Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A ventricular septal defect (VSD) is a hole between the left and right ventricles. A VSD may occur as an isolated anomaly or with a wide variety of intracardiac anomalies, such as tetralogy of Fallot or transposition of the great arteries. This chapter discusses isolated VSD.
Banding of the pulmonary artery as a palliative maneuver was first described in 1952. This decreased left-to-right shunting and as a consequence prevented the development of pulmonary vascular obstructive disease and left-sided volume overload. Until the mid-1960s when primary VSD closure became safer, pulmonary artery banding was the procedure of choice in managing VSDs. The first VSD closure was performed in 1955 by Lillehei and associates at the University of Minnesota, using controlled cross-circulation between the child and parent. Nineteen of the 27 patients who underwent this procedure survived. In 1957, Kirklin and associates at the Mayo Clinic closed a VSD using a heart-lung machine. In 1957, transatrial VSD closure was performed, followed in 1971 by the popularization of primary repair in symptomatic infants by Barratt-Boyes and associates using cardiopulmonary bypass, deep hypothermia, and circulatory arrest.
Surgeons planning VSD surgery must have intimate knowledge of the tricuspid valve, the right ventricular septal anatomy, and the conduction system.
The tricuspid valve has three leaflets: anterior, septal, and posterior. The anterior leaflet is connected via the chordae tendineae cordis to the anterior papillary muscle (located on the anterior right ventricular free wall) and to the septal papillary muscle (sometimes called muscle of Lancisi). The septal papillary muscle is itself part of the septal band of the septomarginal trabecula. The posterior leaflet is attached to the anterior and posterior papillary muscles, and the septal leaflet attaches to the posterior and septal papillary muscles.
The right ventricular septum has five components ( Fig. 117-1 ):
The membranous septum
The atrioventricular (AV) canal or inlet septum
The muscular septum (apical trabecular septum or sinus septum)
The trabecula septomarginalis (septal band and moderator band)
The conal septum (infundibular septum and parietal band)
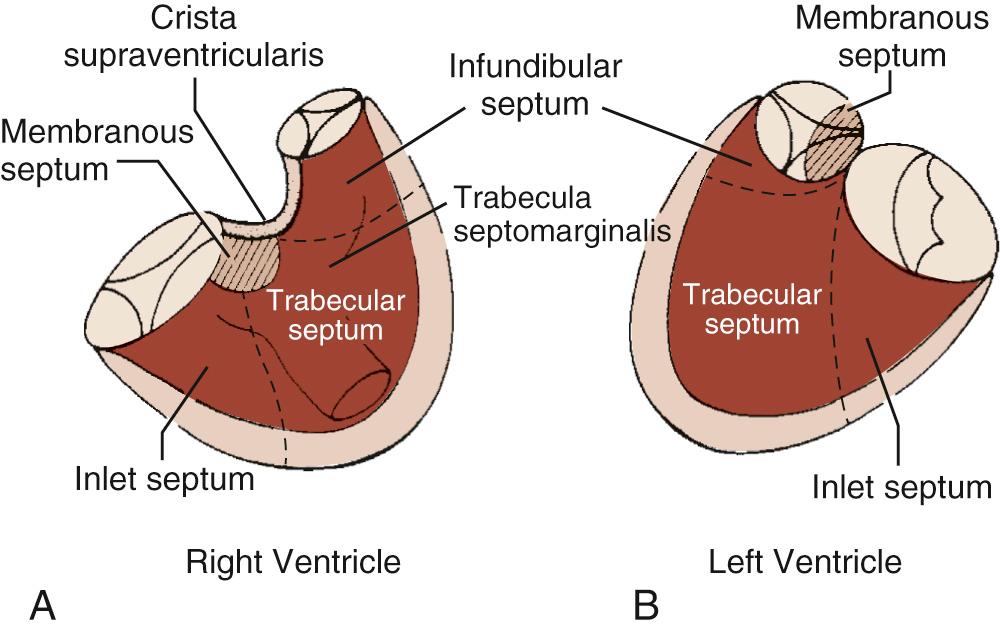
Unlike the left ventricular septum, which is free of any papillary muscle attachment (the mitral valve can be called “septophobic,” whereas the tricuspid valve can be called “septophilic”), the right ventricular septum is where the septal (sometimes called medial) papillary muscle and part of the posterior papillary muscle originate. The septal papillary muscle is a portion of the septal band, which runs along the septum (hence its name). The septal band is a portion of the septomarginal trabecula, which also includes the moderator band. The moderator band links the septum to the anterior papillary muscle (called moderator band because it was erroneously thought to “moderate” the right ventricular free wall—that is, keep in sync with the rest of the ventricle). The membranous septum is the only fibrous component of the septum. It is wedged between the aortic valve, the tricuspid valve, and the mitral valve. Because the tricuspid valve is normally apically displaced vis-à-vis the mitral valve, a portion of membranous septum ends up between the right atrium and the left ventricle, called the AV part of the membranous septum. The portion of membranous septum located between both ventricles is called the interventricular part.
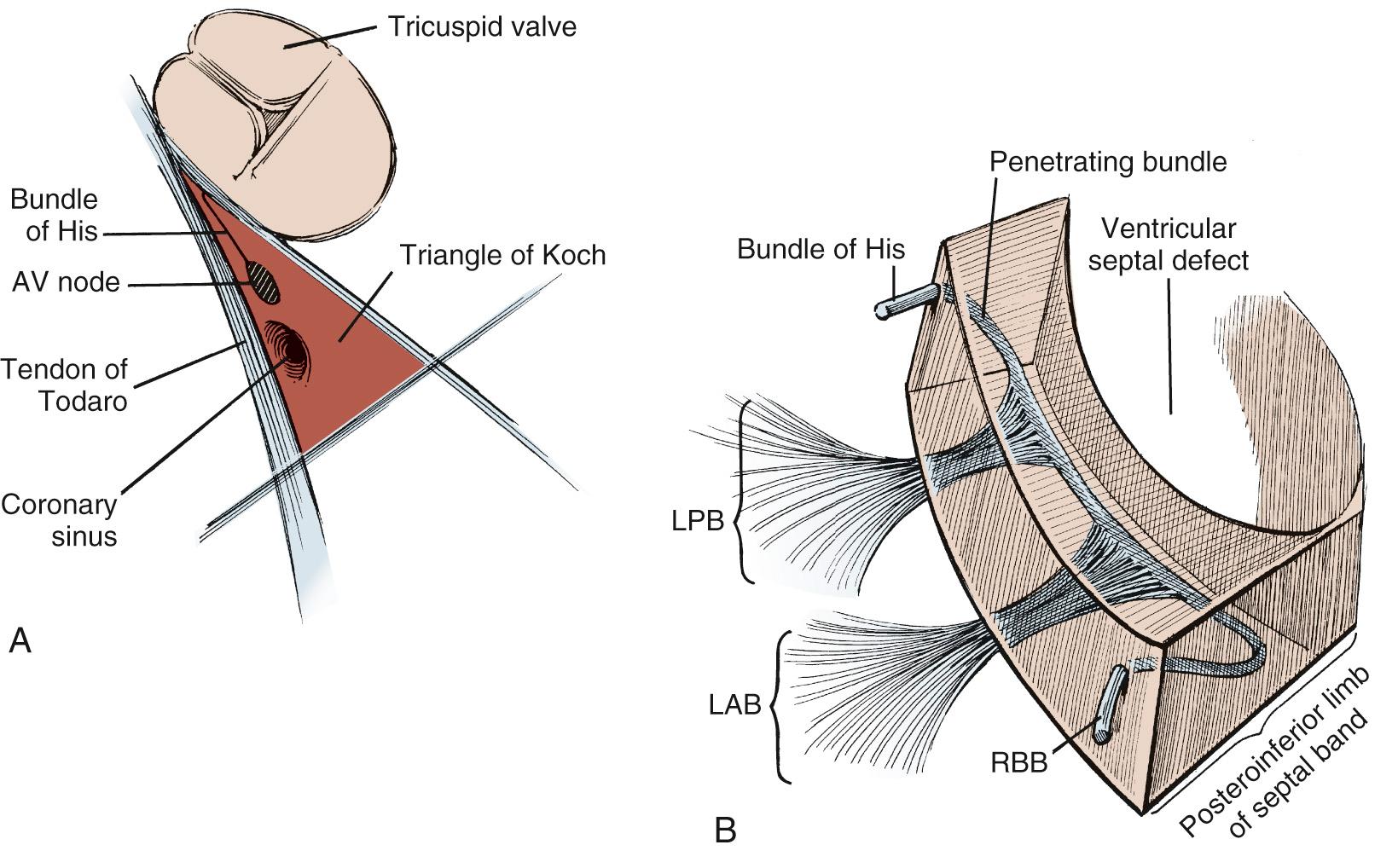
Knowledge of the conduction system of the heart also is critical when approaching VSDs so as to avoid damaging it ( Fig. 117-2 ). The various atrial conduction tracts all converge toward the AV node of Aschoff-Tawara. The AV node is located in the inferior-posterior portion of the membranous septum, just inferior to the anteroseptal commissure of the tricuspid valve. A different description of its location is that it occupies the apex of the triangle of Koch, which is limited by the ligament of Todaro posteriorly, the orifice of the coronary sinus inferiorly, and the tricuspid valve annulus superiorly (see Fig. 117-2 ). From the AV node, the common AV bundle of His descends within the interventricular part of the membranous septum (or, in the case of a membranous VSD, the posteroinferior rim of the VSD), traverses the septum, and then courses along the left ventricular aspect of the septum. It then separates into a right bundle branch, which travels back to the right ventricular surface, as well as a left bundle branch. At the anteroinferior border at the level of the muscle of Lancisi, the right bundle branch descends toward the right ventricular apex.
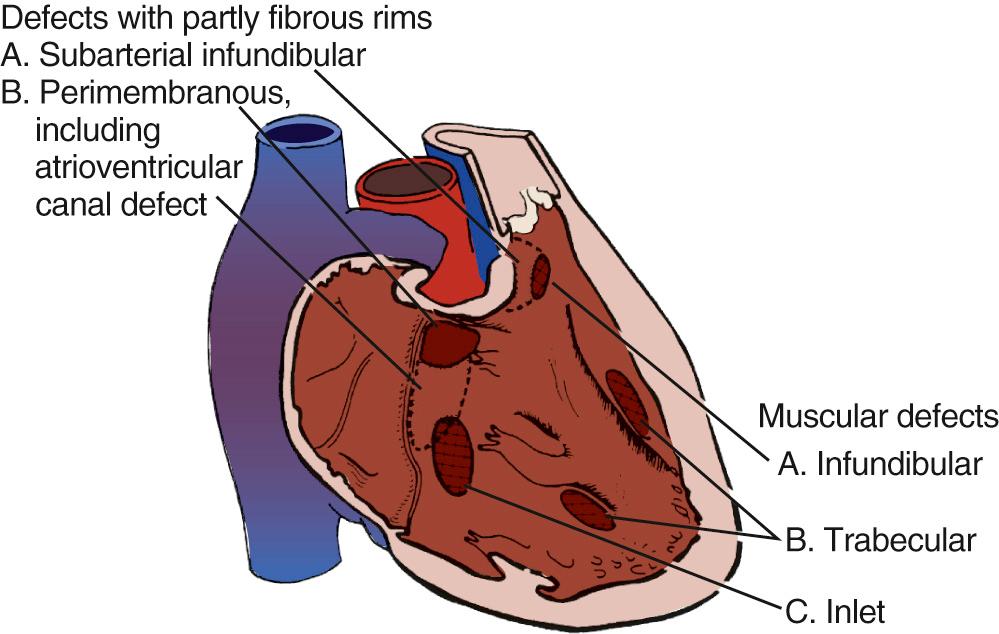
A useful surgical classification of VSDs was initially developed in 1980 by Soto and associates ( Fig. 117-3 ) and then further modified by Van Praagh and associates ( Fig. 117-4 ). Variations of this classification are used in most pediatric cardiac centers. VSDs can be classified as follows:
Conoventricular (or membranous) defects
Conal (or outlet) VSDs
Inlet (or AV canal type) VSDs
Muscular VSDs (single or multiple)
![FIGURE 117-4, Classification of ventricular septal defects (VSDs): atrioventricular canal type; muscular VSDs (anterior [1], midventricular [2], posterior [3], and apical [4]); conoventricular septal defect, which includes paramembranous and malalignment conoventricular septal defects; and conal septal defects. FIGURE 117-4, Classification of ventricular septal defects (VSDs): atrioventricular canal type; muscular VSDs (anterior [1], midventricular [2], posterior [3], and apical [4]); conoventricular septal defect, which includes paramembranous and malalignment conoventricular septal defects; and conal septal defects.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/VentricularSeptalDefectandDoubleOutletRightVentricle/3_3s20B9780323241267001174.jpg)
Conoventricular defects are located between the conal septum and the ventricular septum. They are centered around the membranous septum and comprise 80% of all VSDs. They may be located exclusively in the membranous septum, or they can extend beyond the boundaries of the membranous septum in the inferior, posterior, or anterior direction and are then sometimes called perimembranous or paramembranous VSDs. The prefix peri-, appearing in loan words from the Greek, means “surrounding” (e.g., perimeter). Thus, a truly perimembranous VSD would surround the membranous septum. In contrast, the prefix para-, also from the Greek, means “adjacent to” or “beside” and more accurately reflects the notion of a defect adjacent to the membranous septum. Neither perimembranous nor paramembranous correctly describes the typical defect involving the membranous septum and extending into the adjacent septum. The current recommendation is to call these defects either membranous VSDs or conoventricular defects. Malalignment of the conal septal plane vis-à-vis the ventricular septal plane results in the typical conoventricular defect. The malalignment can be anterior, as seen in tetralogy of Fallot, for example, or posterior, as seen in interrupted aortic arch. In addition to resulting in a VSD, anterior conal septal malalignment also results in right ventricular outflow tract obstruction, whereas posterior malalignment of the conal septum results in left ventricular outflow tract obstruction. Important landmarks in conoventricular septal defects are the anteroseptal commissure of the tricuspid valve inferiorly and the noncoronary cusp of the aortic valve. When the ventricular portion of the membranous septum is entirely absent, the VSD extends to the base of the aortic valve (sometimes called subaortic VSD). The medial papillary muscle (muscle of Lancisi) located at the inferoposterior border of the defect is also an important landmark. Both the septal and the anterior tricuspid valve leaflets are attached to it.
Approximately 8% of VSDs are located in the conal (infundibulum or outlet) septum. They also are called supracristal VSDs. They are either entirely surrounded by muscle (muscular conal VSDs) or limited upstream by the aortic or pulmonary annuli (sometimes called subarterial VSDs).
Inlet VSDs are characterized by the absence of part or all of the AV canal (inlet) septum. These VSDs are located immediately underneath the septal leaflet of the tricuspid valve, with no tissue in between. Approximately 6% of all VSDs are inlet-type VSDs.
Muscular VSDs (10% of all VSDs) are entirely surrounded by muscle. They can occur anywhere in the trabecular portion of the septum and can be isolated or multiple. They are described by their location—that is, anterior, midventricular (between the muscular septum and the septal band), posterior, or apical. When inspected through the left side of the septum, what appeared to be multiple muscular defects often converge into either a single hole or two separate holes.
VSDs are an intrinsic portion of many, if not most, complex cardiac malformations. These VSDs are discussed separately with their respective entities (see other chapters). Almost half of patients who undergo surgical treatment of primary VSD have an associated lesion.
A large patent ductus arteriosus (PDA) is present in approximately 25% of symptomatic neonates and infants with VSDs. This is important to know because preoperative echocardiography may fail to show a PDA in the presence of a large amount of left-to-right shunting. Furthermore, intraoperative transesophageal echocardiography (TEE) is notoriously unreliable in excluding PDAs. Therefore the possibility of a PDA should be kept in mind when approaching a VSD, and if there is any doubt, or if there is a large amount of backflow through the pulmonary arteries on cardiopulmonary bypass, the PDA should be ligated or clipped.
A hemodynamically significant aortic coarctation is present in approximately 10% of cases. Because of the unique pathophysiology of aortic coarctation (more left-to-right shunting across the VSD because of increased afterload caused by the coarctation), these patients usually have presenting symptoms before 3 months of age.
Congenital valvar or subvalvar aortic stenosis, resulting in left ventricular outflow tract obstruction, is seen in approximately 4% of patients requiring an operation for a VSD. The most common type of subaortic stenosis associated with VSDs involves the discrete fibromuscular membrane of the VSD that is located inferior or upstream to it. Congenital mitral valve stenosis is rare and occurs in approximately 2% of patients.
Other significant anomalies include large atrial septal defects (ASDs), right ventricular outflow tract obstruction, vascular ring, and persistent left superior vena cava.
The magnitude and direction of the shunt across a VSD depend on the size of the defect and the pressure gradient across it during the various phases of the cardiac cycle. Large VSDs offer little or no resistance to blood flow and are therefore called nonrestrictive. The right ventricular pressure equals the left ventricular pressure, and the ratio of pulmonary to systemic flow (Qp/Qs) (or shunt) is dependent on the ratio of pulmonary vascular resistance (PVR) to systemic vascular resistance (SVR). On the other hand, small VSDs offer resistance to flow across the defect and are therefore termed restrictive VSDs. The Qp/Qs rarely exceeds 1.5. Moderate-sized VSDs fall between these two categories and the Qp/Qs usually ranges between 2.5 and 3. To a lesser degree, further determinants of shunt magnitude also include the relative compliance of both ventricles and the pressure relationships during the various phases of the cardiac cycle. The size of the VSD—in particular, of muscular VSDs—also may vary during various phases of the cardiac cycle. Because the PVR is elevated during the first few weeks of life, it is unusual to have to close an isolated VSD. As the PVR falls with increasing age, left-to-right shunting increases, necessitating treatment.
Left-to-right shunting at the ventricular level implies increased pulmonary blood flow. Therefore, left ventricular preload is similarly increased, resulting in increased workload for both left and right ventricles. The left atrium is enlarged, and the left atrial pressure is elevated. The left ventricle dilates. The raised left atrial pressure causes many infants with VSD to have an increased accumulation of interstitial fluid in the lungs, resulting sometimes in repeated pulmonary infections. The work of breathing is increased as the lung compliance is decreased. This increases energy expenditure, which, along with the relatively low systemic blood flow, causes these infants to have striking failure to thrive. When pulmonary resistance rises as a result of the development of pulmonary vascular disease, pulmonary blood flow is reduced and the child appears to improve. Unfortunately, further increases in PVR occur, and the classic Eisenmenger complex results. These patients are characterized by fixed pulmonary hypertension, bidirectional shunting, right ventricular hypertrophy, and a normal-sized left ventricle. They are often inoperable and require heart-lung transplantation for further survival.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here