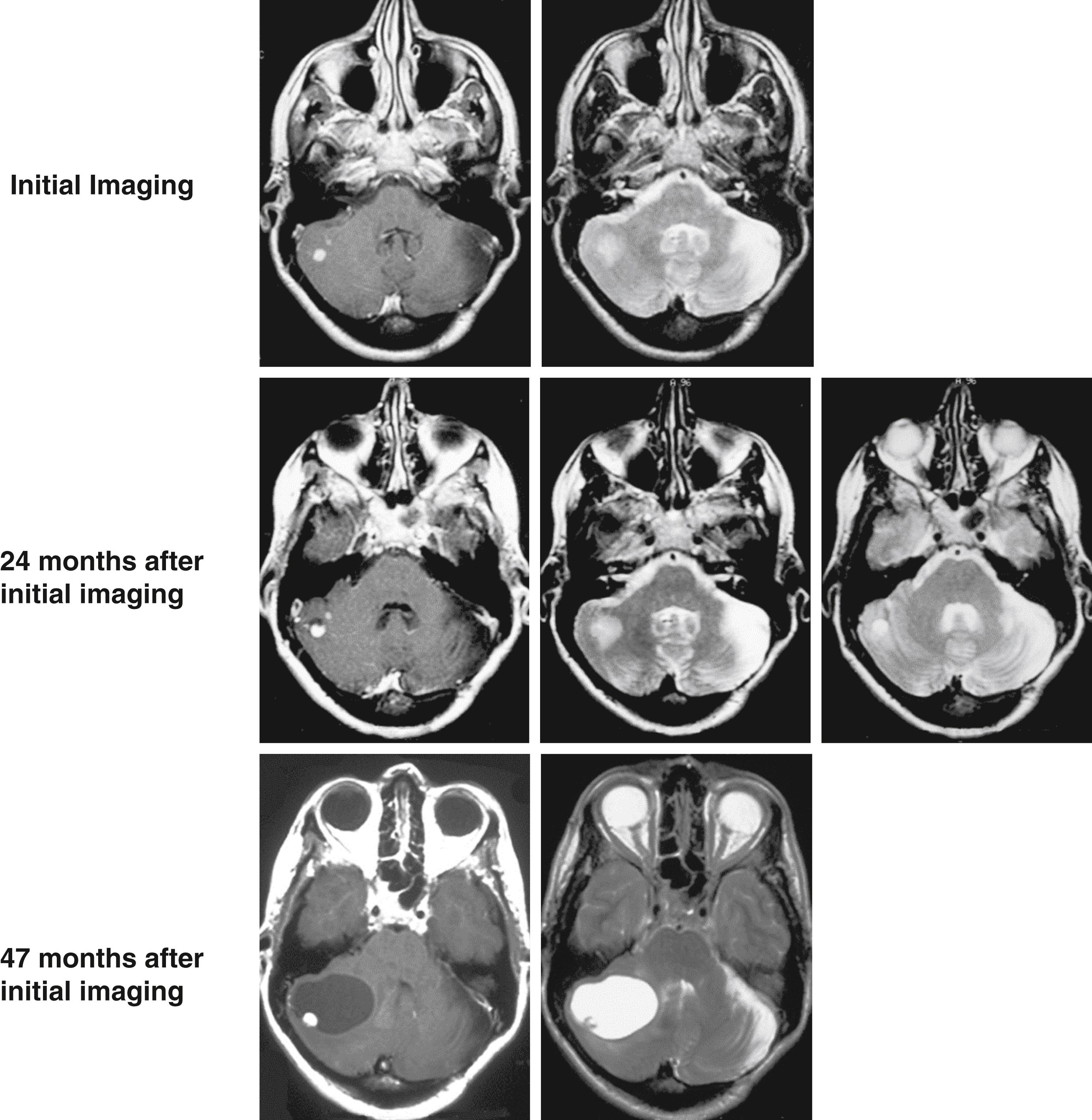
Tumors with extensive vasogenic edema often have high levels of VEGF expression. Blockage of VEGF pathways reduces vascular permeability in vitro. Furthermore, data indicate that glucocorticoids (e.g., dexamethasone) may inhibit both the effects of VEGF on vessel walls and the production of VEGF in tumors. , The use of the anti-VEGF antibody bevacizumab leads to dramatic reduction in gadolinium enhancement and surrounding edema in patients with glioblastoma. , Similar imaging improvements are seen with bevacizumab when used for the treatment of radiation necrosis, which is a disorder characterized by a combination of vasogenic and cytotoxic edema states in which VEGF is implicated. , Other growth factors and cytokines have been shown to alter blood-brain barrier permeability, and their impacts are being studied in the initiation and treatment of cerebral edema.
As described earlier, studies have demonstrated that peritumoral edema results from the extravasation of plasma ultrafiltrate through permeable neoplastic vessels into the interstitial spaces of the tumor. From the tumor, which has an elevated interstitial pressure, the plasma ultrafiltrate moves to the surrounding interstitial spaces of the CNS via bulk (or convective) flow. The excess interstitial fluid expands the extracellular spaces (edema) and is resorbed by the surrounding nervous system tissues and glymphatic system. At any point in time, the volume and or of peritumoral edema is defined by homeostasis between plasma ultrafiltrate extravasation into interstitial spaces and tissue fluid absorption.
With increasing tumor vessel surface area (via neovascularization and/or tumor progression) and/or increased tumor vascular permeability, the amount of plasma ultrafiltrate extravasation will increase and the region of edema will expand until homeostasis is achieved. Over time, the formation of peritumoral gliosis and increased interstitial pressure due to increased plasma ultrafiltrate flow can reduce the capacity for tissue and glymphatic fluid absorption. Ultimately, the rate of fluid extravasation can exceed tissue and glymphatic absorption, and the solid tissue properties of the nervous system will favor the development of a peritumoral cyst (see Fig. 70.3 ). Because the peritumoral cyst represents the path of least resistance, the majority of tumor-derived plasma ultrafiltrate will preferentially flow into it.
Based on the pathophysiology of peritumoral cyst formation, there are important clinical implications. , First, the tumor is the source the interstitial fluid, and complete resection of the tumor mass (not cyst wall) will result in collapse or resolution of edema and the cyst. Second, modalities (radiation therapy) that increase vascular permeability can result in progression of peritumoral edema and cyst, leading to symptom exacerbation. Third, therapies (chemotherapy and steroids) that reduce tumor vascular permeability can reduce edema and cyst size and alleviate associated symptomatology and may have no effect on tumor size or progression. Finally, because the mechanism of peritumoral cyst formation is based on fluid homeostasis, edema or cyst progression often exceeds tumor progression, requiring tumor removal to alleviate cyst growth.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here