Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection and accounts for approximately 20% of deaths worldwide. Despite tremendous advancements in early diagnosis and both surgical and antimicrobial therapies, it remains a leading cause of in-hospital and intensive care unit (ICU) mortality. , Intraabdominal sepsis is the second most prevalent infectious etiology after pneumonia. , Similar to other etiologies of sepsis, early recognition, initiation of antimicrobial therapy, and expedient volume resuscitation are crucial components of initial management of intraabdominal sepsis. Uniquely, rapid and definitive source control is an additional early management priority that differentiates intraabdominal sepsis from other infectious etiologies and is crucial to survival. Failure to achieve early source control is more likely to cause death than refractory infection by a multidrug-resistant pathogen. Independent risk factors for 30-day mortality in severe intraabdominal sepsis include the extent of peritonitis, type of exudate (purulent, fecal or bile), and a nonappendiceal source of infection. In those who require intensive care, studies have shown a mortality rate that exceeds 25%. Recent trends indicate that although inpatient mortality after abdominal sepsis is declining, a large number of “sepsis survivors” develop a clinical trajectory of chronic critical illness (CCI) and suffer dismal long-term outcomes.
Intraabdominal infection (IAI) occurs when bacteria or yeast invade the normally sterile peritoneal cavity. This condition encompasses a variety of pathologic processes ranging from localized appendicitis to feculent peritonitis after colonic perforation. Both bacterial factors and the host innate immune response contribute to the subsequent clinical course and influence the transition from initial infection to sepsis. There is a wide range of disease severity based on the physiologic response of the patient, including systemic inflammatory response syndrome (SIRS), sepsis, and septic shock. These represent a physiologic continuum with a progressively worsening balance of proinflammatory and antiinflammatory responses. Multiple organ dysfunction syndrome (MODS) and multisystem organ failure (MOF) are terms used to describe the development of end-organ dysfunction in septic patients that leads to physiologic imbalances that are not maintainable without organ support interventions.
Several classification systems exist to describe IAIs. These include (1) community acquired versus hospital acquired and (2) complicated versus uncomplicated infections. Complicated infections can be categorized as localized (such as an abscess) or diffuse (peritonitis). Diffuse peritonitis is further classified as primary, secondary, and tertiary. Each of these has a distinct spectrum of infectious organisms ( Table 90.1 ). Therefore treatment regimens vary based on the etiology and extent of infection. Most IAIs (e.g., appendicitis, colitis) are community acquired and, if identified and treated promptly, do not require intensive care. However, some patients will go on to develop sepsis and become critically ill, usually because of delayed presentation or recognition, immunosuppression, or advanced age/frailty. The majority of these patients have polymicrobial intraabdominal infections as a result of inflammatory or ischemic bowel perforation or postoperative complications (i.e., enteric anastomotic leak).
| Primary Peritonitis | Secondary Peritonitis | Tertiary Peritonitis |
|---|---|---|
| Escherichia coli | Bacteroides fragilis | Enterobacter spp. |
| Enterococcus spp. | Escherichia coli | Enterococcus spp. |
| Klebsiella spp. | Klebsiella spp. | Acinetobacter spp. |
| Streptococcus pneumoniae | Clostridium spp. | Pseudomonas spp. |
| Other anaerobe spp. | Staphylococcus spp. | |
| Candida spp. |
Uncomplicated IAIs are those contained within a single organ (e.g., appendicitis, cholecystitis) and rarely lead to critical illness if addressed in a timely fashion. Definitive management is surgical, and antibiotics are not warranted after source control has been achieved. If the infection is contained by the initial host inflammatory response, an intraabdominal abscess will form, which depending on the size, accessibility, and patient status is best treated with abdominal-spectrum antibiotic therapy and image-guided percutaneous drain placement. Abscesses that are not amenable to percutaneous drainage and fail to resolve (or progress) on antibiotic therapy alone may require operative washout and drainage.
In contrast, complicated IAIs extend beyond the source organ into the peritoneal cavity, resulting in a much greater systemic inflammatory response. Complicated infections causing peritonitis can be classified as primary, secondary, or tertiary. In primary peritonitis, formerly referred to as “spontaneous” bacterial peritonitis, the infection does not arise from direct gastrointestinal (GI) tract spillage or contamination and rarely causes critical illness. Primary peritonitis most commonly occurs in patients with ascites caused by advanced cirrhosis, but can also be associated with collagen vascular disease or glomerulopathies. The diagnosis is made with a positive ascites bacterial culture and elevated fluid absolute polymorphonuclear leukocyte (PMN) count. These cases are almost always monomicrobial (most commonly Escherichia coli [~70%] or Klebsiella [~10%]). Treatment consists of antibiotic therapy, and there is no role for operative intervention. Drainage of diffuse ascites as a means of source control is not warranted unless loculated peritoneal fluid collections develop. Device-associated primary peritonitis can occur in patients on peritoneal dialysis. The incidence of these infections is relatively high, at approximately one episode per year of peritoneal dialysis. Peritoneal catheter-related infections are usually monomicrobial, and the most common pathogens are Staphylococcus aureus, Pseudomonas, and Candida . Although rare, these patients can develop methicillin-resistant S. aureus (MRSA), and vancomycin-resistant pathogens are emerging. Treatment includes catheter removal and antibiotics.
Secondary peritonitis is by far the most common presentation in clinical practice and describes infections with an intraabdominal pathology. Causes of secondary peritonitis include perforation of a hollow viscus, bowel ischemic/necrosis, leak of surgical bowel anastomoses, and bowel injury after penetrating trauma. These infections are polymicrobial with, on average, five distinct organisms identified in a peritoneal fluid sample. Not surprisingly, common gut flora are the most frequently encountered bacteria (e.g., E. coli, Klebsiella, Bacteroides ). Treatment includes percutaneous or operative source control and antibiotic therapy.
An infection that recurs or persists after a source control procedure has been performed is considered tertiary peritonitis. These infections are also polymicrobial, but can present an additional management challenge, as there is an increased likelihood of multidrug-resistant organisms (e.g., S. aureus /MRSA, Enterococcus /vancomycin-resistant Enterococcus [VRE], Candida, Pseudomonas ). These patients may have an acute or chronic host defense impairment that has resulted in an inability to clear the index infection. Whether tertiary peritonitis represents a persistent invasive infection versus peritoneal cavity colonization remains controversial.
Regardless of the etiology of peritonitis, clinicians should be aware of the presence of adjuvant substances that decrease the bacterial threshold inoculum necessary to cause an infection and often require removal in order to clear the infection. The most common adjuvant is blood, but other examples include ascites, fibrin, bile, urine, chyle, pancreatic fluid, and platelets. Evacuation and ongoing drainage of these fluid adjuvants (via percutaneous or surgical drainage) are often required for adequate source control. Fibrin promotes bacterial trapping and can isolate bacteria from neutrophils. Iron present in hemoglobin is essential for bacterial growth and reduces phagocyte function. Foreign bodies are also a nidus for persistent infection and may be present in IAIs in the form of prosthetic mesh or nonabsorbable sutures. Clearance of infection most often requires explant of the infected material, especially when the formation of biofilms is suspected.
Most of the bacteria in the gut are commensal flora that generally do not contribute to the pathogenesis of IAI. They play a fundamental role in immunomodulation, maintenance of barrier function, metabolism (enterohepatic circulation), and overgrowth prevention of pathogenic microbes. Loss of intestinal homeostasis, whether by disruption of the gut mucosal barrier or by depleting the normal flora with long-term antibiotic use, is the first step in the development of abdominal sepsis. The vast majority of cases of IAI are bacterial, although intraabdominal candidiasis is not uncommon in critically ill patients and those who are immunocompromised. The microbial density and composition vary across the digestive tract. Therefore the offending pathogens in an IAI can, to some degree, be anticipated if the inciting pathology is known.
The stomach was initially considered to be a “sterile organ” because of its high intraluminal acidity. Helicobacter pylori was discovered in 1982, and for the next few decades, it was believed to be the only bacteria capable of survival in such a low pH secondary to its ability to produce neutralizing urease. The development of culture-independent molecular methods has facilitated the identification and classification of additional gastric bacteria. Although most gastric microbes originate from and are in transit from the oropharynx, the most abundant inhabitants are Streptococcus, Propionibacterium, and Lactobacillus . These bacteria are more prevalent in patients with reduced gastric acidity. In addition to bacteria, Candida commonly colonizes the GI tract and has been associated with the development of gastric ulcers. However, there are relatively small numbers of gastric microbes (<10 4 colony-forming units [CFU]/mL) in healthy subjects.
The proximal small bowel, because of the nearness to the stomach and outflowing acidic juices, contains many of the same bacteria in relatively small numbers. Duodenal bile and pancreatic secretions have additional antibacterial properties. Forward propulsion (peristalsis) also plays a key role in suppressing the flora of the upper GI tract. In the distal small bowel, the more alkaline conditions allow for the growth of enteric gram-negative rods. The terminal ileum represents a transition zone from the jejunum, containing predominantly aerobes, to the colon, containing predominantly anaerobes. There are typically <10 9 CFU/mL of microorganisms immediately proximal to the ileocecal valve.
In the colon, the concentration and variety of enteric flora changes dramatically. The number of anaerobic bacteria outnumber aerobic by a factor of up to 1000:1, with the most abundant being members of the Bacteroides genus. As many as 10 CFU/mL can be found, which contribute to 60% of fecal mass. The stability of the normal flora discourages infection by exogenous microbes and helps prevent overgrowth of pathogenic bacteria already present.
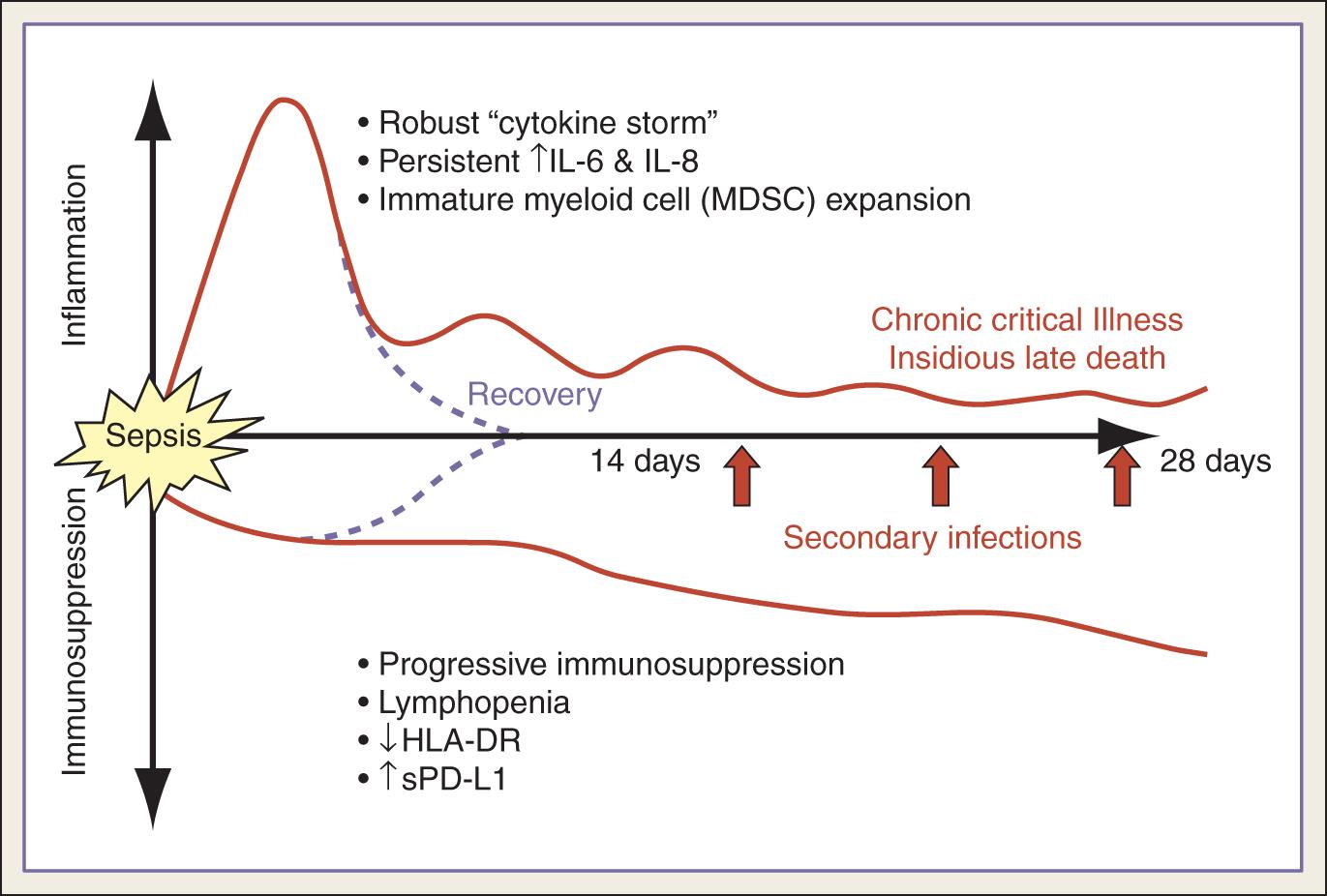
In general, the pathophysiology of intraabdominal sepsis is described as an initial hyperinflammatory phase that lasts several days followed by an immunosuppressive phase ( Fig. 90.1 ). This pathophysiologic state is the result of opposing influences of the host defense and pathogen virulence. Multiple factors contribute to the inflammatory response. Host factors include patient age, functional status, comorbidities, medications (e.g., steroids, immunosuppressants), environment, and genetics. Pathogen factors include the microbial load, virulence, and pathogen-associated molecular patterns (PAMPs). PAMPs are highly conserved molecules essential for bacterial survival that can be detected by the host innate immune response. Examples of PAMPs include lipopolysaccharide (LPS) and porins from gram-negative cell walls, peptidoglycans, lipoteichoic acids, flagellin in bacterial flagella, and bacterial/viral nucleic acids. PAMPs are recognized by Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) and trigger a robust innate immune response which can be beneficial to the host. However, this response can later be counterproductive if it is overly robust or fails to resolve and return to immunologic homeostasis.
Resident flora bacteria occupy the bowel lumen and adhere to the mucosa. Direct penetration of organisms through the mucosa is an abnormal event. However, pathogens such as Shigella, Salmonella, and Campylobacter can invade in this manner. More commonly, bacteria are released in the peritoneal cavity as the result of a perforation of the GI tract. When this occurs, the bacteria must proliferate to cause a clinical infection. To assist with this process, they express specific adherence factors that ultimately make their eradication much more difficult. Adherence factors are one of the many mechanisms bacteria use to achieve increased virulence. Endotoxins are LPSs in the outer membrane of gram-negative bacteria. They are large molecules that provide structural integrity and protect invading organisms from the host immune response. The lipid A domain of LPS is primarily responsible for the initial systemic toxicity in gram-negative bacterial infections. When bacterial cells are lysed, fragments containing lipid A are released into the host’s circulation, causing systemic symptoms such as fever and potentially septic shock. In gram-positive bacteria, lipoteichoic acid and peptidoglycans in the cell walls play an important role in initiating the host innate immune response; however, they can cause an excessive response resulting in the inflammatory sequelae involved in sepsis.
Once the inflammatory response has been initiated, proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α], interleukin [IL]-1, IL-6) are released, which have a multitude of effects. They cause production of toxic mediators such as prostaglandins, phospholipase A 2 , platelet-activating factor, and others, which damage the endothelial lining and subsequently lead to increased capillary leakage. They lead to increased production of adhesion molecules on neutrophils and endothelial cells, resulting in further endothelial injury from release of neutrophil components. These activated neutrophils release nitric oxide, a vasodilator that plays a key role in the development of septic shock. Proinflammatory cytokines from innate immune effector cells also disrupt activated protein C and antithrombin, modulators of coagulation. The culmination of these effects can result in extensive “third-spacing” of intravascular fluid, vasodilatory shock, and MODS/MOF.
After the initial “cytokine storm” triggered by infection, there is a subsequent phase of profound and persistent immunosuppression, which manifests as secondary infections and “sepsis recidivism.” This state of host immunoparalysis is the result of several mechanisms, including apoptotic depletion of immune cells, endotoxin tolerance/impaired cytokine response, the expansion of immature myeloid lineage cells (myeloid-derived stem cells [MDSCs]), and compromised T-cell function and exhaustion (see Fig. 90.1 ). Apoptosis of lymphocytes and antigen-presenting cells (T cells, B cells, and dendritic cells) is considered a hallmark of immunosuppression in sepsis and has shown promise as a new target for sepsis treatment in animal models. Endotoxin tolerance is the severely reduced capacity of a cell to respond to LPS in a second exposure to a toxic stimulus and represents an immune amnesia instead of an antiinflammatory response. T-cell exhaustion develops in the face of persistent antigen exposure and/or inflammation. This prolonged exposure leads to decreased expression of major histocompatibility complex (MHC) class II molecules (human leukocyte antigen [HLA]-DR) on antigen-presenting cells and increased expression of cell surface inhibitory receptors (such as programmed cell death-1 [PD-1]) on CD4 + and CD8 + T lymphocytes. Studies have shown that increased expression of PD-1 in circulating T cells from patients with sepsis correlated with decreased T-cell proliferative capacity and mortality.
More specific to abdominal sepsis is the concept of peritoneal compartmentalization. Based on findings of high concentrations of cytokines (IL-1, TNF-α, IL-6, IL10, interferon-gamma [IFN-γ]) in the peritoneal fluid of patients with peritonitis, studies have suggested that intraabdominal sepsis may result in a cytokine-mediated proinflammatory response that is initially localized to the abdominal compartment and that plasma levels may increase only after saturation of tissues within the peritoneal cavity.
Loss of integrity of the GI tract is the most common cause of IAI and abdominal sepsis. Inciting disease processes range from perforated gastric ulcers to acute appendicitis, diverticulitis, and traumatic injuries. Each of these clinical conditions is discussed in further detail in their respective chapters; however, their significance as common etiologies of abdominal sepsis should be noted. The basic tenets of sepsis management should be applied regardless of the specific cause: timely identification, rapid intravascular volume resuscitation, early initiation of antimicrobial therapy, and definitive source control.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here