Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiogenic shock (CS) is characterized by hypotension and end-organ hypoperfusion as a result of low cardiac output. CS remains the most common cause of death after presentation with a myocardial infarction (MI). This clinical state occurs in up to 10% of patients hospitalized with acute MI and remains the leading cause of death during hospitalization. The incidence of CS has decreased only slightly over time, and the mortality rate remains high, at approximately 50% despite advances in interventional and pharmacological management. For patients with ischemic etiology following MI, early recognition and treatment with revascularization and mechanical circulatory support (MCS) improves prognosis and allows for stabilization while considering options for a durable left ventricular assist device (LVAD) and heart transplantation in selected refractory cases.
CS that occurs as a consequence of acute MI is most commonly secondary to severe left ventricular (LV) dysfunction. This may result from a large-index MI or from acute injury in patients with previous LV dysfunction. In the SHOCK (Should We Revascularize Occluded Coronaries for Cardiogenic Shock) trial, predominant LV failure accounted for four of five of all such cases. Approximately one-third of the patients enrolled in the study had evidence of a previous MI.
Several unique clinical entities may also present with acute hemodynamic collapse. Mechanical complications associated with shock include acute mitral regurgitation related to papillary muscle dysfunction or rupture, ventricular septal rupture (VSR), or free-wall rupture. Right ventricular (RV) failure due to RV infarction in isolation or in combination with LV failure can also present in this manner. The clinician should also be aware of iatrogenic shock that results from inappropriate administration of medications (e.g., β-blockers). Occult hemorrhage due to procedure-related complications or in conjunction with therapy using antithrombotic, antiplatelet, and fibrinolytic agents can also cause hypotension and shock.
Several nonischemic and extracardiac etiologies must be considered in patients with hypotension and suspected CS. Acute myocarditis secondary to infections or toxins can lead to the development of CS within hours of the first signs of illness. Takotsubo cardiomyopathy (also known as stress cardiomyopathy or apical ballooning syndrome) is another cause of acute LV dysfunction, typically in response to emotional or physical stress, and can present as CS. The differential diagnosis should also include acute aortic dissection, which can be associated with acute aortic valve regurgitation, coronary artery dissection, aortic rupture, and tamponade. Cardiac tamponade can also present secondary to a focal myocardial hematoma after cardiac surgery or trauma or from a circumferential pericardial effusion from malignancy, infarction, or infection. A pulmonary embolism may cause volume and pressure overload of the RV and obstruction of RV outflow, which can lead to hemodynamic collapse. Myocardial depression secondary to septic shock must also be excluded. Acute aortic or mitral valve insufficiency from degenerative disease or bacterial endocarditis can also present with the hemodynamic findings of CS.
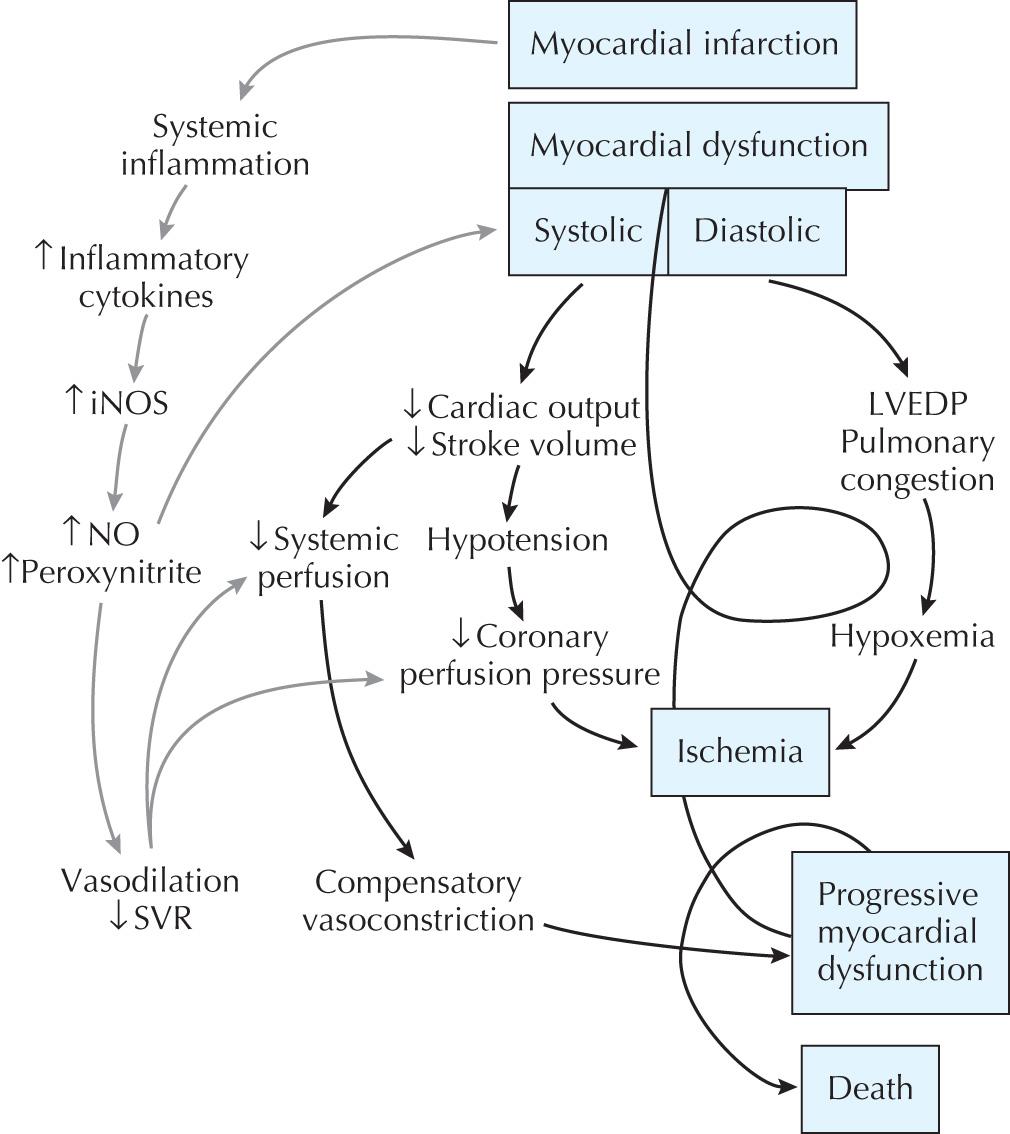
CS is traditionally defined as an unsupported systolic blood pressure of <90 mm Hg with normal to elevated LV filling pressures and evidence of end-organ hypoperfusion. In the setting of acute MI, this is due to plaque rupture and/or thrombosis, which results in acute myocardial dysfunction with decreased cardiac output on the basis of inadequate LV stroke volume. Hypotension can then lead to further reduction in coronary perfusion pressure and further worsening of myocardial ischemia. There may also be cardiac ischemia due to fixed flow-limiting stenoses in nonculprit epicardial coronary arteries remote from the infarct-related vessel. Ischemia thus begets ischemia, which results in a progressive spiral of hemodynamic collapse, culminating with death. In this traditional paradigm of CS, vasoconstriction from falling cardiac output was believed to be the major mechanism by which the neurohormonal system compensates for hypotension. The recognition that many patients have unexpected vasodilation and low systemic vascular resistance in this setting has led to modification of this conceptual design. Observational evidence suggests that inflammatory cytokines, such as interleukin-6, interleukin-1, and tumor necrosis factor-α, are elevated in patients with CS to the same levels seen in patients in a septic state. These findings suggest that MI may result in a systemic inflammatory response syndrome as previously observed with infection or trauma that results in myocardial depression and hypotension independent of ischemic necrosis ( Fig. 24.1 ). These findings are also of importance when considering the diagnostic evaluation of CS patients and optimal therapy (see the following).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here