Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Each of us follows well-established patterns as we go about our daily lives, and in turn we interact with our world through the recognition of patterns: in the people we know, the places we live, and the structures that form our physical environment. Similarly, the specialty of anatomic pathology relies heavily on pattern recognition, especially given the observational nature of this medical discipline. The experienced anatomic pathologist quickly recognizes the pattern of disease and, without dwelling on the initial overview, very often has already moved on instinctively to glean additional qualitative and quantitative information to arrive at a diagnosis. This initial observation forms the basis for this chapter and the basic patterns of pulmonary disease that will be discussed here. In addition, we will explore how specific morphologic findings contribute to the construction of a specific diagnosis or limited differential diagnosis.
The lung is an organ open to the environment, and with every breath it is exposed to a large number of potential injuries. Despite superb dynamics and adaptability, the lung responds to injury with a limited repertoire of inflammatory and reparative reactions. These reactions can be grouped based on their acuity (eg, acute, subacute, and chronic) and by the distribution of abnormalities spatially or in relation to underlying lung anatomy (eg, alveolar space filling, nodule formation). Some lung diseases are characterized by quite subtle changes at the microscopic level, and these are grouped together as a pattern we refer to as minimal changes (at scanning magnification). Through these foundation patterns, we present a practical approach to pulmonary pathology.
A journey through the true three-dimensional microanatomy of the lung, even today, is still mainly an imaginative one. A histology slide prepared from lung tissue presents the lung anatomy in two dimensions. To the inexperienced eye, the structure appears at first overly simple; yet translating what is seen in two dimensions to its corollary in three-dimensional anatomy takes considerable practice and repeated exposure. As a reasonable starting point, a schematic representation may be helpful ( Fig. 3.1 ). This relationship is also very important in correlating the histopathology with the radiologic changes, particularly those seen in high-resolution computed tomography (HRCT) scans. A radiology–pathology correlation of anatomic distribution in lung pathology is summarized in Table 3.1 .
| Histologic | Radiologic (HRCT) |
|---|---|
| Bronchocentric/bronchiolocentric | Centrilobular |
| Bronchovascular | |
| Angiocentric | Bronchovascular (arterial) |
| Interlobular septal (venous) | |
| Pleural/subpleural | Pleural/subpleural |
| Lymphatic | Bronchovascular |
| Interlobular septal | |
| Pleural/subpleural | |
| Peripheral acinar | Subpleural peripheral distribution (paraseptal) |
| Septal | Septal (interlobular septal) |
| Random nodular | Random nodular |
| Parenchymal consolidation | Consolidation |
| Diffuse interstitial | Diffuse interstitial, ground-glass attenuation |
| Mixed and unclassified | Mixed/unclassifiable |
In this chapter we will present these six patterns by describing the essential elements that each of them comprise, followed by a diagnostic algorithm by which individual disease entities within each pattern can be discerned. This pattern-based diagnostic approach is most helpful in the assessment of surgical wedge lung biopsies for nonneoplastic disease—the most difficult and challenging samples in pulmonary pathology. By following the pattern-based approach, we can be certain that all histologic entities from the most common to the most esoteric are acknowledged and, therefore, unlikely to be missed.
Detailed clinical and radiologic features of the specific lung diseases, along with treatment and prognosis, are presented throughout the chapters of this book. Here we will emphasize the pathologic features helpful in narrowing the differential diagnosis and provide cross-references to other chapters for more detailed discussion wherever appropriate.
Lung biopsies are diffusely involved by interstitial and alveolar edema, intraalveolar fibrin, and reactive type II cell hyperplasia
Infections
Sepsis
Shock, trauma
Radiation
Drugs or toxins
Aspiration
Many others (see other chapters)
Edema: interstitial and alveolar
Reactive type II pneumocytes
Hyaline membranes or fibrin deposition in alveoli
Variably present:
Necrosis
Neutrophils
Eosinophils
Siderophages
Vacuolated macrophages
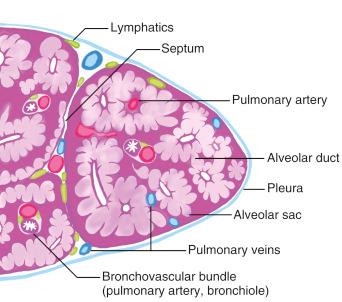
The lung biopsy is involved by varying amounts of alveolar wall edema, intraalveolar edema, and fibrin and has reactive pneumocytes ( Fig. 3.2 ). Acute lung injury typically has a short clinical evolution and often a rapid onset of symptoms. When the pattern of diffuse lung injury is noted, the biopsy should be searched for specific histologic features that will help suggest a more specific etiology. Infectious diseases should always lead the differential diagnosis in Pattern 1, and therefore special stains for organisms are a requirement, along with close correlation with clinical history and microbiologic findings.
Many systemic medical conditions can affect the lung, especially cardiovascular diseases, but also shock, trauma, sepsis, etc. Many of these conditions are rarely biopsied, and when they are, it is to assess the presence, extent, and severity of the lung injury and to exclude the possibility of atypical infection. The earliest changes seen in acute lung injury are interstitial (alveolar septal) edema, followed by intraalveolar edema and fibrin, and then accumulation of cellular alveolar debris. A search for necrosis or granulomas is essential in this setting.
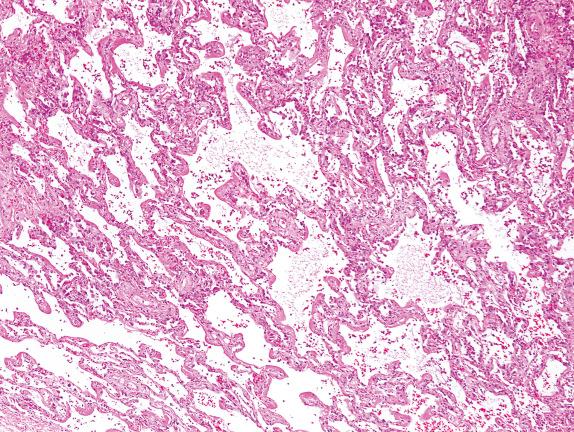
Hyaline membranes are accumulations of proteinaceous alveolar exudates at the periphery of the alveoli. These become adherent to the alveolar septa and are seen to outline the alveolar spaces ( Fig. 3.3 ). The diagnosis of diffuse alveolar damage (DAD) is appropriate when hyaline membranes are present. As the time between the insult and the biopsy becomes longer, the hyaline membranes become more organized (cellular) and distinct (thicker), a phenomenon seen 3 to 7 days after injury. During this phase, fibrin thrombi can be seen in the small pulmonary arteries, and the interstitium shows a mononuclear cell inflammatory infiltrate. When reactive type 2 cell hyperplasia is seen, the injury is usually about 1 week old, and the proliferative phase of DAD has started. This phase is characterized by fibroblastic proliferation, seen mainly in the interstitium, but also in the alveoli ( Fig. 3.4 ). By the late proliferative phase, most hyaline membranes have been reabsorbed. Mitotic activity can be prominent, and immature squamous metaplasia can be seen. Acute lung injury is often a repetitive event (ie, drugs, ongoing infections, or sepsis disease manifesting in the lung), and therefore changes characteristic of the exudative and proliferative phases of DAD can be seen in the same biopsy.
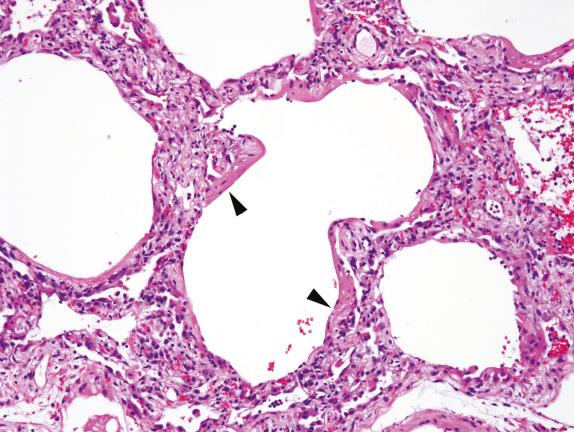
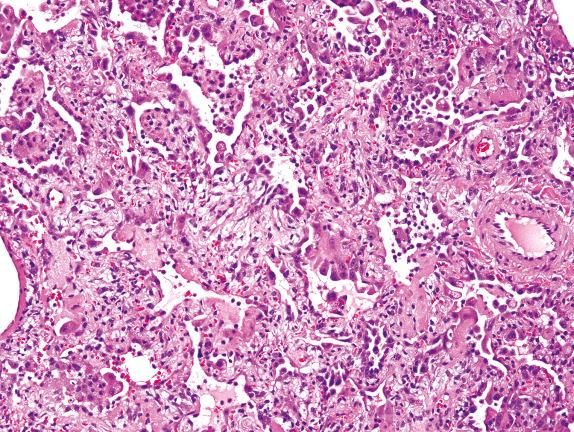
A new entity has recently been described with some similarities to DAD, termed acute fibrinous and organizing pneumonia (AFOP). AFOP lacks hyaline membranes but is rich in fibrinous alveolar exudates ( Fig. 3.5 ). By definition, AFOP lacks evidence of infection or extravascular eosinophils as might be expected of acute eosinophilic pneumonia (EP).
Among lung infections, viral infections are a recognized trigger of DAD ( Chapter 13 ). Bacterial and fungal infections can also occasionally present as DAD, either in the setting of a pneumonia or a systemic infection (sepsis).
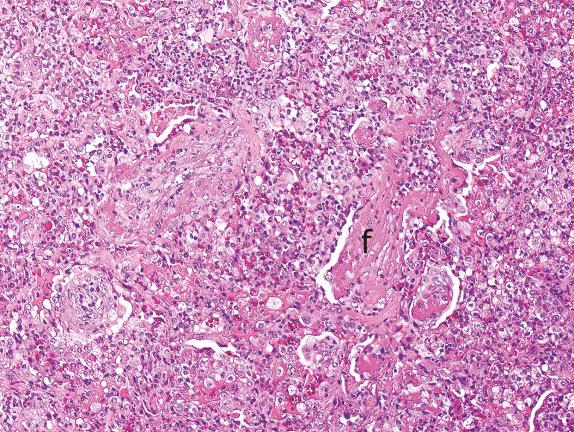
Necrosis in the setting of acute lung injury can be seen in infections, infarction, and acute aspiration. Necrosis can involve lung parenchyma alone or occur with necrosis of airway epithelium ( Fig. 3.6 ).
Influenza, herpes simplex, varicella-zoster, and adenovirus pneumonias are all infections characterized by DAD with necrosis, and some can be easily recognized by characteristic viral cytopathic effects such as intranuclear inclusions (herpes simplex, varicella-zoster) or smudge cells (adenovirus). Some bacterial and fungal infections can also produce necrosis.
Although lung infarction and acute aspiration pneumonia are localized rather than diffuse processes, they can be seen in a background of DAD and demonstrate necrosis. Malignancies can also present with associated areas of DAD, and necrosis can be prominent in these cases.
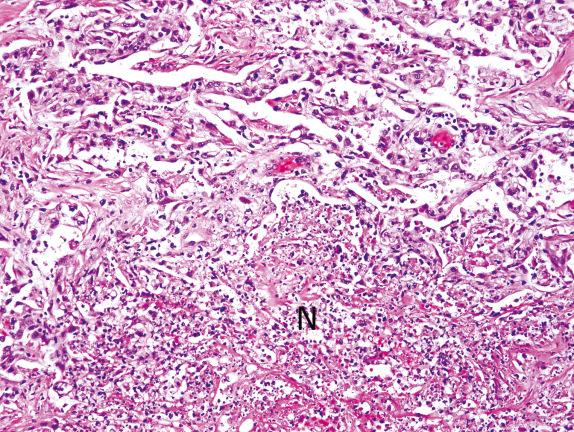
The presence of neutrophils in the alveolar spaces should always raise the possibility of infection (eg, acute bronchopneumonia) ( Fig. 3.7 ). In addition, a careful search for capillaritis is always warranted because this finding, with hemosiderin-laden macrophages, carries strong implications for immediate therapeutic intervention.
Influenza pneumonia can present with fibrinous and focally neutrophilic DAD. Identification of bacteria by Gram or silver stain in predominantly neutrophilic DAD is indicative of bacterial pneumonia, and fungi should also be sought with a silver stain.
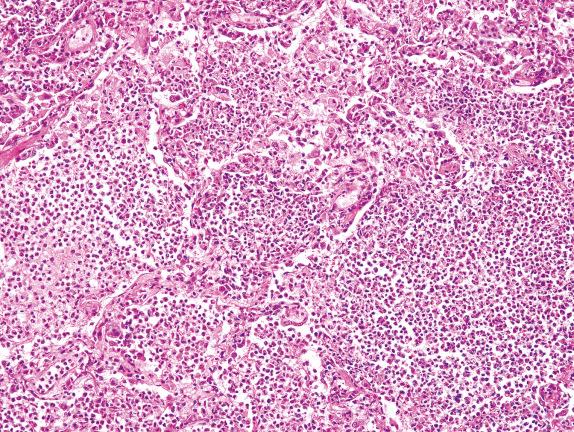
Several different conditions have been described in which the air spaces contain eosinophils. This could represent a manifestation of an idiopathic or a secondary form of EP due to an infection, drug, tumor, or systemic disease. Peripheral blood and bronchoalveolar lavage eosinophils are commonly elevated in these conditions.
EP shows airspaces filled with variable numbers of eosinophils and plump eosinophilic macrophages ( Fig. 3.8 ). Eosinophilic microabscesses and multinucleation of macrophages can also be observed. Atypical type II cell hyperplasia can accompany these changes, raising a concern about viral infection or tumor. If the patient is treated with corticosteroids, eosinophils are reduced considerably.
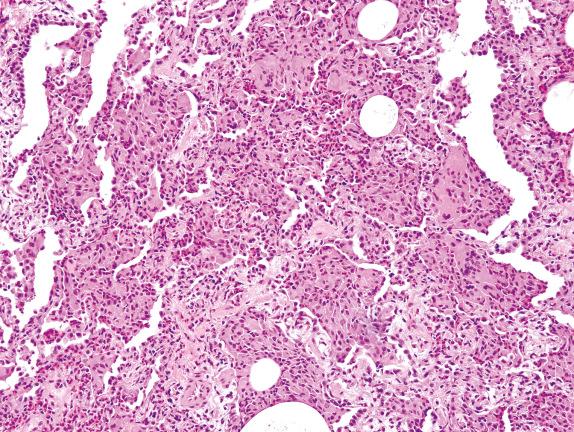
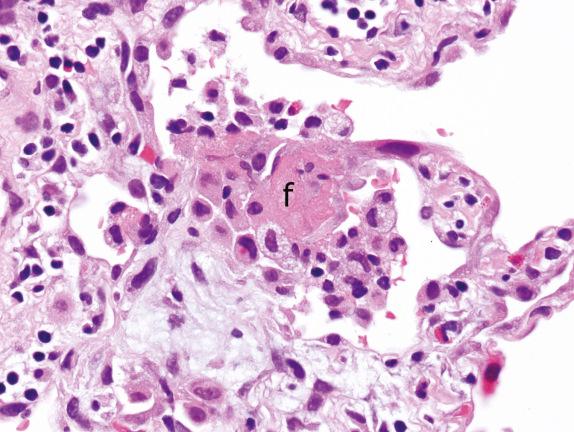
Identification of hemosiderin-laden macrophages (with coarsely granular, brown-golden, and refractile pigment) in the setting of Pattern 1 raises a differential diagnosis to include lung infarction, drug toxicity, and a condition referred to as diffuse alveolar hemorrhage (DAH). DAH of immune origin shows fresh hemorrhage and hemosiderin-laden macrophages in the alveolar spaces ( Fig. 3.9 ) and often small-vessel vasculitis. Attention should be given to distinguish it from the artifactual hemorrhage, which appears as fresh hemorrhage without associated siderophages, fibrin, or capillaritis. It must also be distinguished from a hemorrhagic infectious pneumonia. The presence of intraalveolar macrophages may raise the diagnosis of desquamative interstitial pneumonia (DIP). However, this diagnosis should never be made in the presence of acute injury and fibrin.
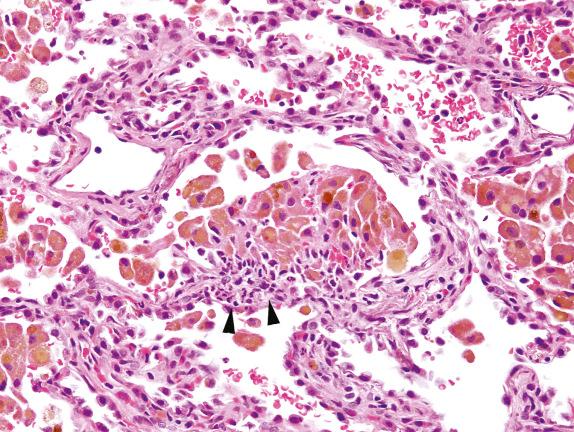
The presence of intraalveolar macrophages with prominently vacuolated cytoplasm and associated alveolar fibrin accumulation ( Fig. 3.10 ) should raise the differential diagnosis of drug toxicity. Drug toxicity in the lung has many manifestations, from DAD to fibrosis ( Chapter 19 ). Cytotoxic drugs often cause DAD, most commonly bleomycin, busulfan, and carmustine. An antiarrhythmic drug, amiodarone, is well recognized to cause pulmonary toxicity, characterized microscopically by acute and organizing lung injury with vacuolated macrophages.
Lung biopsies in which there is variable fibrosis
Fibrosis and microscopic honeycombing
Usual interstitial pneumonia (UIP)
Connective tissue diseases
Rheumatoid arthritis (RA)
Progressive systemic sclerosis (PSS)
Mixed connective tissue disease (MCTD)
Dermatomyositis/polymyositis (DM/PM)
Sjögren’s syndrome (SS)
Hypersensitivity pneumonitis
Chronic eosinophilic pneumonia
Erdheim-Chester disease
Sarcoidosis (advanced)
Chronic drug reactions
Healed infectious pneumonias, DAD, and other inflammatory processes,
Prominent bronchiolization and/or bronchiolocentric scarring
Pulmonary Langerhans cell histiocytosis
Respiratory bronchiolitis
Connective tissue diseases
Chronic hypersensitivity pneumonitis
Small-airway diseases
Chronic aspiration
Intraalveolar vacuolated cells
Chronic obstruction
Drug toxicity
Metabolic diseases
Hermansky-Pudlak syndrome
Hyaline membranes
Connective tissue diseases (acute, subacute)
Acute exacerbation of IPF
Intraalveolar siderophages
Chronic cardiac congestion/chronic interstitial pulmonary edema
Chronic alveolar hemorrhage
Pneumoconiosis
The lung architecture is distorted by varying degrees of collagen deposition, giving the biopsy an overall eosinophilic appearance ( Fig. 3.11 ). A large number of lung diseases can lead to permanent structural lung remodeling with fibrosis, very often with alveolar loss. As with the other patterns presented, radiologic findings are extremely helpful for suggesting specific diagnoses.
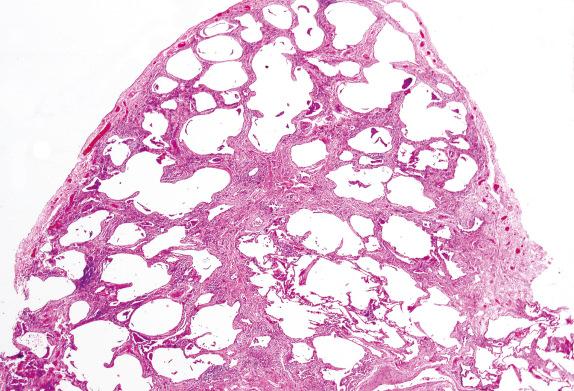
Usual interstitial pneumonia (UIP) is the prototype of pulmonary fibrosis with so-called temporal heterogeneity. Temporal heterogeneity refers to the variegated appearance of the lung biopsy in UIP, where areas of advanced fibrosis are seen adjacent to entirely normal lung, with interspersed areas of active fibroblastic proliferation known as fibroblastic foci. The UIP pattern tends to have fibrosis at the periphery of the lung lobules, with centrilobular (ie, peribronchovascular) sparing ( Fig. 3.12 ). Honeycomb changes eventually develop.
When a UIP pattern is identified and no cause can be found (eg, chronic hypersensitivity, systemic connective tissue disease), the process is considered idiopathic, and the clinical term for this condition is idiopathic pulmonary fibrosis (IPF). HRCT typically shows abnormalities mostly present at the periphery of the lung and in the lung bases in IPF.
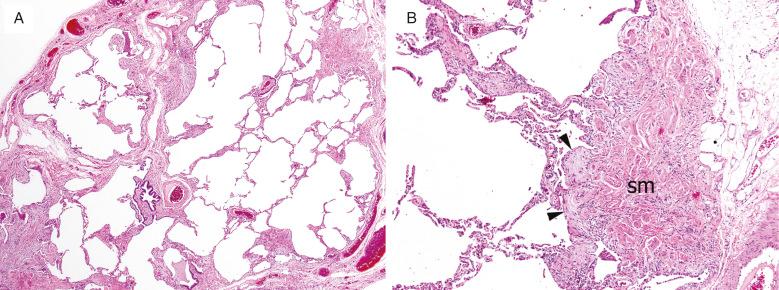
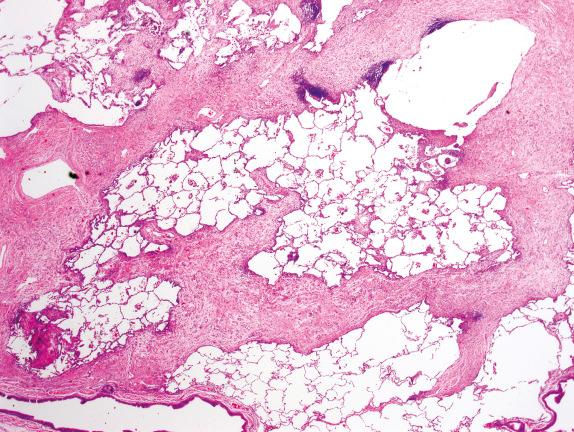
Honeycombing is the final common pathway for many types of severe lung injuries, and is characterized by focal or diffuse replacement of alveolar architecture by cystic spaces surrounded by thick fibrous septa, with mucus- or air-filled airspaces. Thick, irregular bundles of smooth muscle can be present around these cysts within areas of fibrosis, presumably due to progressive parenchymal collapse with incorporation of native airway and vascular smooth muscle into the fibrosis. Honeycombing is not specific for UIP/IPF and can develop with other interstitial lung diseases as well ( Fig. 3.13 ). Comparing Figs. 3.13 and 3.15 , the subtle details differentiating honeycombing and peribronchial metaplasia and fibrosis are seen, as described later.
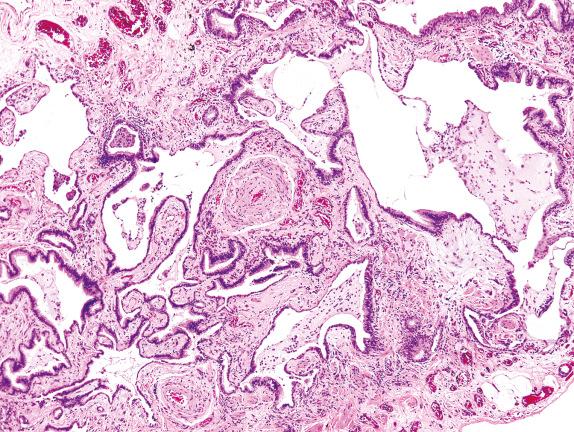
Nonspecific interstitial pneumonia (NSIP) was described in 1994 by Katzenstein and Fiorelli as a constellation of disorders with an overall significantly better prognosis than UIP (90% survival at 5 years). Patients with NSIP were often found to have resolving infection, collagen vascular disease (CVD), or hypersensitivity pneumonitis. Many of the CVDs have an NSIP pattern of lung injury.
On scanning magnification, biopsies with an NSIP pattern show preserved lung architecture with interstitial fibrosis having a “temporally homogeneous” appearance. Three forms of NSIP were originally proposed: a cellular inflammatory form, a mixed inflammatory and fibrotic form, and a purely fibrotic form ( Fig. 3.14 ).
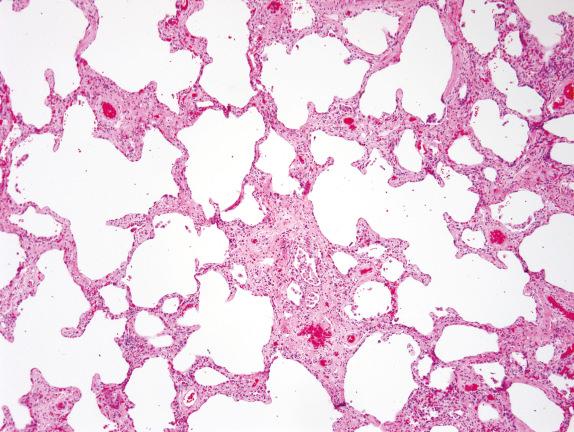
Peribronchiolar metaplasia ( Fig. 3.15 ), also referred to as bronchiolization or Lambertosis, consists of a proliferation of cuboidal epithelial cells over a scaffolding of interstitial fibrosis. It is considered a nonspecific reactive/reparative reaction to chronic injury of the terminal and respiratory bronchioles, often due to cigarette smoking or various inhaled agents or toxins. Connective tissue diseases, chronic hypersensitivity pneumonitis, small-airway diseases with constrictive bronchiolitis, and chronic aspiration can also show prominent peribronchiolar metaplasia, and this finding can occur idiopathically as an isolated abnormality. Many of these disorders also show other histologic features of airway disease such as mucostasis, bronchiolar smooth muscle hyperplasia, and the presence of variable numbers of lightly pigmented airspace macrophages within bronchiolar lumens and in the immediate surrounding alveoli. Langerhans cell histocytosis also classically affects the small airways, producing bronchiolocentric stellate scars.

Accumulation of intraalveolar macrophages in the airspaces is a nonspecific phenomenon. Intraalveolar macrophages characterized by fine vacuolation in their cytoplasm can be observed with drug toxicity (ie, amiodarone), chronic obstruction (“golden pneumonia”), genetic storage diseases, and the Hermansky-Pudlak syndrome (HPS). HPS is a group of autosomal-recessive genetic disorders associated with Golgi apparatus abnormalities. The largest cohort of HPS (400–500 individuals) resides in northwest Puerto Rico. The phenotype is characterized clinically by oculocutaneous albinism, platelet storage pool deficiency, and variable tissue lipofuscinosis. Histopathologically, broad zones of fibrosis are seen, either pleural based or centered on the airways. Alveolar septal thickening is present and associated with prominent, clear, and vacuolated type II pneumocytes ( Fig. 3.16 ).
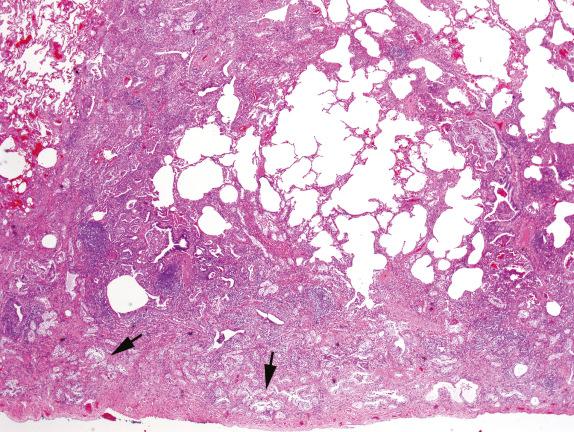
Chronic passive congestion, chronic immune-mediated alveolar hemorrhage, pneumoconiosis, and idiopathic hemosiderosis can present in lung biopsies with fibrosis and accumulation of intraalveolar siderophages. Both hemosiderin and exogenous iron in siderosis stain with Prussian blue, but hemosiderin lacks the black cores within particles characteristic of iron oxide seen in exogenous siderosis.
The presence of hyaline membranes in a lung biopsy dominated by fibrosis suggests an acute event superimposed on a chronic lung disease with scarring. When the background fibrosis is peripherally accentuated and associated with honeycombing (UIP pattern), then the acute process may be an exacerbation of the underlying disease ( Fig. 3.17 ).
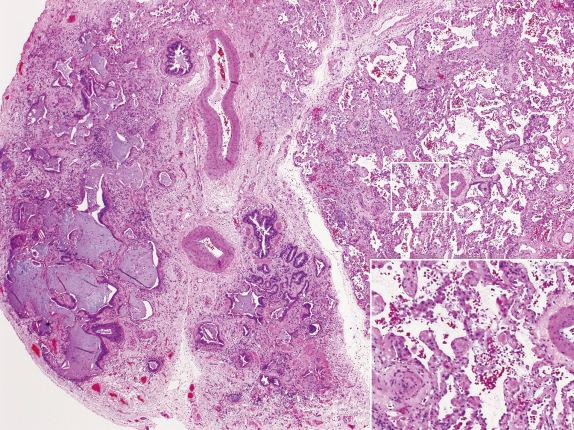
Acute exacerbations of IPF are characterized clinically by the sudden development of acute respiratory distress and new alveolar infiltrates in radiologic studies in a patient being followed for IPF. On microscopic examination, acute lung injury is seen, superimposed on background fibrosis that can be highlighted by a Masson trichrome stain. An acute infectious process or drug reaction superimposed on an unrelated chronic lung disease is also in the differential diagnosis.
Other patterns of fibrosis can help suggest an underlying disease. For example, fibrosis in sarcoidosis will follow the subpleural areas, interlobular septa, and bronchovascular bundles. In Erdheim-Chester disease, a distinctive pattern of subpleural and septal fibrosis with lymphatic distribution is seen ( Fig. 3.18 ).
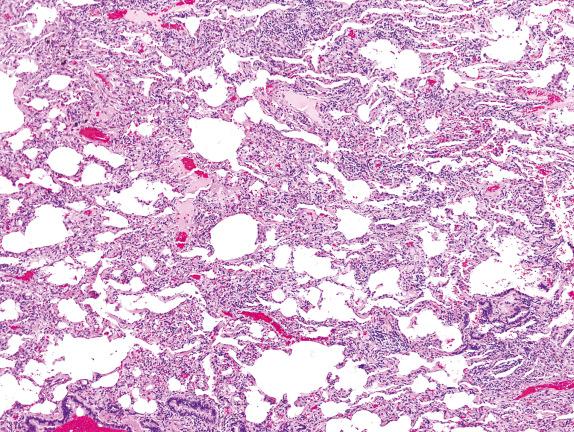
The lung is involved by varying types and distributions of chronic inflammation ( Fig. 3.19 ). Inflammatory infiltrates occupy the interstitium and are composed primarily of mononuclear inflammatory cells (lymphocytes, plasma cells, and histiocytes).
The lung biopsy is dominated by variable amounts of chronic inflammation and reactive type II pneumocyte hyperplasia
Hypersensitivity pneumonitis
Nonspecific interstitial pneumonia (NSIP)
Lymphoid interstitial pneumonia (LIP)
Lymphoma
Diffuse lymphoid hyperplasia
Follicular bronchiolitis
Collagen vascular diseases
Infections
Drug reactions
Aspirations
Mononuclear inflammatory cell infiltrates
Inflammatory infiltrate in the alveolar wall
Preserved lung architecture
Lymphocytes, plasma cells, plasmacytoid lymphocytes, histiocytes
Lymphoid aggregates/germinal centers
Mature lymphocytes
Invasion into bronchial epithelium
Spatial distribution: along airways, bronchovascular sheaths, lymphatics, or subpleurally
Other findings
Viral inclusions
Granulomas and multinucleated giant cells
Food particles
Eosinophils and macrophages
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here