Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
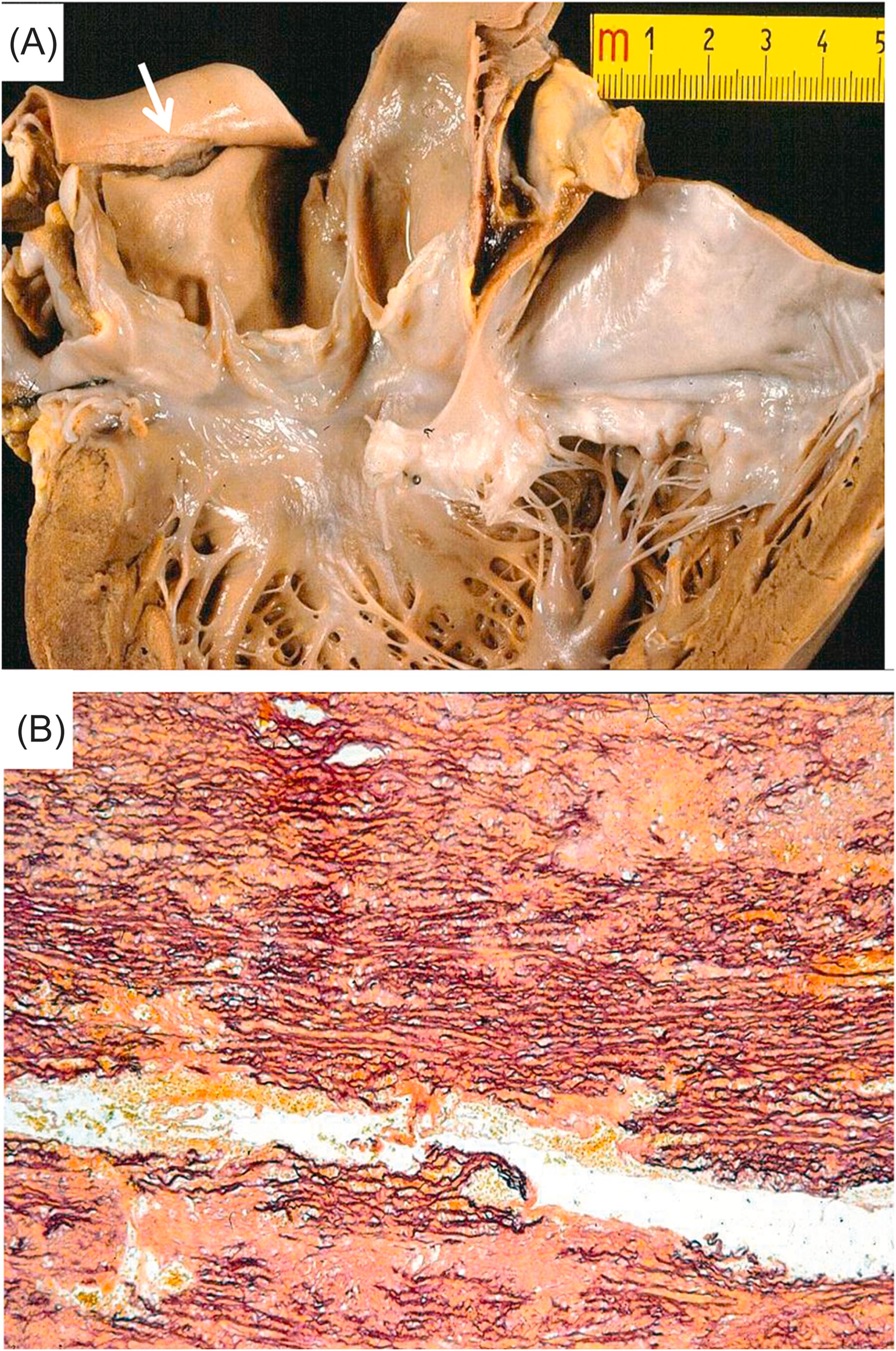
(A) Arrhythmogenic cardiomyopathy: the arrhythmic killer is the fibrofatty replacement of the right ventricle
(B) Hypertrophic cardiomyopathy: myocardial disarray is the substrate of the fatal tachyarrhythmia
(C) The histology of coronary vasospasm: transient ST segment elevation, reperfusion, and onset of ventricular fibrillation
Sudden, unexpected death cuts short human life with cardiac arrest. It has been proven that electrical instability of the heart often provides the final common pathway. Cardiac arrest is usually ascribable to an abrupt fatal arrhythmia. A large spectrum of both congenital and acquired cardiovascular diseases represents the organic substrate of life-threatening electrical dysfunction. Rarely the event is a mere functional disorder. The underlying abnormality is frequently concealed and discovered with surprise at postmortem examination. Nonetheless, it has been proven that most diseases, although asymptomatic, are potentially detectable in life with proper electrophysiologic or imaging investigations.
In this chapter, we deal not only with the causes and mechanisms of cardiovascular sudden death but also briefly with the in vivo diagnosis of hidden heart defects at risk of sudden death, for possible prevention. Sudden infant death syndrome (SIDS) has been excluded from this review.
We define sudden death as a natural, unexpected fatal event occurring within 1 h of the beginning of symptoms, in an apparently healthy subject, or in one whose disease was not so severe that it would predict such an abrupt outcome (1).
The definition includes some fundamental issues that need discussion:
Sudden death is a mode of dying; in other words, it is a symptom, not a disease. The morbid conditions causing it are frequently missed in vivo, so postmortem examination is mandatory to unveil a concealed substrate.
Sudden death occurs in the natural history of several diseases. Other causes, such as occult trauma, drowning, choking, drug addiction, or iatrogenic disorders should be excluded. In this regard, previous definitions of sudden death included the term “witnessed” to reinforce the reliability of a natural occurrence. However, most sudden deaths occur during sleep and are not witnessed. Therefore, this strict definition would exclude many natural deaths. For this reason, we do not require that death be witnessed in our definition. However, we strongly recommend toxicologic tests in such cases, in addition to autopsy.
In the definition, the time elapsing between the onset of final symptoms and death has been controversial, varying from a few minutes (instantaneous death) to 24 h. An interval of 24 h is too long because the concept of sudden death would then include many noncardiovascular diseases (e.g., cerebral stroke, fulminant bronchopneumonia, pancreatitis, adrenal apoplexy), in which cardiac arrest is secondary and usually delayed. “Instantaneous death,” representing most sudden cardiac deaths, should be confined only to cases of abrupt irreversible cardiac arrest with immediate interruption of cerebral blood flow and loss of consciousness. Most authors are inclined to adopt a time of 1 h, thus, an interval of 1 h seems a wise compromise .
Although in most cases the underlying defect is clinically covert or poorly defined, there are sudden deaths that occur during the natural course of patently evident cardiac diseases. This is the case, for instance, of symptomatic ischemic heart disease (before or after myocardial infarction) or cardiomyopathies. Although sudden death may occur unexpectedly in an individual, thus fitting the definition, it is predicted by a yearly rate in a population affected by these morbid conditions and according to defined parameters. Risk stratification of sudden death is a major challenge in cardiovascular medicine.
Cardiac arrest may be reversible in the setting of resuscitative maneuvers, which are becoming more prompt, sophisticated, and effective. The term “aborted sudden death” has been introduced to refer to this situation and to patients who suffer but survive cardiac arrest. In terms of pathophysiology, the condition is equivalent to sudden death, although the difference is obviously immense. Many patients, whose cardiac arrests are aborted, subsequently develop overt myocardial infarction. Nevertheless, the majority of aborted sudden deaths are not followed by an acute myocardial infarction. It should be stressed that sometimes a cardiac rescue is achieved, but irreversible cerebral damage follows the circulatory arrest, leading thereafter to a delayed cerebral death. This death, in all respects, should be considered sudden and cardiac in origin.
There is general agreement that sudden death is a major issue in industrialized countries, but variability of definition and diagnosis, as well as factors such as missing information, lack of autopsy investigation, and different classifications of causes make epidemiologic evaluation difficult .
In the United States, Gillum estimated that more than 350,000 people die suddenly each year of cardiovascular causes. The annual incidence of sudden death in people between 35 and 74 years of age was 191/100,000/year in men and 57/100,000/year in women. Almost half of sudden deaths occur in people with known coronary artery disease.
Other studies from selected regions of the United States suggested similar rates of sudden cardiac death, despite different definitions, populations, and time periods. In all, the incidence of sudden death is two to three times higher in men than in women, mostly paralleling the male/female ratio in ischemic heart disease. The proportion of sudden ischemic heart disease deaths is always 50%–60%, except in the Framingham study in which results were lower (23%), probably because of exclusion of people with known coronary artery disease . Recently, an overall estimated incidence in the population of 0.1%–0.2% year was reported, with total events in the United States of 300,000 sudden cardiac deaths .
As far as sudden deaths occurring in countries other than the United States, data are less well documented. However, international reports show the same patterns, except in Finland , where rates are considerably higher than in the United States, again paralleling an increased rate of coronary artery disease mortality in that country. The total sudden death events in Italy are nearly 50,000–60,000 deaths per year.
Sudden cardiac death is uncommon in children and adolescents. Several studies have estimated that the rate in those between 1 and 20 years of age is from 1.3 to 8.5/100,000 patients per year . This would amount to at least 600 juvenile sudden deaths annually in the United States .
The incidence and causes of sudden death in a young adult population have not been well defined. In young adults (<35 years), the incidence varies from 0.5 to 8/100,000/year in the reported epidemiological studies. Cardiomyopathies, myocarditis, premature coronary artery disease, congenital coronary artery anomalies, and channelopathies play a major causative role ( Fig. 11.1 ). Shen and colleagues published the results of a 30-year population-based study, reporting in 54 young adults (20–40 years of age) with nontraumatic sudden death; all were residents of Olmsted County, Minnesota. An overall cohort incidence rate of 6.2/100,000 annually was calculated. Of the 54 cases who died suddenly, 19 were women (4.1/100,000 population annually) and 35 were men (8.7/100,000 population annually).
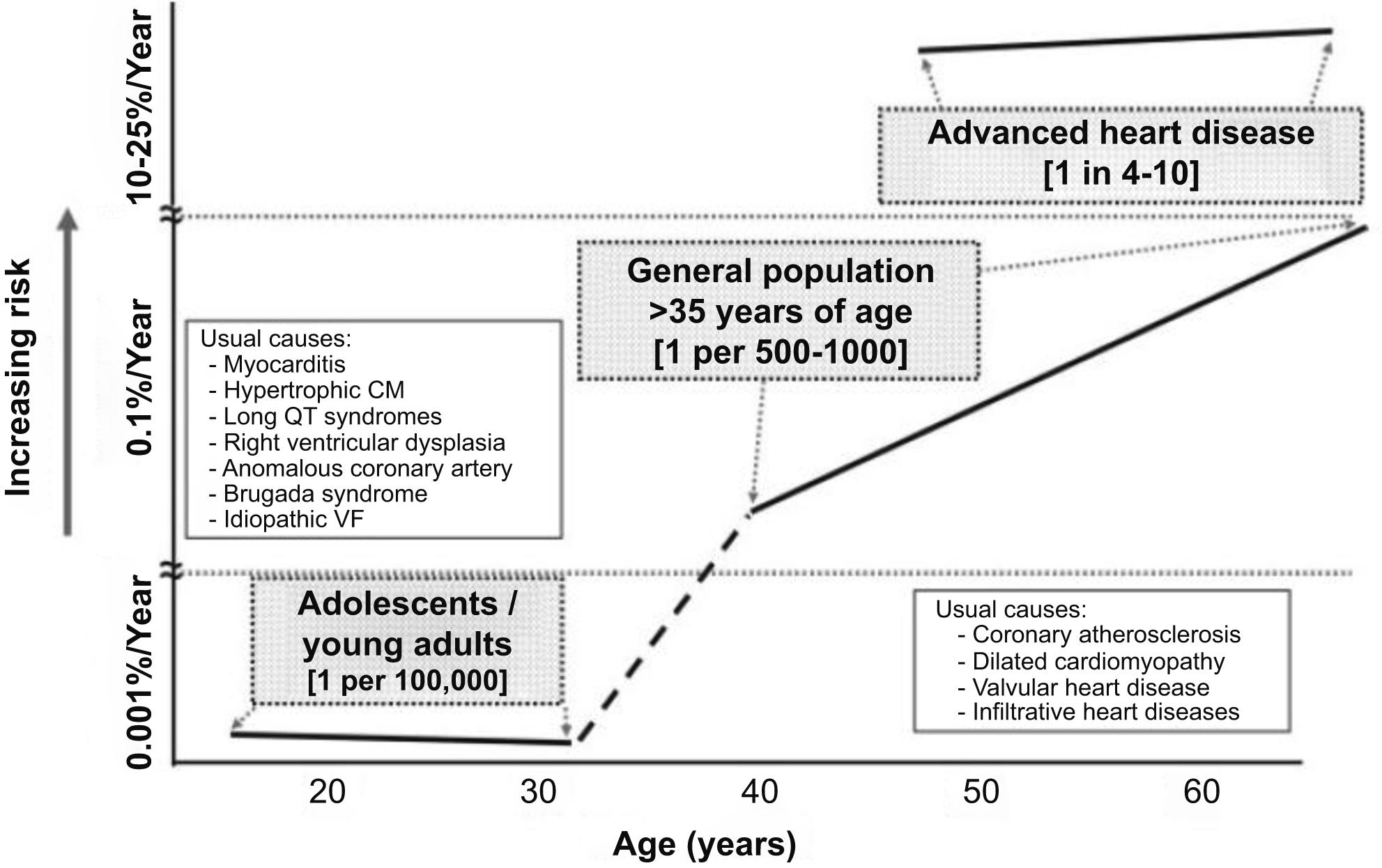
In the prospective study carried out in the Veneto Region, North East Italy, which holds a mean 4,379,900 overall population, young population (12–35 years old) was 1,386,650 and young athletes 112,790, according to Italian Census Bureau & Sports Medicine database 1978–1999 . The cumulative incidence of sudden death was 1/100,000/year. Among the nonathletic young people, the incidence was 0.9/100,000/year, whereas in the athletic it was 2.3/100,000/year. Thus the incidence of sudden death in athletes was 2.5-fold, clearly indicating that effort is a major risk factor in people affected by hidden morbid entities, simply by unmasking them.
With age, the incidence of sudden death in the general population is increasing, mostly due to coronary atherosclerosis but also to degenerative valve disease, such as mitral valve prolapse and aortic stenosis . However, coronary atherosclerosis may account for premature sudden death as early as at 20–35 years of age . Overall, incidence of sudden death is 1 out of 1000/year during the first year of life (because of SIDS), 0.01 out of 1000/year in adolescents and young adults (<35 years), 2 out of 1000/year in adults and 200 out of 1000/year in the elderly ( Fig. 11.1 ). There are two ages of peak of incidence of extrauterine sudden death, one between 1 and 12 months (SIDS) and another over 45 years (coronary artery disease). The risk of coronary sudden death is 4–7 times greater in males compared to females in the young adult—middle-aged population because of the hormonal protection in females . This does not exclude that classical coronary risk factors are a predictor of events even in females (cigarette smoking, obesity, diabetes, hypertension, and oral contraceptives) . Blood pressure levels and left ventricular hypertrophy have also been associated with a higher risk of sudden death .
The rate increases in higher-risk subgroups. For instance, the sudden death incidence is 1%–2%/year in people with coronary risk profile, 5%/year in those with a prior coronary event, 15%/year in those with congestive heart failure and ejection fraction <35%, 25%/year in cardiac arrest survivors. The combination of prior myocardial infarction, low ejection fraction, and ventricular tachycardia accounts for a risk of nearly 35%/year ( Fig. 11.2 ). In such conditions implantable cardiac defibrillator is strongly recommended for prevention of sudden death.
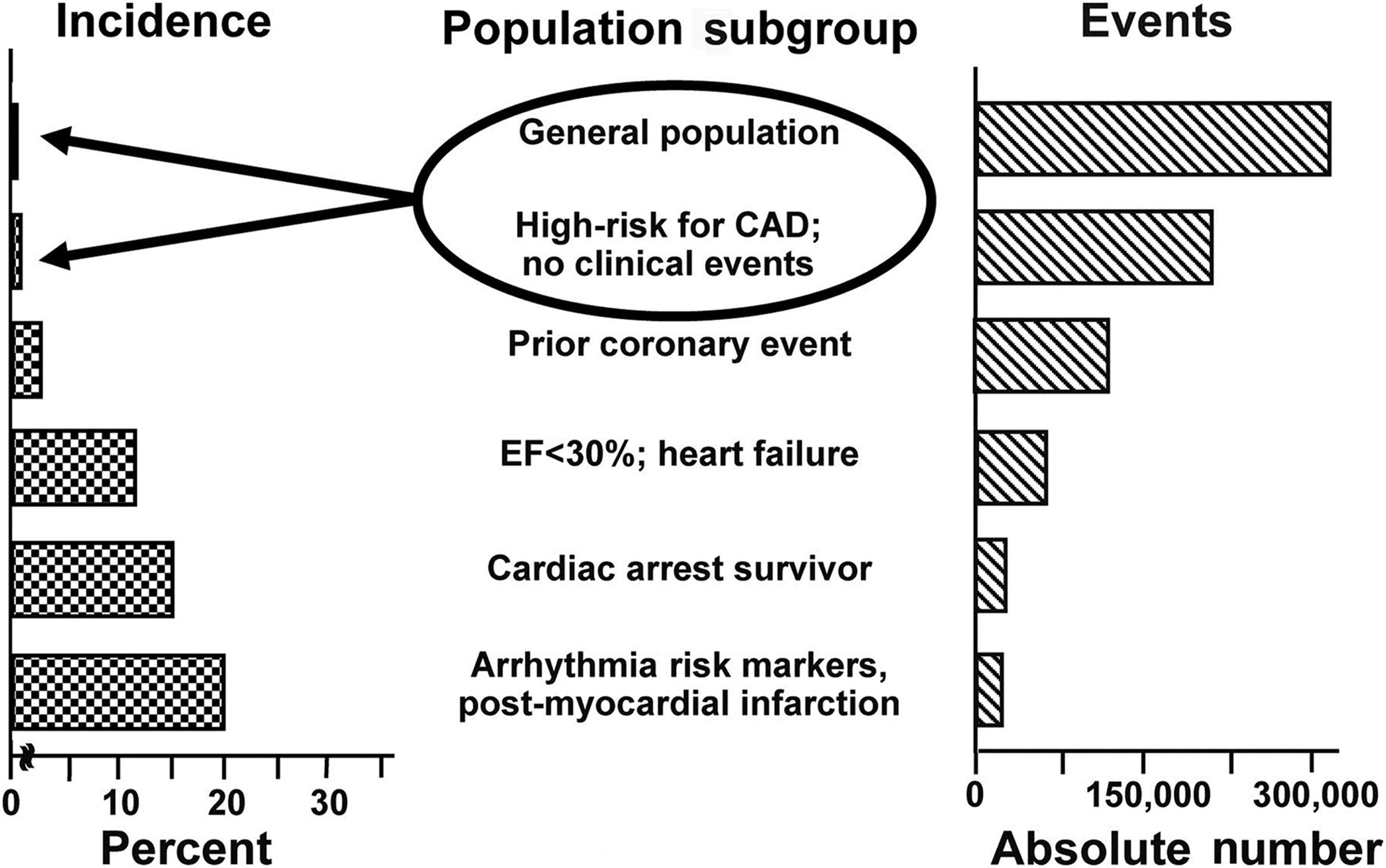
A recent systematic review of the publications dealing with the incidence of sudden cardiac death in the United States clearly shows that very few studies have reported estimates from primary data and that the stated definitions of sudden cardiac death and sudden cardiac arrest are not standardized across the medical community . The major issue in interpreting epidemiologic data on sudden death, besides a lack of standardization in death certificate coding, is the variability of its definition. This includes differences in the time interval between symptom onset and death, the location of death, inclusion or exclusion of unwitnessed deaths, and consideration of the unexpected nature of death . Time intervals used to define sudden death vary from 1 to 24 h between the time of collapse and the loss of vital signs . In fact, the onset of symptoms is often difficult to define because patients have been unwell for days or weeks before sudden death. Noteworthy is that studies using a shorter time period in definition (1 h or less) report a lower incidence of sudden death than those using a wider definition (up to 24 h), in which most deaths might be clinically classified as due to myocardial infarction or stroke .
Most authors accept that sudden unexpected deaths should exclude those occurring in hospitals. More recently, the definition has been extended to include deaths that occur in emergency rooms because of an increasing number of emergency services and prolonged life-support efforts . As indicated, many studies exclude unwitnessed death because the exact times of symptom onset and death cannot be established . The probability of unwitnessed sudden death is higher in the setting of a shorter survival from symptom onset, among people living alone, deaths occurring at home or during sleep, and those without a history of known heart disease. As a consequence, exclusion of unwitnessed sudden deaths seriously biases a study by underrepresenting these conditions. On the other hand, inclusion of such sudden deaths implies a need to obtain detailed postmortem examinations and to interview clinicians and relatives about previous illnesses and symptoms. In this particular subgroup, as stressed earlier, the study should include toxicologic examination to rule out deaths due to drug addiction, excess alcohol intake, homicide, or suicide.
Finally, because as many as 50% of people who die suddenly have a previous history of heart disease, physicians tend to ascribe sudden death to coronary artery disease without further investigation, recording that on death certificates .
The final mechanism of death is cardiac arrest, but by no means all sudden deaths are cardiac or cardiovascular in nature. The heart may stop beating as a result of acute cerebral or respiratory failure. Thus, when considered as a primary loss of vital function, sudden death may be defined as cerebral, respiratory, or cardiovascular.
This occurs in the setting of abrupt brain apoplexy, usually a consequence of a hypertensive hemorrhage with ventricular flooding, or of a subarachnoid hemorrhage secondary to rupture of a congenital aneurysm on the circle of Willis ( Fig. 11.3 ), or of an extensive stroke with hemispheric infarction after embolic occlusion of a carotid or cerebral artery . The resulted acute cerebral edema involves the brain stem, where cardiorespiratory centers are located, leading to a sudden loss of autonomous central respiratory function. The heart then stops, usually with a progressive sinus bradycardia up to asystole.
In cerebral embolism, with the exception of paradoxical embolism, the source is the left side of the heart or the aorta and the embolus may be thrombotic, septic, or neoplastic. Thus although the primary morbid disease is cardiovascular, the mechanism of sudden death due to cerebral embolism is cerebral and not cardiac, because sudden death is a symptom rather than a disease, and it is classified according to the primary loss of vital function.
Sudden death may occur during epileptic seizures associated with respiratory chest muscle paralysis. Patients with epilepsy have a 20-fold increased risk of sudden death compared with the general population . Sudden unexpected death in epilepsy is defined as “the sudden, unexpected, witnessed or unwitnessed, nontraumatic, and nondrowning death of a patient with epilepsy with or without evidence of a seizure, excluding documented status epilepticus, and postmortem examination does not reveal a structural or toxicological cause of death” . Clearly, the abrupt dysfunction is cerebral in origin, whereas respiratory failure is secondary. In some instances, the occurrence of bradycardia in association with apnea suggests involvement of cardiorespiratory reflexes. However, there are cases of witnessed sudden death in subjects with a history of epilepsy, in whom the final episode does not occur associated with a convulsive seizure and in whom postmortem examination rules out structural cardiovascular causes of death. It is a matter of debate whether cardiac arrhythmia is the primary cause of death in this setting. The underlying mechanisms are still not elucidated, but recent studies suggest the possibility that a genetic channelopathy might contribute to both epilepsy and cardiac disease to increase the incidence of death via a lethal cardiac arrhythmia. Moreover, antiepileptic drugs might induce lengthening of the QT interval and lead to fatal arrhythmia .
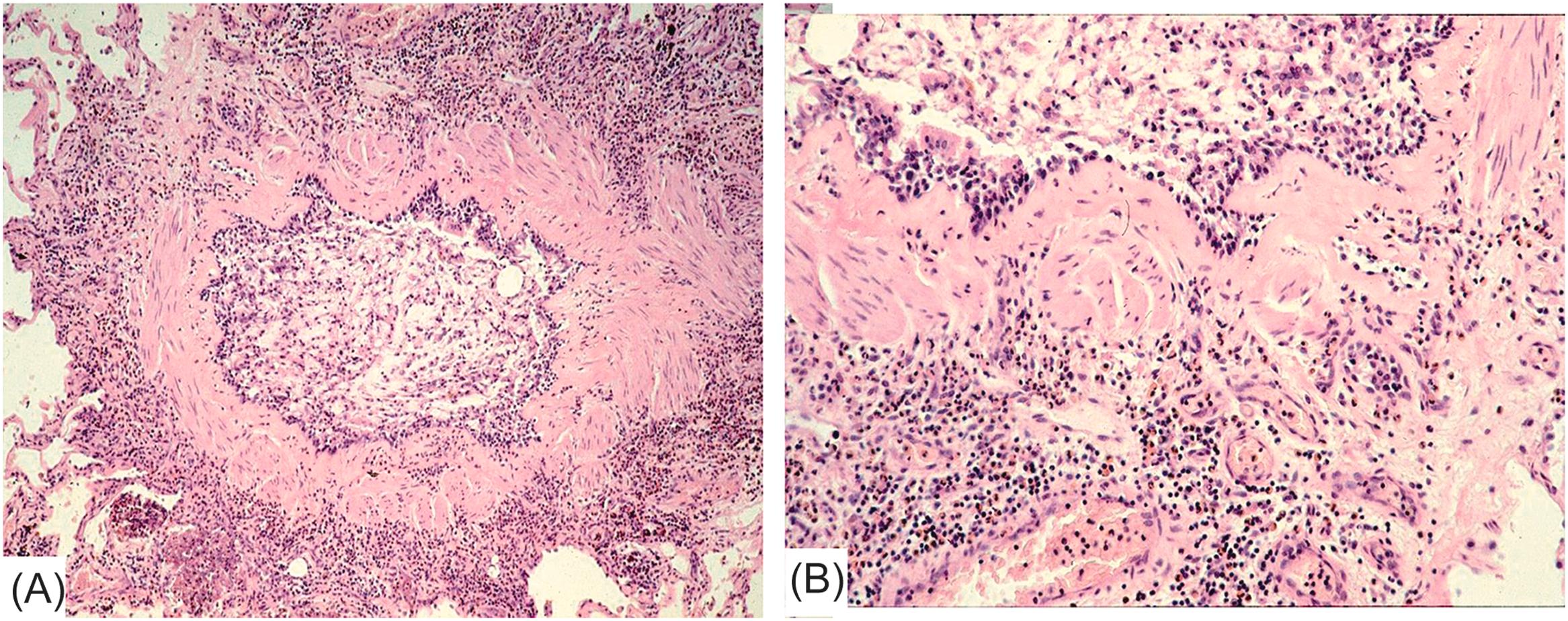
In this instance, an abrupt airway obstruction accounts for an acute failure of ventilation and alveolar gas exchange, producing hypoxia, cyanosis, and final heart standstill. Accidental events such as tracheal obstruction following inhalation of a foreign body account for most of these cases, which should not strictly be considered natural sudden deaths. The sudden obstruction may be spontaneous in the setting of a congenital anomaly (tracheal or bronchial stenosis) or be the result from acquired conditions such as glottal edema complicating grippe and allergic bronchial asthma . In the latter condition, airway obstruction is peripheral and diffuse. Amine release by inflammatory infiltrates in the bronchial wall explains the associated bronchospasm. Histology discloses eosinophils, typical thickening of bronchial basal membrane, and plugs in the bronchial lumen caused by hypersecretion of the mucinous glands ( Fig. 11.4 ). The latter aggravates airway obstruction .
In cardiovascular or cerebral sudden deaths, immediate loss of consciousness occurs, whereas in airway obstruction, only ventilatory function is jeopardized and circulation, including brain perfusion, is preserved during the attack. This implies that consciousness is maintained for minutes during desperate inspiratory efforts, until severe deoxygenation and asystole occur. In other words, the victim is the witness to his impending death.
By cardiovascular, we mean heart and great arteries within the pericardial cavity. The causes of cardiocirculatory arrest may reside in the aorta or the pulmonary artery or in one of the major cardiac structures, the integrity of which is essential for regular heart function. Thus coronary arteries ensure adequate myocardial blood perfusion; normal myocardium guarantees not only an ordered contractility but also homogeneous electric impulse propagation; valves and endocardium allow unimpeded blood transit and no reflux during cardiac cycles; and the conduction system maintains electrical stability through a regular pacemaking function and impulse transmission, with delay at the specialized atrioventricular (AV) junction allowing the sequence of atrial and ventricular systoles. Of course, to cause instantaneous death, dysfunction of any of these structures must be fulminant.
As far as pathophysiology is concerned, cardiac arrest takes place through two mechanisms.
The heart and circulatory functions are suddenly impeded by mechanical factors. This is the case, for instance, of aortic or cardiac rupture into the pericardial cavity, which produces hemopericardium and cardiac tamponade ( Fig. 11.5 ). Death occurs because blood cardiac tamponade impairs diastolic ventricular filling rather than because of hypovolemia, which is, instead, the mechanism with aortic rupture into the pleural or the retroperitoneal space.
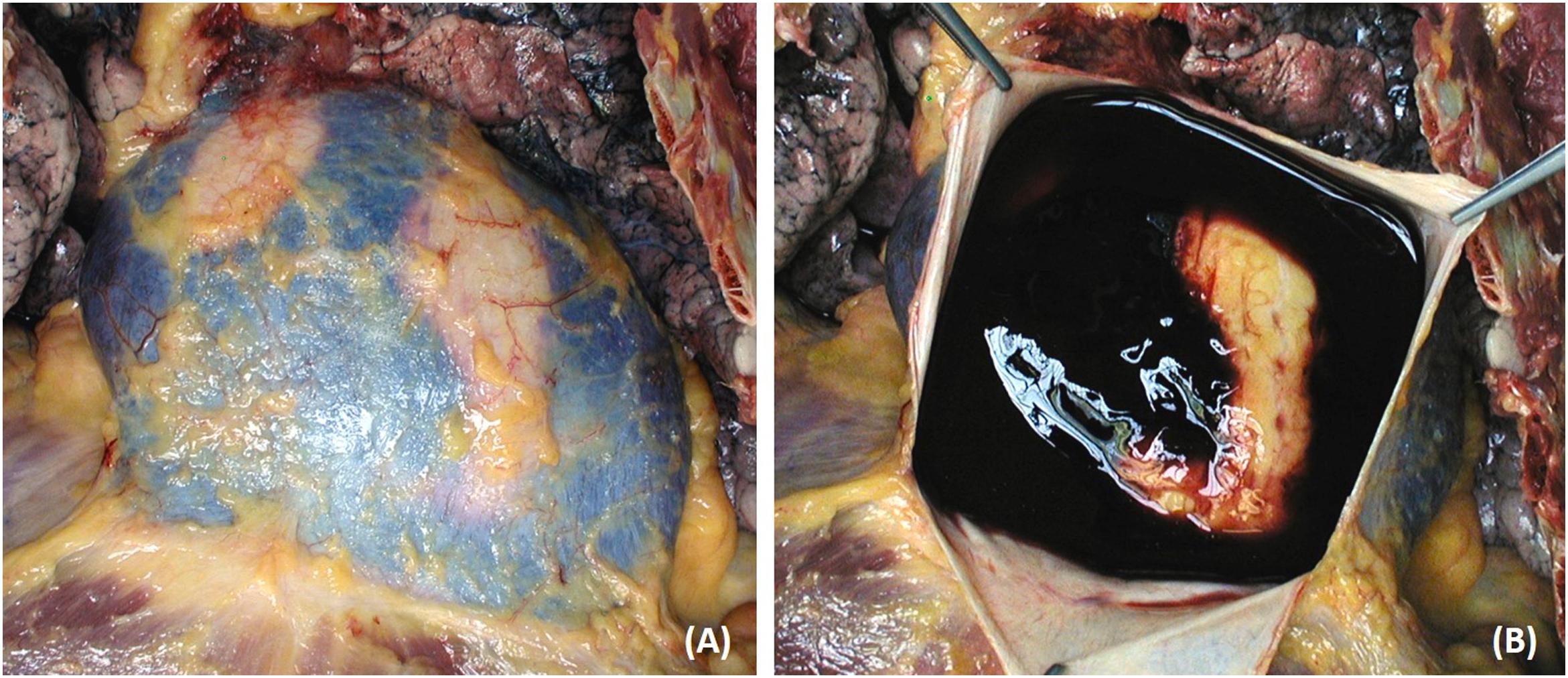
Rupture of a mitral papillary muscle or of chordae tendineae, in the setting of myocardial infarction, mitral valve prolapse, or infectival endocarditis may induce severe mitral incompetence and acute pulmonary edema.
Sudden, spontaneous hemorrhage may occur elsewhere, such as in the gastrointestinal tract, and may be so quick and massive as to induce loss of consciousness, shock, and cardiac arrest .
Shock, defined as a discrepancy between the capacity of the circulatory system and the blood content, may also develop without hemorrhage in the setting of septic fulminating syndrome (Waterhouse–Friderichsen) or allergic (bee sting) conditions with anaphylaxis. The fall of blood pressure may be so severe to account for fulminant multiorgan failure and death within minutes or hours.
Another cause of mechanical cardiovascular sudden death is a saddle pulmonary thromboembolism, which abruptly blocks blood transit ( Fig. 11.6 ) . Apart from conditions such as orthopedic trauma or constrained postoperative position, in which the risk of pulmonary embolism is well known, such catastrophic events may happen in young women taking combined estrogen/progesterone therapy because of ovarian dysfunction or for contraception, and among passengers of long-distance travels . It is noteworthy that cardiac rupture, acute heart failure with pulmonary edema, and cardiogenic shock in the setting of myocardial infarction are now all considered major events that are separate from sudden death.
This is by far the most common pathophysiologic mechanism of sudden cardiac death. Heart rhythm is suddenly upset with severe impairment of cardiac ventricular filling and emptying. As a consequence, cardiac output decreases, cerebral blood flow drops, and irreversible brain damage and death result. Ischemic myocardial injury, whether recent (myocardial necrosis) or past (myocardial fibrosis), is the most frequent substrate for an abrupt electrical disorder. However, arrhythmias also occur in the absence of ischemia (e.g., cardiomyopathy, conduction system abnormalities, channelopathies). There are cases indeed in which complete postmortem examination, including gross and histological investigations, fails to identify a cause of sudden death. These morbid entities will be considered separately. Nonetheless, if an extracardiac cause is excluded, these fatalities should be regarded by definition as cardiac, considering that an arrhythmic cardiac arrest was the final mechanism of death (see below).
Tracings from Holter-monitored sudden deaths or electrocardiograms (ECGs) recorded at resuscitative maneuvers revealed the following patterns during cardiac arrest :
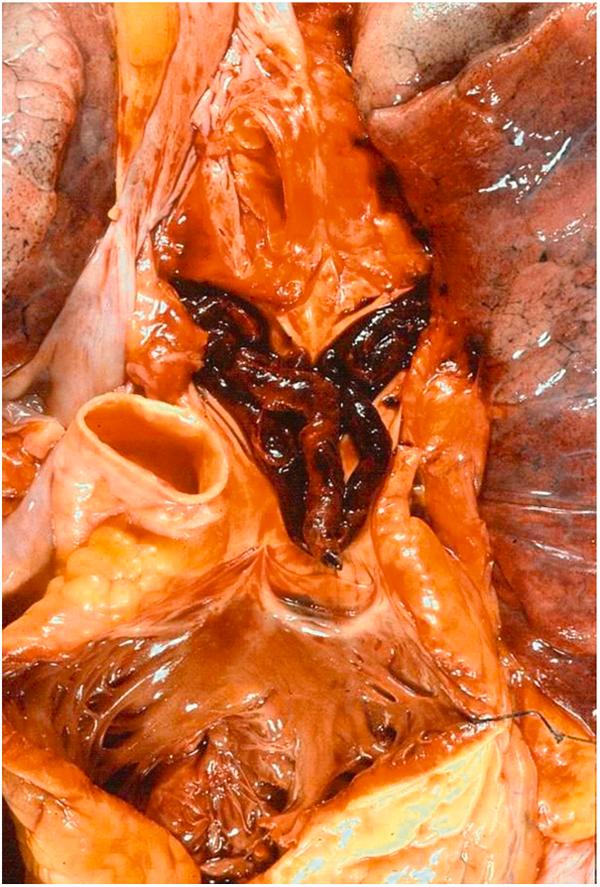
Ventricular fibrillation (nearly 70%) : Ventricular electrical activity consists of as many as 400–500 nonsynchronized beats per minute (bpm). This high frequency does not leave time enough for diastolic filling and systolic emptying and ventricular contractility is transformed in a vermicular, ineffective motion (“heart delirium”) ( Fig. 11.7 ).
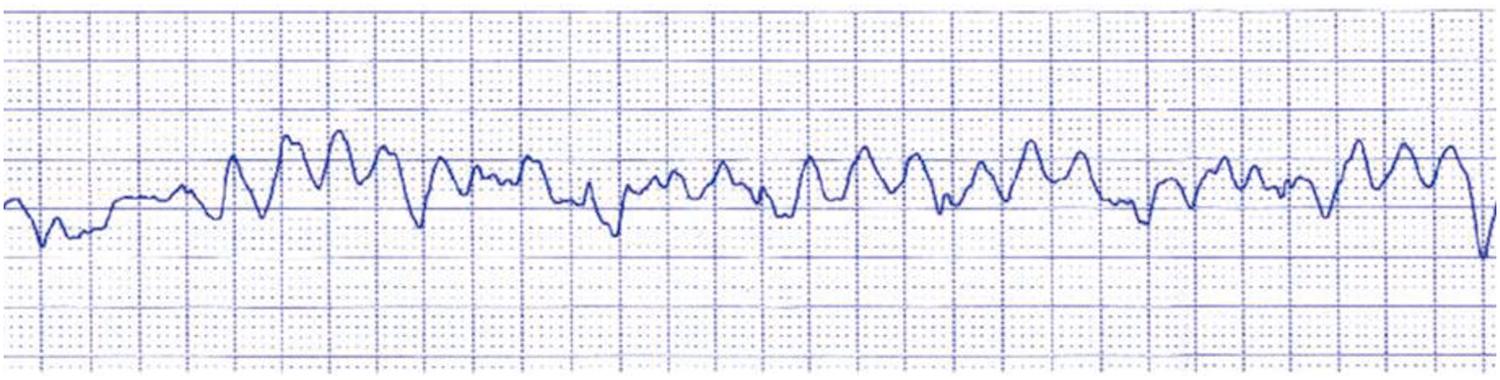
Ventricular tachycardia (nearly 10%) : The ventricular rhythm, despite its high frequency (up to 250 bpm), is regular and presents as monomorphic, large, wide, and aberrant QRS complexes. The high rate does not allow enough blood flow to enter and exit the ventricles, so blood pressure drops and collapse occurs, often in already depressed left ventricular function. In this setting, ventricular tachycardia often degenerates into ventricular fibrillation.
Asystole (nearly 15%) : There is a standstill of ventricular activity either because of sinus node arrest or complete AV block. No QRS complexes are registered on the ECG, and that corresponds hemodynamically to absent ventricular systole. At echo, the heart appears still.
Electromechanical dissociation (nearly 5%) : Also known as pulseless electric activity (PEA), it is defined as the presence of spontaneous, normal cardiac electric activity in the absence of stroke volume sufficient to maintain pressure within the arteries . No pressure exists in the arterial system, although the ECG regularly records QRS complexes. PEA is usually secondary to cardiac tamponade or pulmonary embolism. However, conditions do exist, especially in the setting of acute and widespread myocardial ischemia, in which the phenomenon is primary because the myocardium becomes unexcitable . This is an odd phenomenon consisting of a split between myocardial excitation and contraction (true electromechanical dissociation). Despite a regular onset and spread of electrical activity, no ventricular contraction follows.
By far cardiovascular sudden death is the most frequent occurrence, followed by deaths induced by respiratory or cerebral causes . In our experience (the Veneto Region study project of Juvenile Sudden Death), cardiovascular sudden death accounted for 94% of the collected cases, followed by cerebral (4%) and respiratory (2%) cases ( Table 11.1 ) .
| Mechanisms of sudden death | N | % |
|---|---|---|
| Cerebral | 26 | 4 |
| Respiratory | 17 | 2 |
| Cardiovascular | 652 | 94 |
| Mechanical —38(6%) | ||
| Arrhythmic—614(94%) | ||
| TOTAL | 695 | 100 |
In cardiovascular sudden death, arrhythmic cardiac arrest is the main pathophysiologic mechanism (94%, Table 11.1 ). Mechanical cardiac arrest caused by aortic rupture or pulmonary thromboembolism is more frequent in the elderly. Table 11.2 presents our experience of all causes of cardiovascular sudden death in the young in the Veneto Region of Italy up to 2014 . Note that 18% of sudden cardiac deaths were unexplained (“sine materia”). These findings have been recently confirmed.
| Cardiovascular causes of sudden death | N | % |
|---|---|---|
| Obstructive coronary atherosclerosis | 125 | 19 |
| Arrhythmogenic cardiomyopathy | 60 | 9.2 |
| Mitral valve prolapse | 47 | 7.2 |
| Conduction system abnormalities | 33 | 5.0 |
| Congenital coronary anomalies | 30 | 4.6 |
| Myocarditis | 78 | 12 |
| Hypertrophic cardiomyopathy | 61 | 9.3 |
| Aortic rupture | 21 | 3.2 |
| Dilated cardiomyopathy | 22 | 3.4 |
| Nonatherosclerotic, acquired coronary artery disease | 15 | 2.3 |
| Aortic stenosis | 9 | 1.4 |
| Pulmonary thromboembolism | 12 | 2.0 |
| Congenital heart disease | 15 | 2.3 |
| Other | 7 | 1.0 |
| Sine materia | 117 | 18.0 |
| TOTAL | 652 | 100 |
Excitation of the ventricular myocardium occurs via the His bundle, bundle branches, and Purkinje network, with final, rapid fiber-to-fiber conduction in the working myocardium. It is a wavefront phenomenon leading to quick, complete ventricular myocardial excitation, which corresponds to the QRS complex on the ECG (depolarization) so that the whole myocardium contracts almost simultaneously (systole). The muscle then becomes electrically refractory, which corresponds to the ST segment—T wave of the ECG (repolarization), and relaxation occurs (diastole). Ventricular arrhythmias may develop when electrical activity originates spontaneously from the ventricular myocardium and not from the regular excitation through conduction pathways. Clinical forms of ventricular arrhythmias include premature ventricular beats, nonsustained ventricular tachycardia (duration less than 30 s), sustained ventricular tachycardia (duration more than 30 s), or ventricular fibrillation.
Premature ventricular beats or ventricular tachycardia may arise from the right or left ventricle or from the ventricular septum. The source may be constant, giving rise to uniform QRS complexes, or variable, producing polymorphic QRS complexes.
The following three main mechanisms explain the onset of ventricular arrhythmias and influence the ventricular fibrillation threshold:
Automaticity : Foci of ventricular myocardium spontaneously depolarize faster than the pacemaker. For instance, this mechanism may explain the arrhythmogenesis in acute focal myocarditis.
Reentry : The wavefront phenomenon of ventricular depolarization is disturbed by an interposition of archipelago-like necrotic or fibrotic tissue, which delays or blocks the conduction undirectionally within surviving scattered myocytes. The delayed impulse propagation reaches adjacent ventricular myocardium at the end of its phase of depolarization-repolarization when it is newly excitable. This explains the onset of ventricular arrhythmias, which according to the ventricle origin may show right or left branch block morphology. Therefore, intraventricular reentry occurs between the two pathways, with different electrophysiologic properties. This mechanism has been proved in conditions such as acute myocardial infarction or chronic ischemic heart disease with myocardial fibrosis and in cardiomyopathies such as arrhythmogenic right ventricular cardiomyopathy. Patients with chronic ischemic heart disease have been proved to have higher risk of arrhythmic SD when ejection fraction is less the 35% as to require implantable cardioverter-defibrillator (ICD) . These guidelines have been questioned.
Triggered activity : Because of the proarrhythmic effect of some drugs or a congenital abnormality of repolarization with ion channels defects, myocytes may be triggered after depolarization. This manifests as ventricular arrhythmias, mainly in the shape of torsade de pointes (polymorphic ventricular tachycardia).
Spontaneous rupture of the aorta may occur in the setting of inflammatory and degenerative disease.
Once a major cause of death, now syphilis rarely reaches the tertiary stage to involve the aorta with mesoaortitis and to develop saccular thoracic aneurysms at risk of rupture. They involve mostly the ascending aorta and the aortic arch .
“Mycotic” aneurysms are a consequence of a localized bacterial aortitis and may develop unexpectedly in patients with septicemia, even in the absence of valve endocarditis . Bacteria enter the vessel wall through the vasa vasorum or through atherosclerotic ulcer of the intima, settle and destroy the tunica media, leading to abscess and aneurysm formation. Such aneurysms may be located in the ascending aorta within the pericardial sac so that their rupture leads to cardiac tamponade and instantaneous death ( Fig. 11.8 ).
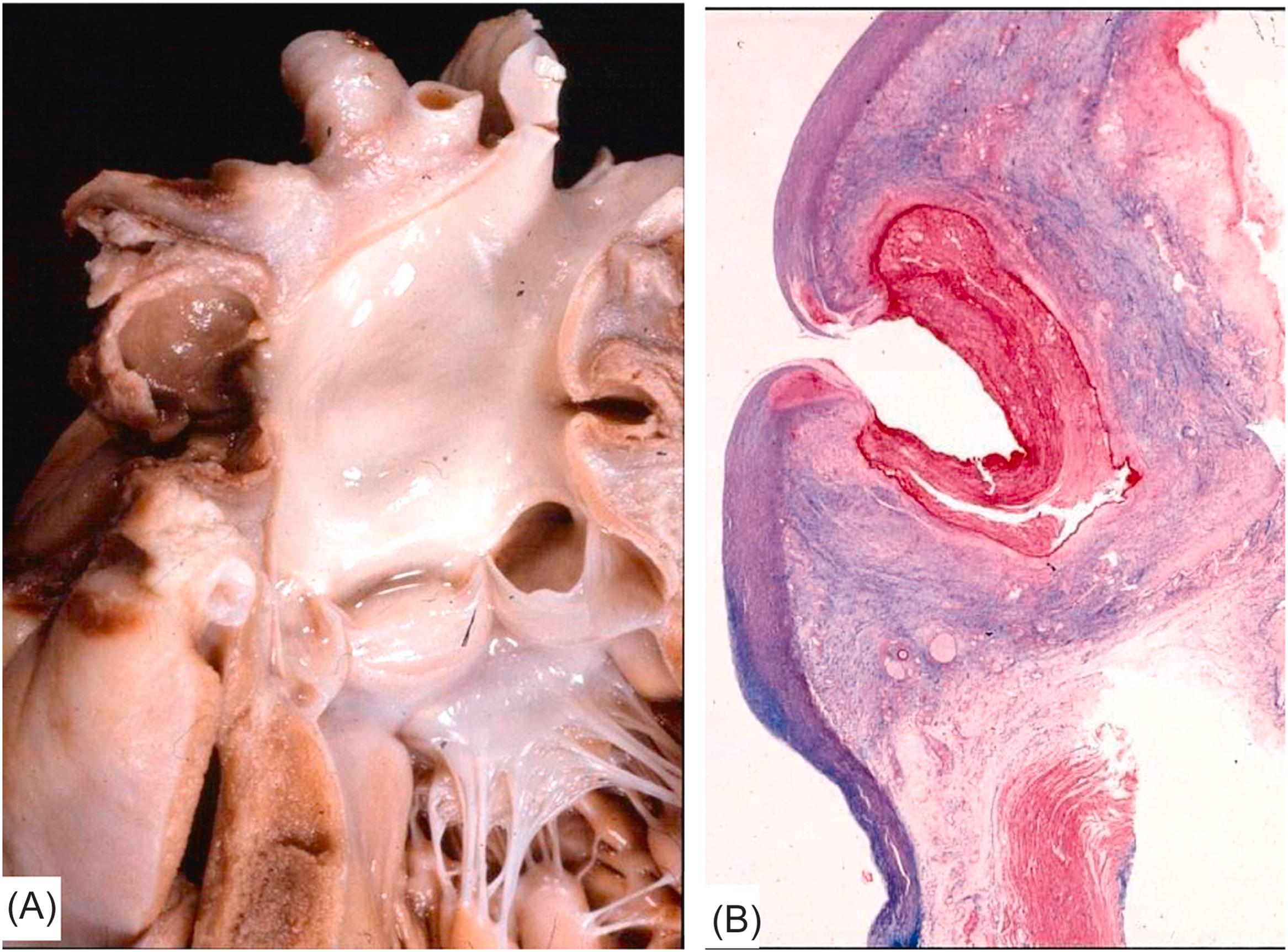
The dissection usually occurs as a “bolt from the blue.” Tearing of the intima is followed by splitting of the media. The dissection may involve the entire aorta (type I), only the ascending intrapericardial aorta (type II), or only descending thoracic aorta (type III) . The location in the outer media, close to the adventitia, makes the dissection prone to external rupture. Hemopericardium and cardiac tamponade occur when rupture is into the pericardial cavity. Hemothorax, particularly of the left pleural cavity, develops when rupture involves the descending thoracic aorta leading to fatal hemorrhagic shock. Retrograde dissection toward the aortic root may give rise to other mechanisms of sudden death: the coronary ostia may be involved with abrupt obstruction, myocardial ischemia, and ventricular fibrillation, with or without evidence of a myocardial infarction. Acute aortic valve incompetence resulting from dehiscence of the cusps commissure may induce ventricular volume overload and pulmonary edema. Retrograde dissecting hematoma may also extend to the atrial septum through the aortoatrial space and may spread toward the AV node and thereby creating atrionodal discontinuity with the onset of AV block and cardiac asystole .
Aortic dissection in the elderly is mostly a complication of systemic hypertension , and the intimal tear is a consequence of a hypertensive peak. Whether in hypertensive subjects the tunica media exhibits significant changes in terms of medionecrosis, severe disruption of elastic fibers, loss of smooth muscle cells, and accumulation of proteoglycan pools (cystic medial necrosis) is a controversial issue . When aortic dissection occurs in the young, these abnormalities are a constant microscopic feature .
In women, pregnancy is a well-known risk factor for aortic dissection. A genetic or congenital anomaly underlies aortic dissection in the young (e.g., familial dissecting aneurysms , Marfan syndrome , Loeys–Dietz syndrome , Ehlers–Danlos syndrome , isthmal coarctation , and bicuspid aortic valve ).
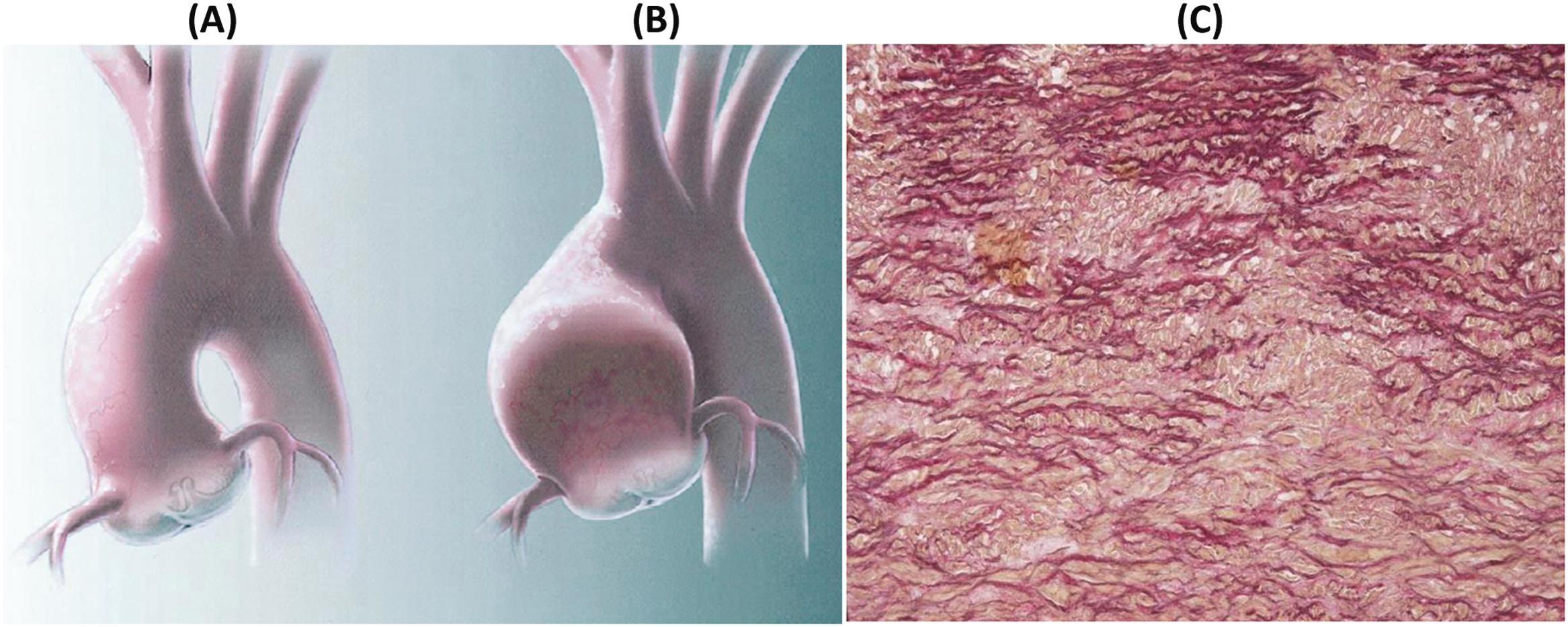
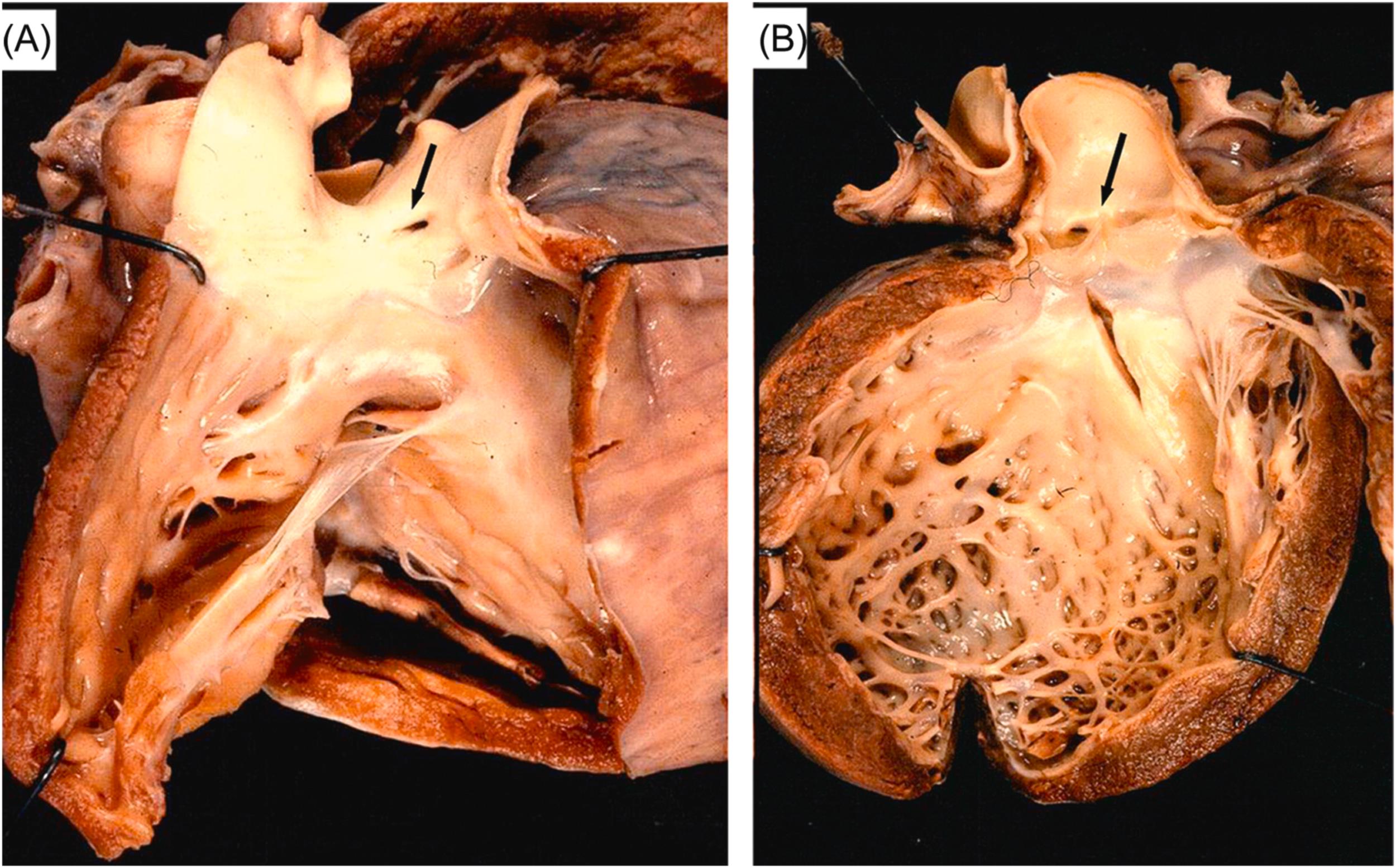
The genetic defect in Marfan syndrome maps to chromosome 15q21.3 . The disease is familial in the majority of patients, whereas 30% of cases are sporadic. The defective gene encodes fibrillin-1, which is the major constituent of microfibrils of the extracellular matrix and regulates local transforming growth factor (TGF)-β activity since mutations in fibrillin-1 leads to activation of TGF-β pathway . Hearts of Marfan patients who die suddenly because of aortic dissection (usually type I or II, with rupture within the pericardial cavity) exhibit typical cardiovascular features consisting of mitral valve prolapse, annuloaortic ectasia, with or without fusiform aneurysm of the ascending aorta, and aortic incompetence ( Fig. 11.9 ). Nonetheless, aortic dissection in Marfan syndrome may also be observed without dilatation of the aorta, its occurrence being unpredictable on clinical grounds .
Several gene mutations have been associated recently with syndromic and nonsyndromic forms of aneurysms and dissections, including TGF-β receptor 1 and 2 (TGFBR1, TGFBR2) and SMAD3 in Loeys–Dietz syndrome , ACTA2, MYH1, and MYLK. These genes encode either structural proteins associated with connective tissue, members of the TGF-β pathway, or components or regulators of the vascular smooth muscle cells .
The association between an isolated bicuspid valve and aortic dissection is not incidental. Indeed, the rate of bicuspid aortic valve among those with aortic dissection is significantly higher than in the normal population (13% vs 1%) . The rupture involves a severely degenerated ascending aorta, with or without dilatation, in the setting of a normally pliable bicuspid valve ( Figs 11.10–11.12 ). Dissections have been reported among the offspring of individuals with a bicuspid aortic valve, which is now regarded a familial dominant Mendelian disorder with incomplete penetrance . Considering the frequency of a bicuspid aortic valve among the general population , the risk of dissection is low. Most probably, medionecrosis is present only in a subset of patients with a bicuspid aortic valve. Echocardiographic monitoring of the aortic root in young individuals with this anomaly may detect progressive aortic dilatation as a marker of underlying vessel wall degeneration and impending rupture . Abnormal aortic elasticity and impaired aortic stiffness evaluation by echocardiography may identify patients at risk of dissection . One may wonder whether a bicuspid aortic valve and medionecrosis are the phenotypic expressions of the same genetic disease or simply a congenital heart disease complex in which the maldevelopment involves either the aortic valve or the wall, which both derive from the neural crest .
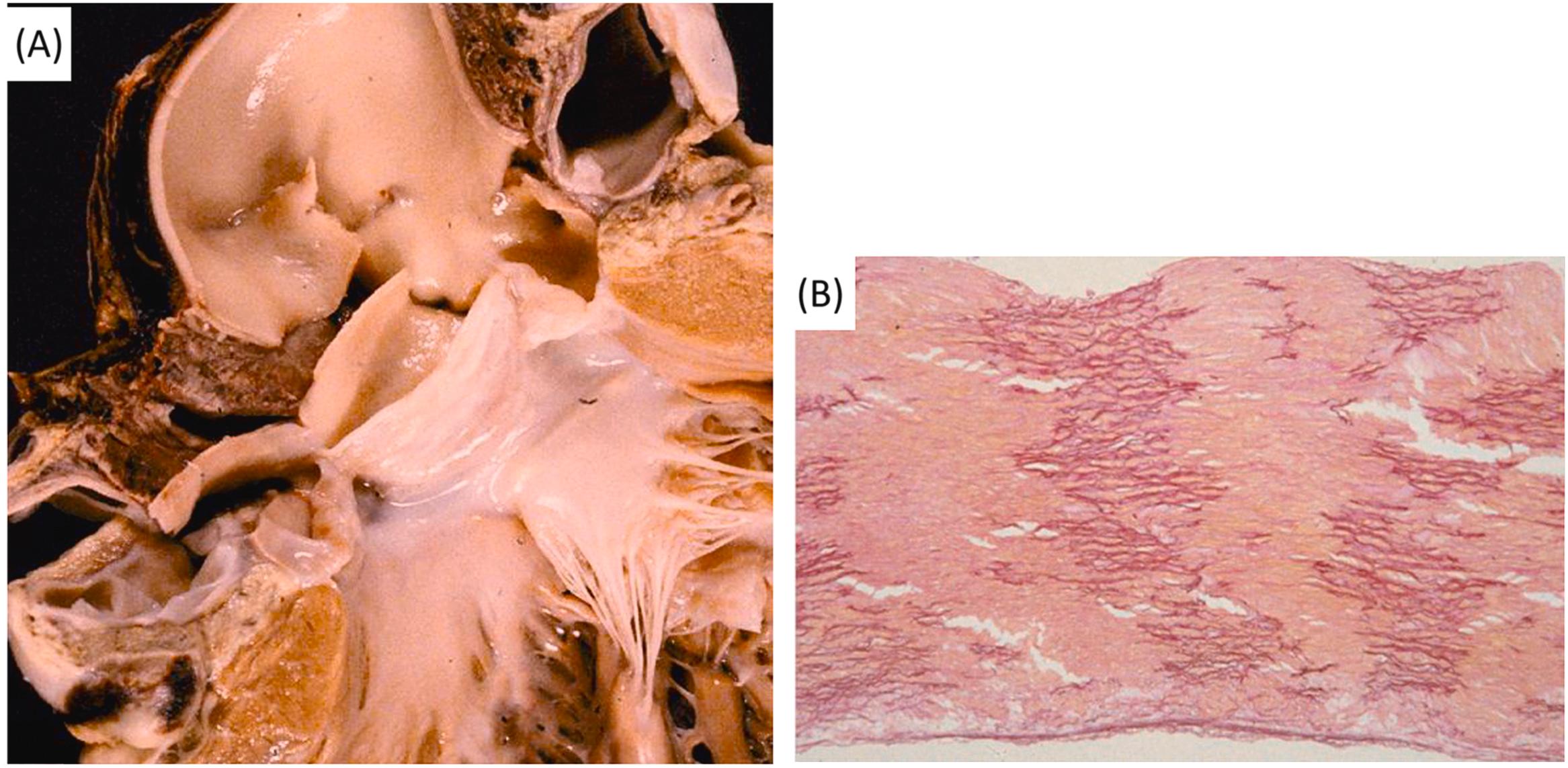
This seems to be the case in isthmal coarctation (the so-called adult coarctation), associated with a bicuspid aortic valve in 40%–50% of cases, and in which aortic dissection occurs in its natural history ( Fig. 11.13 ). An equal severity of medionecrosis in spontaneous aortic rupture has been reported in patients with Marfan syndrome or isolated bicuspid aortic valve or isthmal coarctation, with or without a bicuspid valve . A relationship between the development of the ascending aorta–aortic valve and the neural crest has been proved by experimental embryology .
Bicuspid aortic valve also occurs as a component of known genetic syndromes, in particular Turner syndrome , or as a part of nonsyndromic familial aortic aneurysms. Bicuspid aortic valve is considered to have a significant genetic etiologic component on the basis of observed familial clustering . Linkage studies have demonstrated associations between bicuspid aortic valve and nonsyndromic familial aortic aneurysms in chromosomal regions 5q, 13q, and 18q . The most significantly associated genes are NOTCH1, GATA5, and TGFBR2, TGFBR1 .
Table 11.3 reports cases of spontaneous aortic rupture in the Juvenile Sudden Death project from the Veneto Region of Italy. Aortic dissection displayed mostly a congenital or genetic milieu.
| Type, risk factors | N |
|---|---|
| Mycotic aneurysm | 2 |
| Hypertension, dissecting aneurysm | 2 |
| Isthmic coarctation, dissecting aneurysm | 2 |
| Bicuspid aortic valve and isthmic coarctation, dissecting aneurysm | 2 |
| Isolated bicuspid aortic valve, dissecting aneurysm | 6 |
| Marfan syndrome, dissecting aneurysm | 2 |
| Pregnancy, dissecting aneurysm | 2 |
| Idiopathic, dissecting aneurysm | 3 |
| TOTAL | 21 |
Aortic rupture in the setting of an atherosclerotic aneurysm is a frequent event in the elderly. Such aneurysms are located more frequently in the abdominal infrarenal aorta. They develop as a consequence of aortic medial atrophy and degeneration with wall thinning. Medial atrophy may be caused by intimal atherosclerosis, but the cause of both wall thinning and intimal atherosclerosis remains cardioversial . Matrix metalloproteinase (collagenase, elastase), released by monocyte-macrophages and smooth muscle cells in atherosclerotic plaques, play a pivotal role in the pathogenesis and progression of atherosclerosis and related complications (aortic aneurysm and rupture), contributing to vascular remodeling with tunica media atrophy and disrupting extracellular matrix components . An abdominal atherosclerotic aortic aneurysm exceeding 5 cm in diameter is considered at high risk of impending rupture and sudden death and is an indisputable indication for surgery or interventional angiology, because of the high wall tension according to the Laplace law [ T = p × r /2 s ( T =tension, p =pressure, r =radius, and s =wall thickness)] .
Although instantaneous deaths complicating myocardial infarction in intensive care units are usually ascribable to cardiac rupture, and although historically most cases reported in Lancisi's “De subitaneis mortibus” and Morgagni's “De Sedibus” were explained by this complication, death under these circumstances is not in keeping with the definition of an unexpected event, and now is not listed among sudden deaths. The patient is usually hospitalized and already diagnosed as having a myocardial infarction.
Sudden cardiac death in the setting of ischemic heart disease is almost invariably arrhythmic. Clinical studies conducted in patients rescued from cardiac arrest, including ECG recordings, enzyme test, coronary angiography, and follow-up demonstrate that aborted sudden death occurs in a variable framework that includes the following conditions.
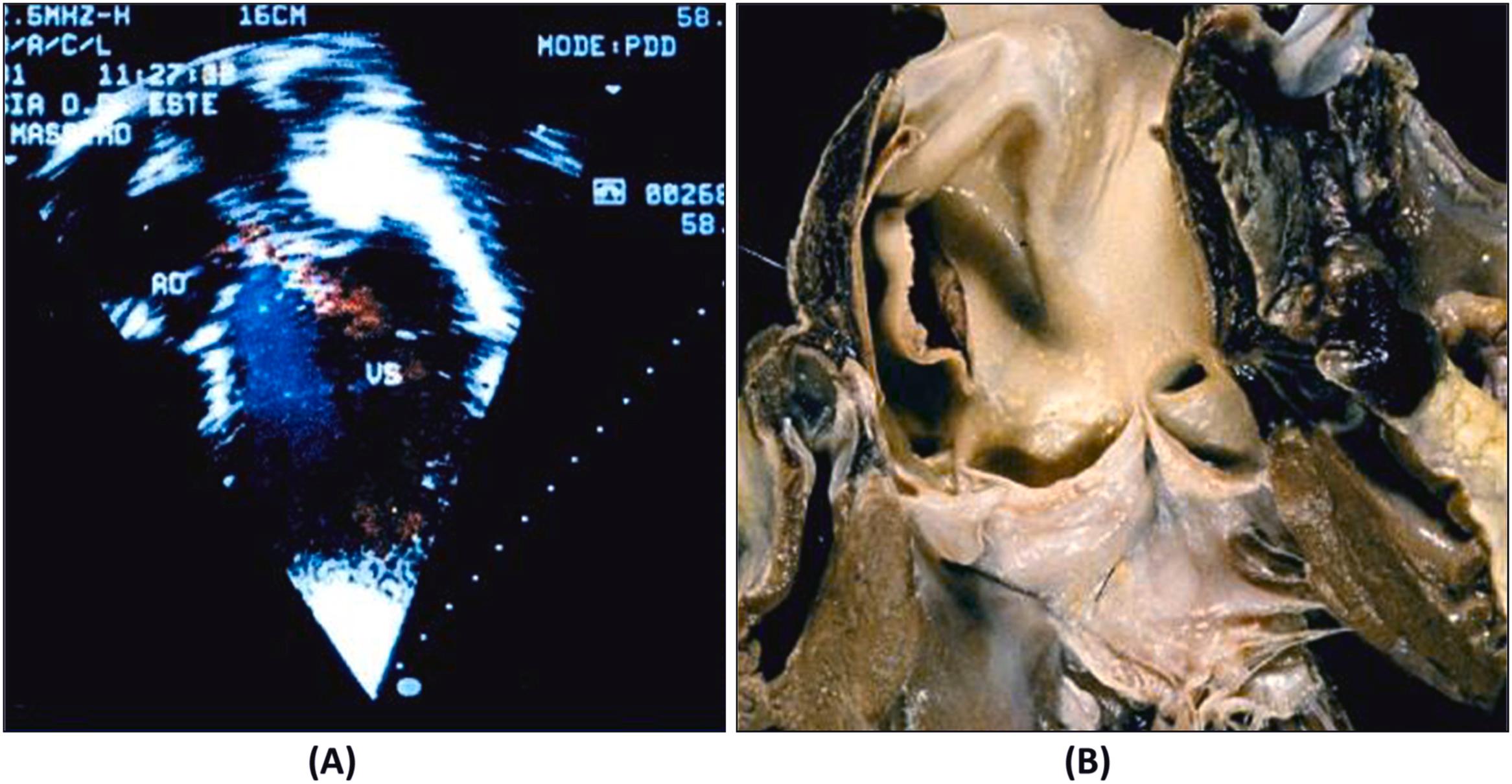
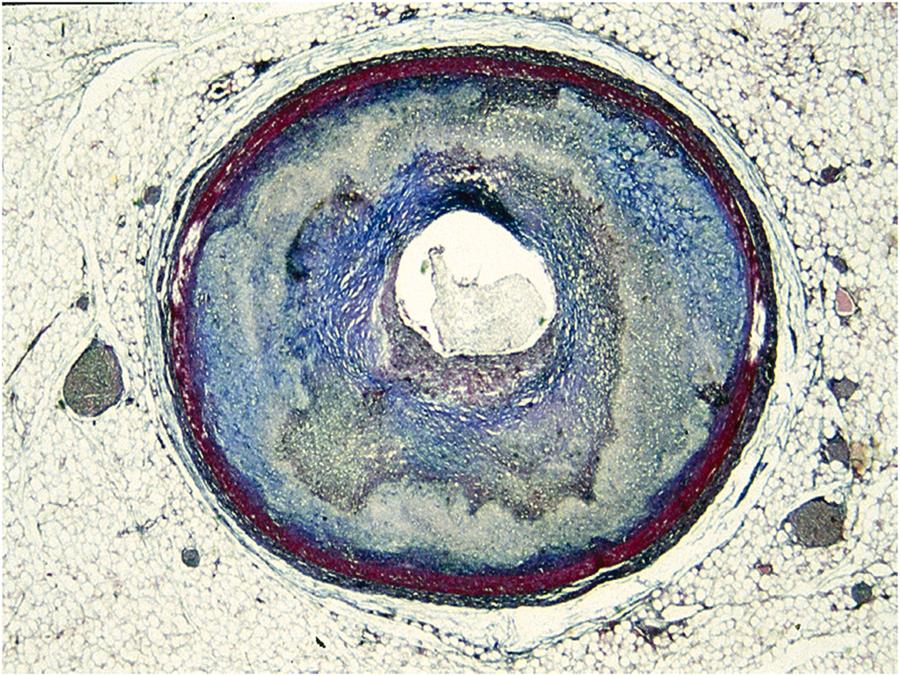
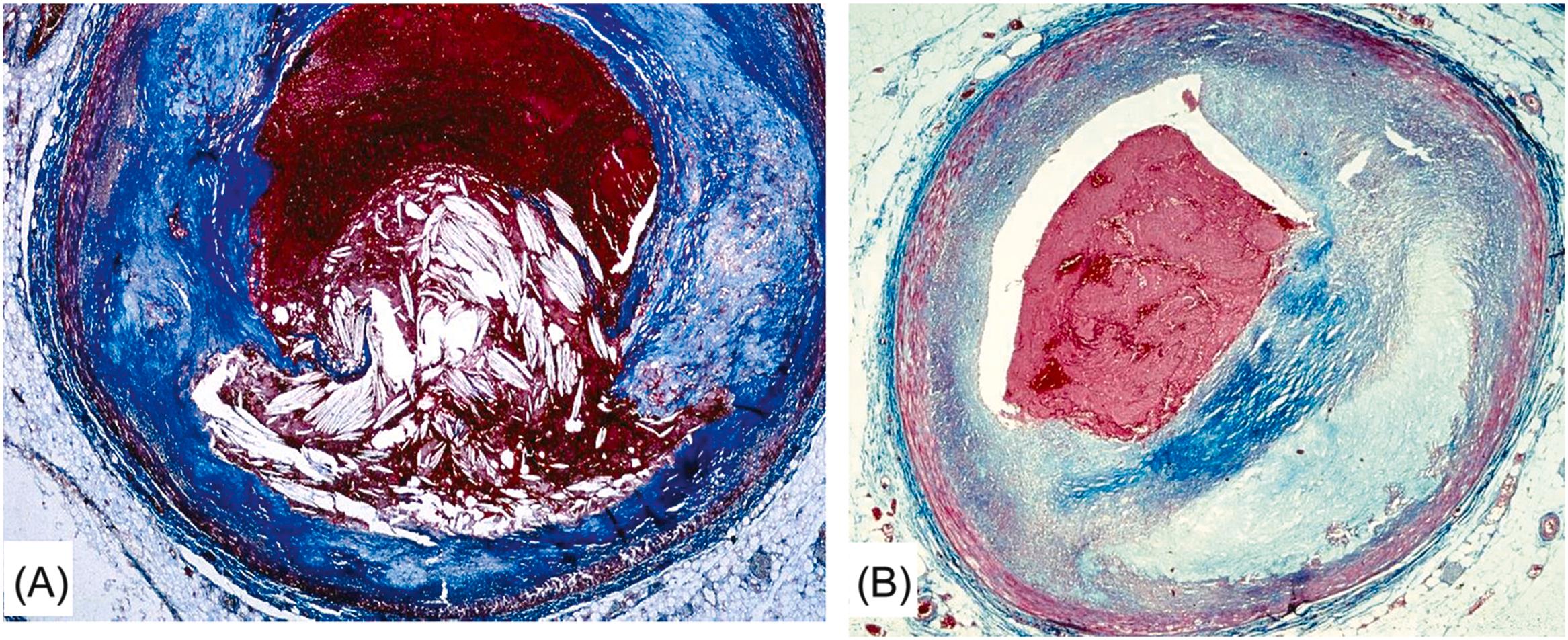
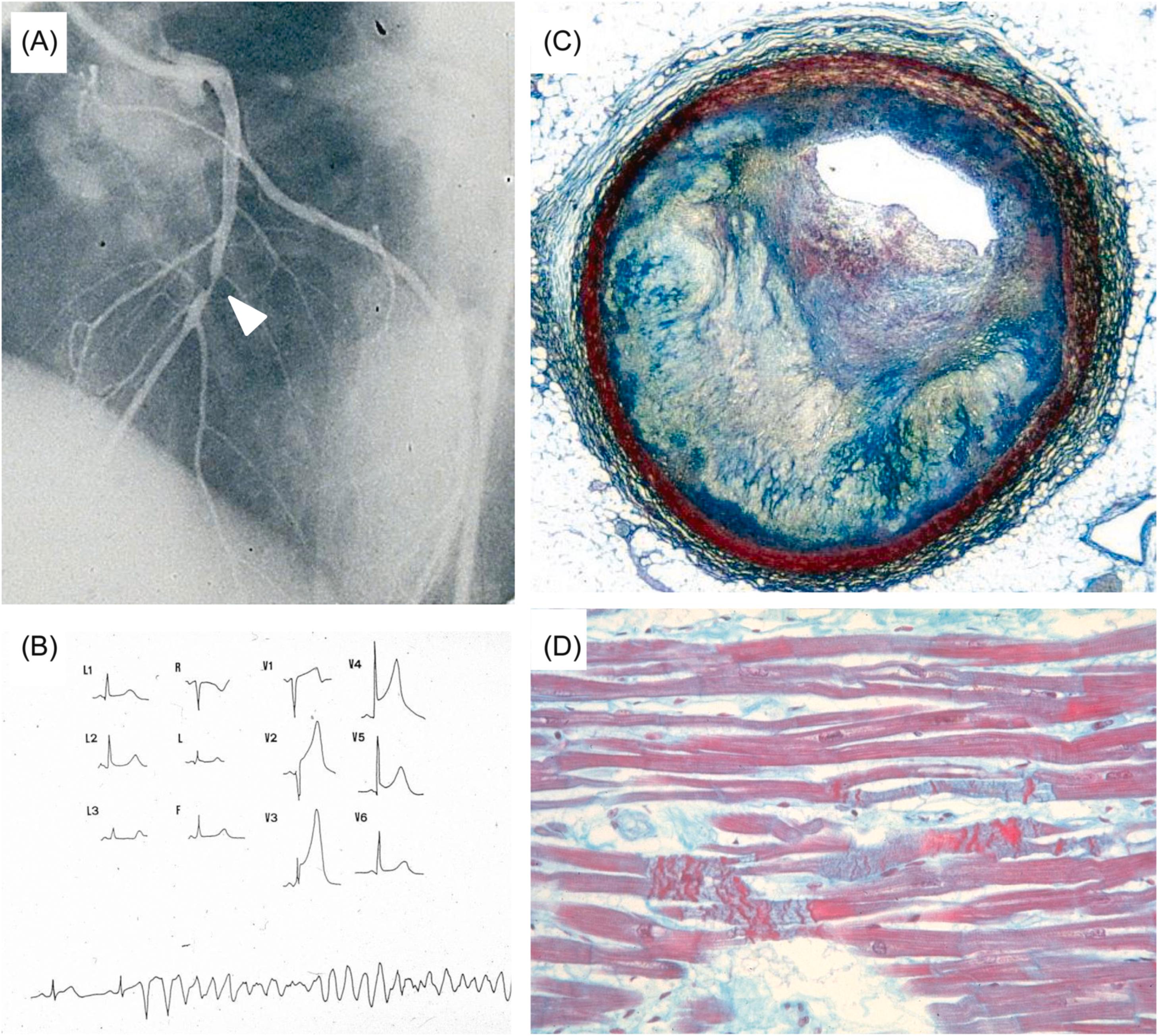
Arrhythmic cardiac arrest may be the earliest manifestation of an acute myocardial infarction. Nearly 40% of patients who are resuscitated after the arrest develop overt signs of a myocardial infarction, with ST segment elevation, Q wave and serum enzyme increase . Most deaths related to myocardial infarction, either first or recurrent episode, occur within the first hour of the onset of an anginal attack and are a consequence of an ischemic, life-threatening arrhythmia. Fatal outcome usually precipitates before the patient arrives to an intensive coronary care unit. The coronary artery pathology consists of single-, double-, or triple-vessel coronary atherosclerotic disease and usually includes a thrombotic occlusion of a coronary segment, which produces a sharp interruption of regional myocardial blood flow . Immediate coronary angiography in survivors of out-of-hospital cardiac arrests disclosed significant coronary artery disease in 70% of patients ( Fig. 11.14 ) and coronary artery occlusion in nearly half . Thrombus may precipitate upon a ruptured atherosclerotic plaque or an endothelial erosion ( Fig. 11.15 ). Rupture affects plaque with lipid-rich cores and thin fibrous caps. Erosion is usually the consequence of inflammation and may involve a fibrocellular plaque devoid of lipids . Acute myocardial damage accounts for automaticity or, most probably, for slow conduction, unidirectional blocks, reentry circuits, and onset of ventricular tachyarrhythmias. The arrhythmias recorded by ECG at the time of cardiac arrest are high-frequency ventricular tachycardia or ventricular fibrillation.
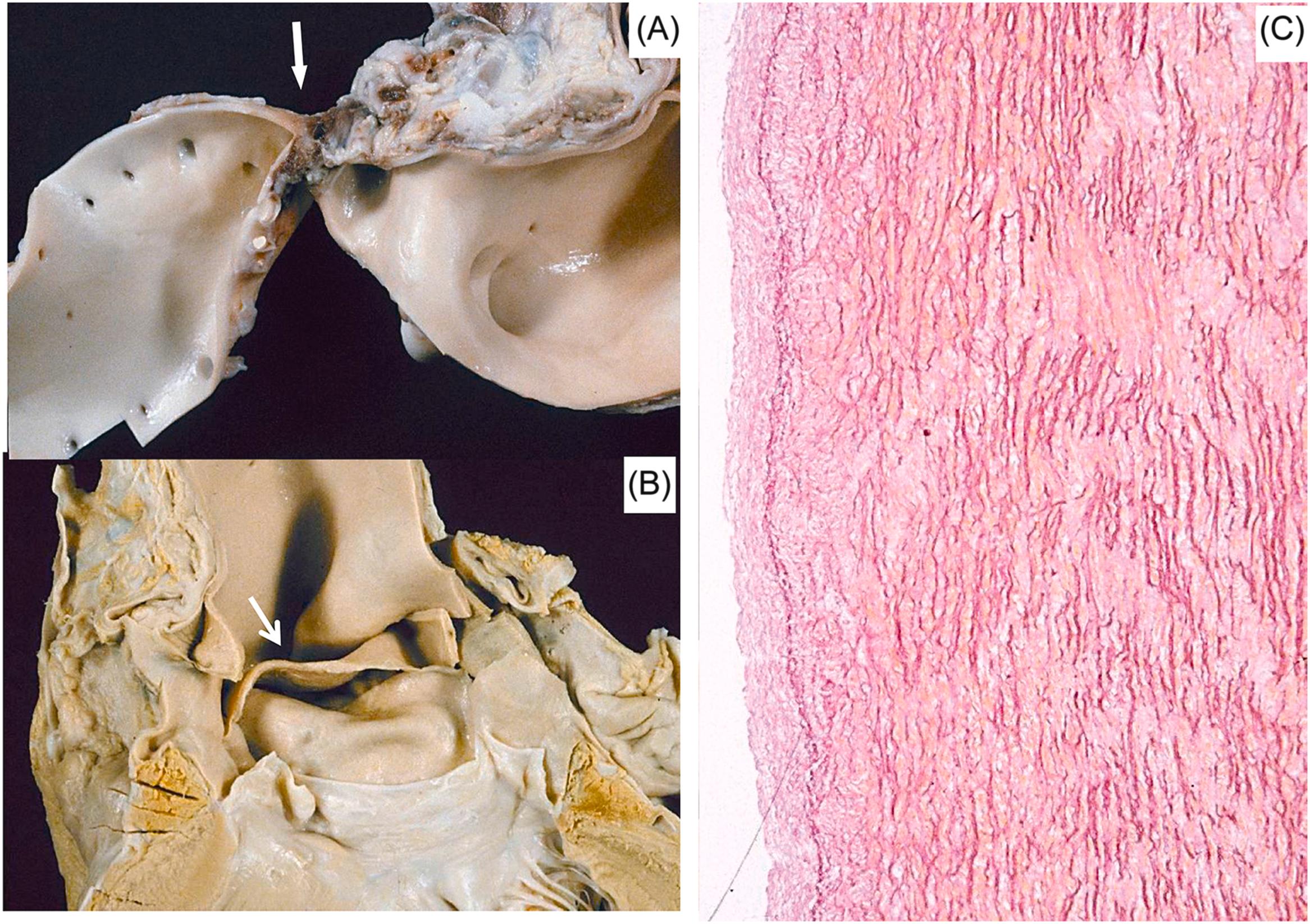
If the coronary thrombosis affects the right coronary artery in a right dominant circulation or the left circumflex artery in a left dominant pattern, blood supply to the sinoatrial and AV nodes may be jeopardized . In those circumstances, cardiac arrest may result in asystole caused either by sinus arrest or AV block. Although conducting tissues are resistant to ischemia, temporary dysfunction of the origin and transmission of the electric impulse may occur, prolonged enough as to be fatal.
It is worth stressing that myocardial infarction, although clearly diagnosed in vivo by ECG abnormalities (ST segment elevation or depression, inverted T wave, and Q wave) and enzyme elevation (Troponin T, CK, and CKMB), may not be obvious pathologically because of the short time elapsing between onset of symptoms and death, even if the triphenyl tetrazolium chloride (TTC) test or other gross histochemical techniques are employed. Even the histology of the myocardium in early infarction may be negative as far as classical findings are considered (e.g., wavy fibers, coagulative necrosis, granulocyte infiltrates) . A thorough microscopic examination, however, allows detection of very early damage in terms of contraction band necrosis or anisotropism of cross striations, which both indicate breaking of the sarcolemma barrier and flooding sarcoplasm with increase of Ca 2+ inflow. However, the specificity of these lesions is questionable because they may also be explained as reperfusion damage after resuscitation or thrombolysis.
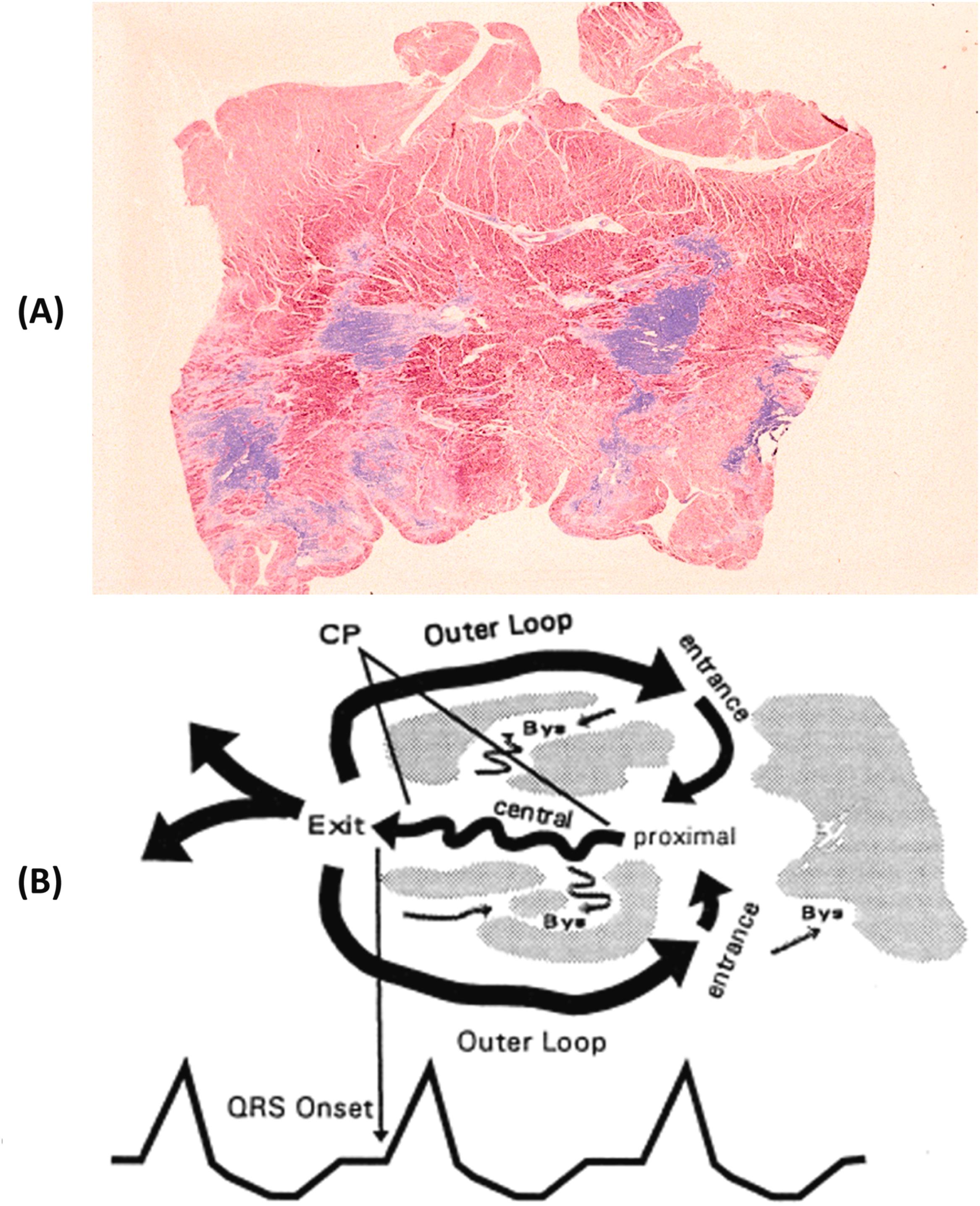
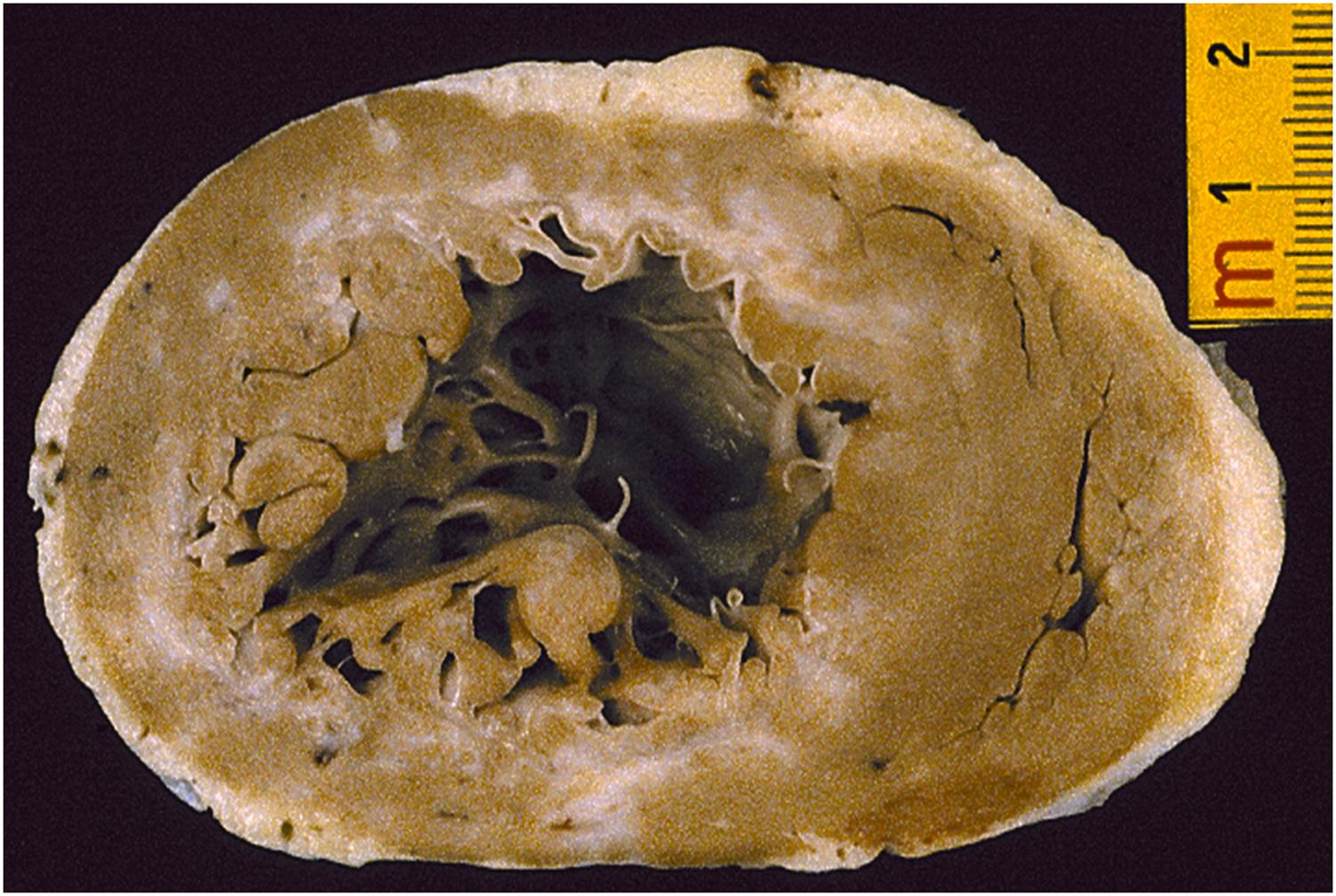
Life-threatening ventricular tachyarrhythmias arise in patients with myocardial scars from previous infarction and result from a slowing of conduction and the onset of reentry circuits at the border between normal myocardium and scar , as well as a zigzag propagation of the impulse within the fibrotic area ( Fig. 11.16 ) . Postmortem studies of sudden cardiac death reported an incidence of healed myocardial infarction in up to two-thirds of adult cases ( Fig. 11.17 ) . Neurovegetative influences, with sympathetic stimulation, whether elicited from emotion or effort, may have a triggering role . Late potentials detected by signal-averaged ECG may reflect this intraventricular conduction defect and the risk of reentrant ventricular arrhythmias, and they represent an adverse prognostic clinical marker for ventricular electrical instability. Warning ventricular arrhythmias, such as premature ventricular beats with QRS on T or ventricular tachycardia (nonsustained or sustained), may be recorded by 24-h Holter monitoring and be induced by programmed ventricular stimulation. Decreased left ventricular ejection fraction less than 35%, mitral incompetence, left ventricular aneurysm, and residual myocardial ischemia are all poor predictors of survival and help to define risk stratification of sudden death, which, by the way, remains the main mode of death after a myocardial infarction in the long term . However, scars with even high EF may be enough to trigger for life-threatening ventricular arrhythmias ( Fig. 11.18 ) . Sudden death is a Damocle’s sword for those who have suffered a myocardial infarction, caused either by recurrence of a coronary thrombosis or by the arrhythmogenicity of myocardial scars .
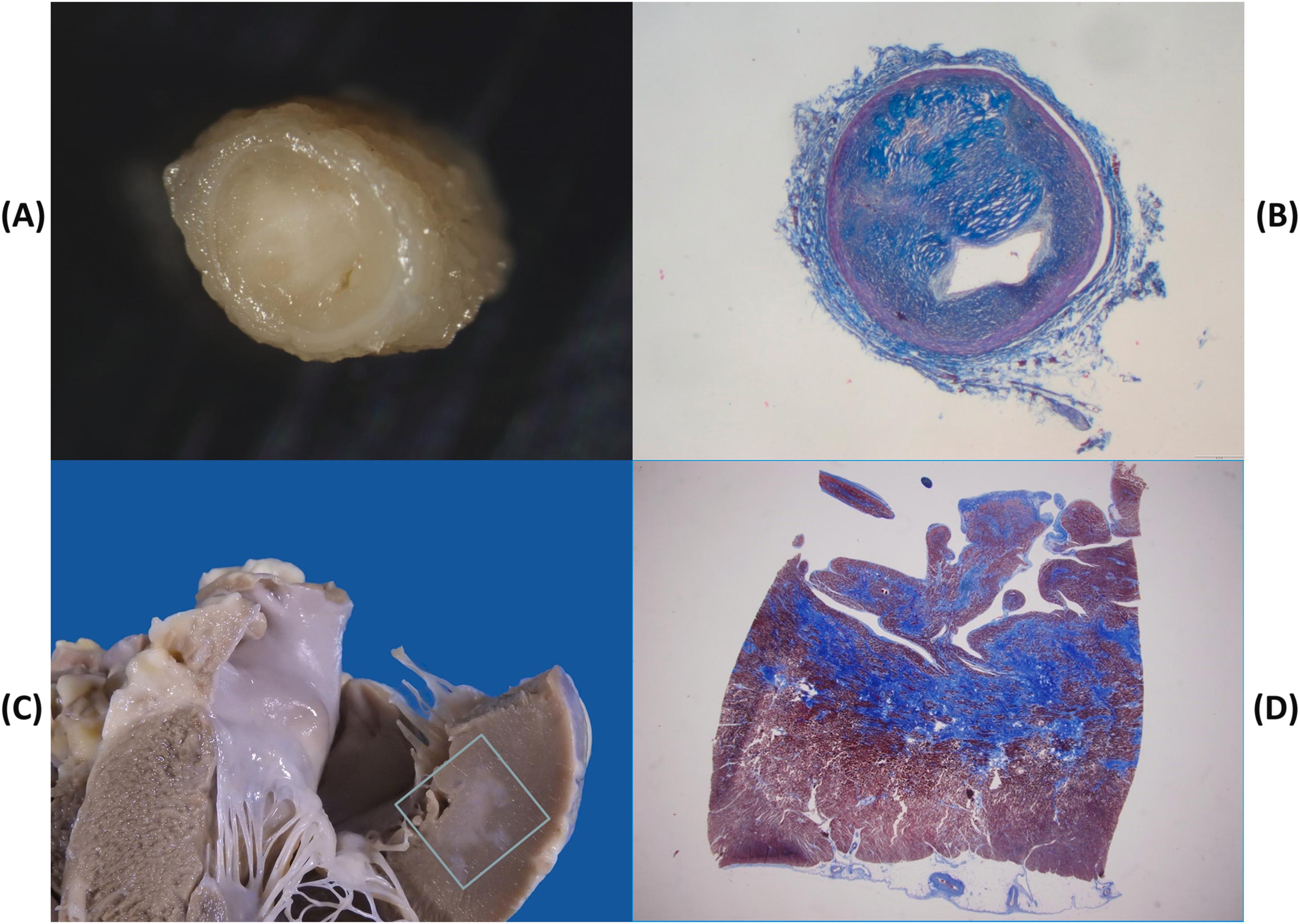
Temporary myocardial ischemia, occurring in the setting of stable (on effort) or unstable (at rest), or variant (vasospastic) angina pectoris, may trigger fatal arrhythmias in the absence of overt myocardial damage, or, more probably, in the presence of patchy contraction band necrosis sufficient to account for slow conduction unidirectional block and reentry phenomena . Vasospasm may be the mechanism of sudden death in the young with premature obstructive coronary atherosclerosis, in whom occlusive thrombosis or a healed myocardial infarction are rarely observed . Indeed, whereas coronary sudden death in adult and elderly patients is mostly associated with an occlusive or mural thrombus in the setting of a multivessel disease post infarction scar , coronary sudden death in young patients is rarely precipitated by coronary thrombosis . Rather, the pathology usually consists of a single obstructive plaque, located at the first segment of the left anterior descending coronary artery. The plaque is mainly fibrocellular and devoid of atheroma, fissuring, or thrombosis . Preservation of the tunica media, absence of thrombosis, recent intimal smooth muscle cell proliferation of contractile type and frequent occurrence of unexpected death at rest are all features in keeping with a transient ischemic episode, most probably attributable to coronary vasospasm ( Fig. 11.19 ). This has been proven to occur during Holter monitoring . The observation of contraction band necrosis in the myocardium related to the obstructed artery also favors a transient ischemia followed by reperfusion injury.
Another feature, that distinguishes sudden coronary death in the young from that in the adult, is the rarity of a previous myocardial infarction in the former .
The normal pattern of the coronary arterial tree is characterized by two coronary arteries, left and right, which arise close to the sinotubular junction of the ascending aorta from left and right anterior aortic sinuses respectively, facing the pulmonary trunk. They arise perpendicular to the aortic root with widely patent ostia and have no relation to the pulmonary trunk in their proximal course ( Fig. 11.20A ). A separate origin from the right aortic sinus of the conal artery and the right coronary artery or of the left circumflex branch and the left anterior descending coronary artery from the left aortic sinus separately, should be considered variants of normal. Right dominant, left dominant, and balanced patterns of the coronary artery circulation are also normal individual variations. The main stems of the coronary arteries run in the subepicardium.
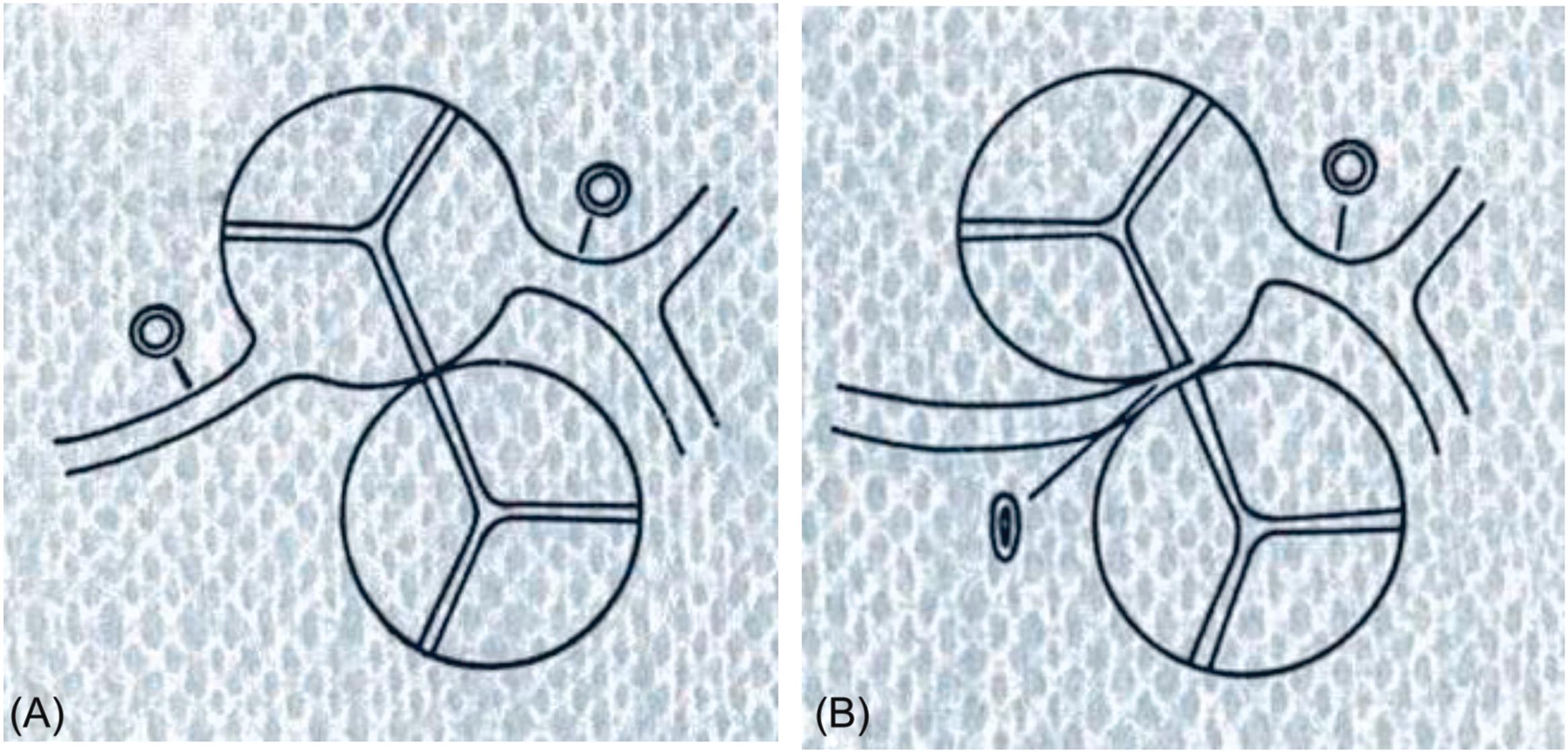
Coronary artery anomalies observed in patients who died suddenly present with an anomalous origin or anomalous course or both.
Before addressing the usual congenital malformations of the coronary arteries, a peculiar situation of coronary ostial obstruction is that observed in cases of hyperelastosis of the aortic wall . In this condition, the coronary ostium and the intramural course demonstrate severe obstruction. This malformation may be observed in supravalvular aortic stenosis (Williams syndrome ).
Anomalous origin of a coronary artery from the pulmonary trunk, usually the left coronary stem ( Fig. 11.21 ), is symptomatic in early infancy because of coronary blood steal from the aorta to the pulmonary artery, which results into severe myocardial ischemia and necrosis . Sudden death may occur, although the usual clinical manifestation is progressive congestive heart failure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here