Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Arterial and venous thromboses are major causes of morbidity and mortality. Arterial thrombosis is the most common cause of acute myocardial infarction (MI), ischemic stroke, and limb gangrene, whereas deep vein thrombosis can lead to pulmonary embolism, which can be fatal. Most arterial thrombi are superimposed on disrupted atherosclerotic plaque, which exposes thrombogenic material in the plaque core to the blood. This material then triggers platelet aggregation and fibrin formation, which results in the generation of a platelet-rich thrombus that can temporarily or permanently occlude blood flow.
In contrast to arterial thrombi, venous thrombi rarely form at sites of obvious vascular disruption. Although they can develop after surgical trauma to veins, or secondary to indwelling venous catheters, venous thrombi usually originate in the valve cusps of the deep veins of the calf or in the muscular sinuses, where they are triggered by stasis. Sluggish blood flow in these veins reduces the oxygen supply to the avascular valve cusps. Endothelial cells lining these valve cusps become activated and express adhesion molecules on their surface. Tissue factor-bearing leukocytes and microparticles adhere to these activated cells and induce coagulation. In addition, webs of DNA released from activated neutrophils, so-called neutrophil extracellular traps, also contribute to thrombosis by providing a scaffold that binds platelets and promotes their activation and aggregation. Local thrombus formation is exacerbated by reduced clearance of activated clotting factors as a result of impaired blood flow. If the calf vein thrombi extend into more proximal veins of the leg, thrombus fragments can dislodge, travel to the lungs, and produce a pulmonary embolism.
Arterial and venous thrombi are composed of platelets and fibrin, but the proportions differ. Arterial thrombi are rich in platelets because of the high shear in the injured arteries. In contrast, venous thrombi, which form under low shear conditions, contain relatively few platelets and are predominantly composed of fibrin and trapped red cells.
Antithrombotic drugs are used for prevention and treatment of thrombosis. Targeting the components of thrombi, these agents include: (a) antiplatelet drugs, which inhibit platelets; (b) anticoagulants, which attenuate coagulation; and (c) thrombolytic agents, which induce fibrin degradation ( Fig. 41.1 ). With the predominance of platelets in arterial thrombi, strategies for prevention or treatment of arterial thrombosis focus mainly on antiplatelet agents, although in the acute setting, they often include anticoagulants and thrombolytic agents. Anticoagulants are the mainstay of prevention and treatment of venous thromboembolism because fibrin is the predominant component of venous thrombi. Antiplatelet drugs are less effective than anticoagulants in this setting because of the limited platelet content of venous thrombi.
Anticoagulants are available in parenteral and oral forms. Parenteral anticoagulants include unfractionated heparin, low-molecular-weight heparin (LMWH), fondaparinux, a synthetic pentasaccharide, and parenteral direct thrombin inhibitors (bivalirudin, hirudin analogs and argatroban). Oral anticoagulants include vitamin K antagonists (VKAs), such as warfarin, and the direct oral anticoagulants (DOACs); dabigatran etexilate, which inhibits thrombin, and rivaroxaban, apixaban and edoxaban, which inhibit factor Xa.
This chapter focuses on anticoagulant drugs. The reader is referred to Chapter 42 (Antiplatelet Agents) for a comprehensive presentation of antiplatelet therapy.
A sulfated polysaccharide, heparin is isolated from mammalian tissues rich in mast cells. Most commercial heparin is derived from porcine intestinal mucosa.
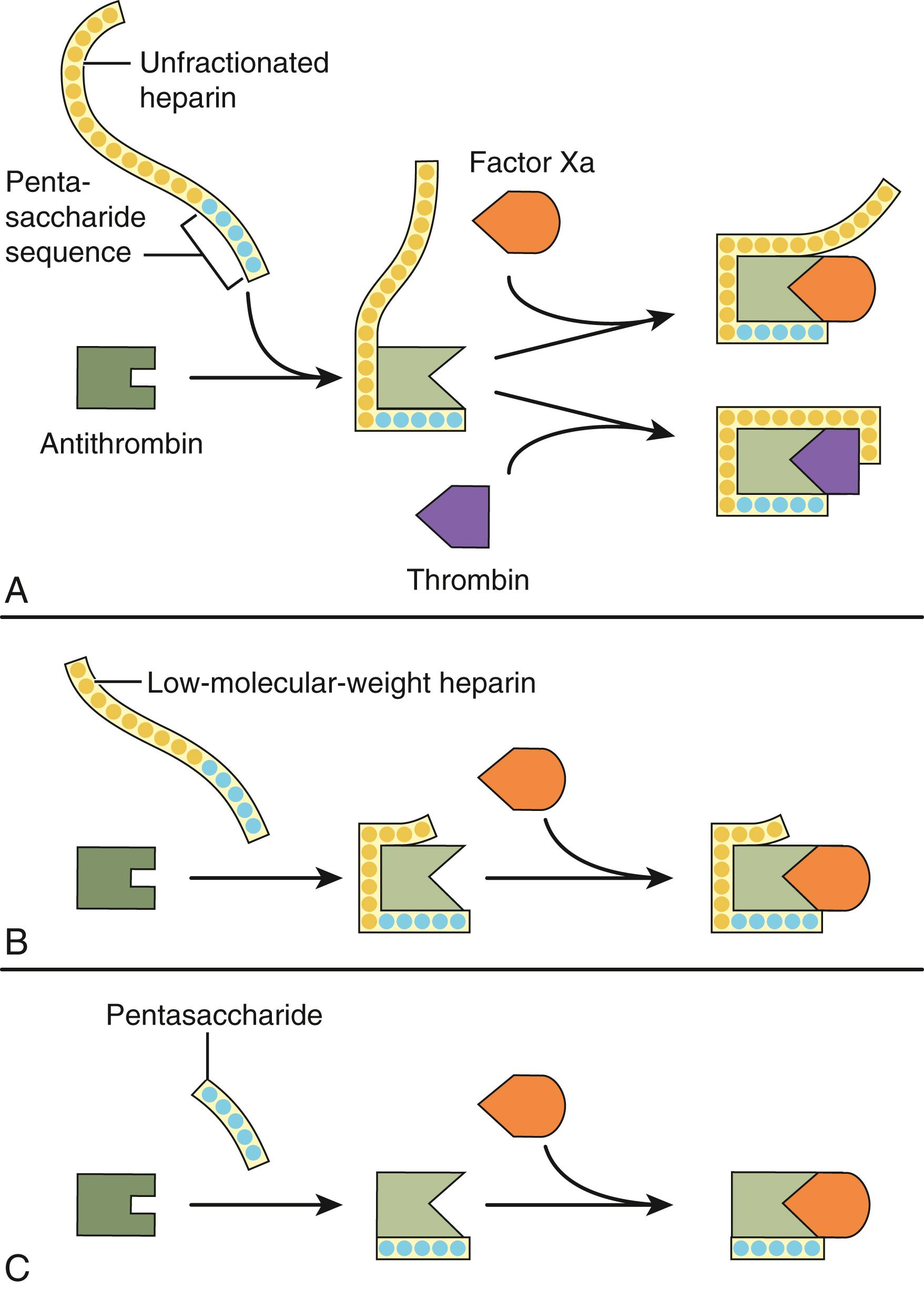
Heparin acts as an anticoagulant by activating antithrombin (previously known as antithrombin III) and accelerating the rate at which antithrombin inhibits clotting enzymes, particularly thrombin and factor Xa. To activate antithrombin, heparin binds to the serine protease inhibitor via a unique pentasaccharide sequence that is found on one third of the chains of commercial heparin ( Fig. 41.2 ). The remainder of the heparin chains that lack this pentasaccharide sequence have little or no anticoagulant activity. Once bound to antithrombin, heparin induces a conformational change in the reactive center loop of antithrombin that renders it more readily accessible to its target proteases. This conformational change enhances the rate at which antithrombin inhibits factor Xa by at least two orders of magnitude but has little effect on the rate of thrombin inhibition by antithrombin. To catalyze thrombin inhibition, heparin serves as a template that binds antithrombin and thrombin simultaneously. Formation of this ternary complex brings the enzyme in close apposition to the inhibitor, thereby promoting the formation of a stable covalent thrombin–antithrombin complex. Only pentasaccharide-containing heparin chains composed of at least 18 saccharide units (which corresponds to a molecular weight of 5400) are of sufficient length to bridge thrombin and antithrombin together. With a mean molecular weight of 15,000, and a range of 5000 to 30,000, most of the chains of unfractionated heparin are long enough to produce this bridging. Consequently, by definition, heparin has equal capacity to promote the inhibition of thrombin and factor Xa by antithrombin and is assigned a 1:1 ratio of anti–factor Xa to anti-factor IIa (thrombin) activity.
Heparin induces the release of tissue factor pathway inhibitor (TFPI), a factor Xa–dependent inhibitor of tissue factor-bound factor VIIa, from the endothelium. TFPI may contribute to the antithrombotic activity of heparin. Longer heparin chains induce the release of more TFPI than shorter chains.
Heparin must be given parenterally. It is usually administered subcutaneously when given for prophylaxis and by continuous intravenous infusion when used for therapeutic purposes. If heparin is given subcutaneously for treatment of thrombosis, the dose of heparin must be high enough to overcome the limited bioavailability associated with this method of delivery.
In the circulation, heparin binds to the endothelium. Heparin binding to endothelial cells explains its dose-dependent clearance. At low doses, the half-life of heparin is short because it rapidly binds to the endothelium. With higher doses of heparin, the endothelium is saturated and the half-life is longer. Because of this phenomenon, the plasma half-life of heparin ranges from 30 to 60 minutes with bolus intravenous doses of 25 and 100 units/kg, respectively. Clearance of heparin is mainly extrarenal; heparin binds to macrophages, which internalize and depolymerize the long heparin chains and secrete shorter chains back into the circulation.
Once the endothelium is saturated, heparin enters the circulation where it binds plasma proteins other than antithrombin, a phenomenon that reduces the anticoagulant activity of heparin. Some of the heparin-binding proteins in plasma are acute-phase reactants whose levels are elevated in ill patients.
Since the levels of heparin-binding proteins in plasma vary from person to person, the anticoagulant response to fixed or weight-adjusted doses of heparin is unpredictable. Consequently, coagulation monitoring is essential to ensure that a therapeutic response is obtained. This is particularly important when heparin is administered for treatment of established thrombosis because a subtherapeutic anticoagulant response renders patients at risk for recurrent thrombosis, whereas excessive anticoagulation increases the risk for bleeding.
The interaction of heparin with plasma proteins also contributes to the phenomenon of heparin rebound. Defined as the reappearance of anticoagulant activity after adequate neutralization of heparin with protamine, heparin rebound may contribute to excessive bleeding after cardiac surgery. This phenomenon likely reflects the slow release of protein-bound heparin after circulating heparin is neutralized by protamine. Heparin rebound can be managed by administration of additional protamine by intravenous bolus or as a low-dose continuous infusion.
Heparin therapy can be monitored using the activated clotting time (ACT), activated partial thromboplastin time (aPTT) or anti-factor Xa level. The ACT, which is less sensitive than the aPTT and can be measured at the bedside, is used to monitor the high doses of heparin given during PCI or cardiac or vascular surgery. The aPTT is the test most often used to monitor therapeutic doses of heparin. If anti-factor Xa levels are measured, the therapeutic level ranges from 0.3 to 0.7 units/mL.
For prophylaxis, heparin is usually given in fixed doses of 5000 units subcutaneously two or three times daily. With these low doses, coagulation monitoring is unnecessary. In contrast, monitoring is essential when the drug is given in therapeutic doses. Fixed-dose or weight-based heparin nomograms are used to standardize heparin dosing and to shorten the time required to achieve a therapeutic anticoagulant response.
Heparin has pharmacokinetic and biophysical limitations ( Table 41.1 ). The pharmacokinetic limitations reflect heparin’s propensity to bind in a pentasaccharide-independent fashion to cells and plasma proteins. Heparin binding to endothelial cells explains its dose-dependent clearance, whereas binding to plasma proteins results in a variable anticoagulant response and can lead to heparin resistance.
| Limitations | Mechanism |
|---|---|
| Poor bioavailability at low doses | Limited absorption of long heparin chains |
| Dose-dependent clearance | Binds to endothelial cells |
| Variable anticoagulant response | Binds to plasma proteins whose levels vary from patient to patient |
| Reduced activity in the vicinity of platelet-rich thrombi | Neutralized by platelet factor 4 released from activated platelets |
| Limited activity against factor Xa incorporated in the prothrombinase complex and thrombin bound to fibrin | Reduced capacity of the heparin-antithrombin complex to inhibit factor Xa bound to activated platelets and thrombin bound to fibrin |
The most common side effect of heparin is bleeding. Other complications include thrombocytopenia, osteoporosis, and elevated levels of transaminases.
The risk for heparin-induced bleeding increases with higher heparin doses. Concomitant administration of antiplatelet or fibrinolytic agents increases the risk for bleeding, as does recent surgery or trauma. Heparin-treated patients with serious bleeding can be given intravenous protamine sulfate to neutralize the heparin. Protamine sulfate, a mixture of basic polypeptides isolated from salmon sperm, binds heparin with high affinity, and the resultant protamine–heparin complexes are then cleared. Typically, 1 mg of protamine sulfate neutralizes 100 units of heparin. Anaphylactoid reactions to protamine sulfate can occur, and drug administration by slow intravenous infusion is recommended to reduce the risk for these problems.
Heparin can cause thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is an antibody-mediated process that is triggered by antibodies directed against neoantigens on PF4 that are exposed when heparin binds to this protein. These antibodies, which usually are of the IgG subtype, bind simultaneously to the heparin–PF4 complex and to platelet Fc receptors. Such binding activates the platelets and generates platelet microparticles. Circulating microparticles are prothrombotic because they express anionic phospholipids on their surface and can bind clotting factors, thereby promoting thrombin generation.
The clinical features of HIT are illustrated in Table 41.2 . Typically, HIT occurs 5 to 14 days after initiation of heparin therapy, but it can manifest earlier if the patient has received heparin within the past 3 months. Even a 50% decrease in the platelet count from the pretreatment value should raise the suspicion of HIT in those receiving heparin. HIT is more common in surgical patients than in medical patients and, like many autoimmune disorders, occurs more frequently in females than in males.
| Features | Details |
|---|---|
| Thrombocytopenia | Platelet count of 100,000/μL or less or a decrease in platelet count of 50% or more |
| Timing | Platelet count falls 5–10 days after starting heparin |
| Type of heparin | More common with unfractionated heparin than LMWH. Rare with fondaparinux. |
| Type of patient | More common in surgical patients than medical patients; more common in women than men |
| Thrombosis | Venous thrombosis more common than arterial thrombosis |
HIT can be associated with thrombosis, either arterial or venous. Venous thrombosis, which manifests as deep vein thrombosis and/or pulmonary embolism, is more common than arterial thrombosis. Arterial thrombosis can manifest as ischemic stroke or acute MI. Rarely, platelet-rich thrombi in the distal aorta or iliac arteries can cause critical limb ischemia (see Ch. 40 , Disorders of Coagulation: Hypercoagulable States).
The diagnosis of HIT is established using enzyme-linked assays to detect antibodies against heparin–PF4 complexes or with platelet activation assays. Enzyme-linked assays are sensitive, but can be positive in the absence of any clinical evidence of HIT. The most specific diagnostic test is the serotonin release assay. This test is performed by quantifying serotonin release when washed platelets loaded with labeled serotonin are exposed to patient serum in the absence or presence of varying concentrations of heparin. If the patient serum contains the HIT antibody, heparin addition induces platelet activation and subsequent serotonin release.
Management of HIT is outlined in Table 41.3 . Heparin should be stopped in patients with suspected or documented HIT, and an alternative anticoagulant should be administered to prevent or treat thrombosis. The agents most often used for this indication are parenteral direct thrombin inhibitors (such as argatroban or bivalirudin) or parenteral or oral factor Xa inhibitors (such as fondaparinux, danaparoid or rivaroxaban). The alternative anticoagulant should be continued until the platelet count is normal or the diagnosis of HIT is excluded. Warfarin should be avoided in HIT patients because it can trigger skin necrosis and thrombosis. These complications occur because warfarin lowers the levels of protein C and many HIT patients already have low levels of this anticoagulant protein because of increased thrombin generation.
|
|
|
|
|
Consisting of smaller fragments of heparin, LMWH is prepared from unfractionated heparin by controlled enzymatic or chemical depolymerization. The mean molecular weight of LMWH is 5000, one-third the mean molecular weight of unfractionated heparin. LMWH has advantages over heparin ( Table 41.4 ) and has replaced heparin for most indications.
| Advantage | Consequence |
|---|---|
| Better bioavailability and longer half-life after subcutaneous injection | Can be given subcutaneously once or twice daily for both prophylaxis and treatment |
| Dose-independent clearance | Simplified dosing |
| Predictable anticoagulant response | Coagulation monitoring is unnecessary in most patients |
| Lower risk of heparin-induced thrombocytopenia | Safer than heparin for short- or long-term administration |
| Lower risk of osteoporosis | Safer than heparin for extended administration |
Like heparin, LMWH exerts its anticoagulant activity by activating antithrombin. With a mean molecular weight of 5000, which corresponds to about 17 saccharide units, at least half of the pentasaccharide-containing chains of LMWH are too short to bridge thrombin to antithrombin ( Fig. 41.2 ). However, these chains retain the capacity to accelerate factor Xa inhibition by antithrombin because this activity is largely the result of the conformational changes in antithrombin evoked by pentasaccharide binding. Consequently, LMWH catalyzes factor Xa inhibition more than thrombin inhibition. Depending on their molecular weight distribution, the anti-factor Xa to anti-factor IIa ratios of the various LMWH preparations range from 2:1 to 9:1.
Although usually given subcutaneously, LMWH also can be administered intravenously if a rapid anticoagulant response is needed. LMWH has pharmacokinetic advantages over heparin. These advantages reflect the fact that shorter heparin chains bind less avidly to endothelial cells, macrophages, and heparin-binding plasma proteins. Reduced binding to endothelial cells and macrophages eliminates the rapid, dose-dependent, and saturable mechanism of clearance characteristic of unfractionated heparin. Instead, the clearance of LMWH is dose independent and its plasma half-life is longer. Based on measurement of anti-factor Xa levels, LMWH has a plasma half-life of about 4–6 hours. LMWH is cleared almost exclusively by the kidneys, and the drug can accumulate in patients with renal insufficiency.
LMWH exhibits about 90% bioavailability after subcutaneous injection. Because LMWH binds less avidly to heparin-binding proteins in plasma than heparin, LMWH produces a more predictable dose response and resistance to LMWH is rare. With a longer half-life and more predictable anticoagulant response, LMWH can be given subcutaneously once or twice daily without coagulation monitoring. These properties render LMWH more convenient than unfractionated heparin. Capitalizing on this feature, studies in patients with venous thromboembolism have shown that home treatment with LMWH is as effective and safe as in-hospital treatment with continuous intravenous infusions of heparin. Out-patient treatment with LMWH streamlines care, reduces healthcare costs, and increases patient satisfaction (see Ch. 148 : Acute Lower Extremity Deep Venous Thrombosis: Presentation, Diagnosis, and Medical Treatment).
In most patients, LMWH does not require coagulation monitoring. If monitoring is necessary, anti-factor Xa levels must be measured because LMWH has little effect on the aPTT. Therapeutic anti-factor Xa levels with LMWH range from 0.5 to 1.2 units/mL when measured 3 to 4 hours after drug administration. When LMWH is given in prophylactic doses, peak anti-factor Xa levels of 0.2 to 0.5 units/mL are desirable.
Indications for LMWH monitoring include renal insufficiency and obesity. LMWH monitoring in patients with a creatinine clearance of 50 mL/min or less is advisable to ensure that there is no drug accumulation. Although weight-adjusted LMWH dosing appears to produce therapeutic anti-factor Xa levels in patients who are overweight, this approach has not been extensively evaluated in those with morbid obesity. It may be advisable to monitor the anticoagulant activity of LMWH during pregnancy because dose requirements can change, particularly in the third trimester. Monitoring also should be considered in high-risk settings, such as in pregnant women with mechanical heart valves who are given LMWH for prevention of valve thrombosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here