Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We thank Margot M. Bartelings for support and useful comments while preparing the anatomy and embryology part of the manuscript and for her help in making the morphologic photographs, and Ron Slagter for help in preparing the figures.
The reported incidence of congenital heart disease (CHD) depends on the number of trivial lesions included. For moderate to severe CHD that will require specialized care, numbers are stable at 6 per 1000 live births. In contrast, the population prevalence , in particular of adults with moderate to severe lesions, is increasing. , In 2000 the reported population prevalence was 5.8 per 1000 people, with 11.9 per 1000 children and 4.1 per 1000 adults being affected. The prevalence of severe defects, including tetralogy of Fallot (TOF), was 1.45 per 1000 children and 0.38 per 1000 adults. Of importance, between 1985 and 2000, the prevalence of severe CHD increased by 85% in adults but only by 22% in children. In 2010 the estimated population prevalence of CHD in the United States was 7.85 per 1000 people (13.21 per 1000 children and 6.16 per 1000 adults), and the prevalence of severe defects was 1.66 per 1000 children and 0.68 per 1000 adults.
A clear change in mortality in CHD has been observed, with better survival in infancy and a trend toward more deaths occurring at an older age. Survival to adulthood increased from 81% for individuals born between 1970 and 1974 to 88.6% for those born in 1990 to 1992. Improvements in survival are driven by a decreased mortality in moderate and severe forms of CHD in childhood, including TOF, truncus arteriosus, atrioventricular septal defect (AVSD), transposition of the great arteries (TGA), and univentricular hearts. For individuals born between 1990 and 1992, survival to the age of 18 was 78.1% for TOF, 76.5% for AVSD, 70.7% for TGA, and 49.1% for univentricular hearts. This is likely the result of earlier surgical interventions and improved surgical techniques and outcomes. Nevertheless, improved survival is not exclusively a function of lower mortality in infancy. The increasing number of patients with repaired congenital heart disease joining the adult population requires the training of electrophysiologists with special interest in adult CHD because potentially life-threatening ventricular arrhythmias (VAs) and sudden cardiac deaths (SCDs) can still occur late after surgery.
The leading causes of mortality in contemporary adults with CHD is chronic heart failure (42%), followed by pneumonia (10%) and SCD, which still accounts for 7% of all deaths, despite a more liberal use of implantable defibrillators in the current era. , The incidence of SCD in repaired CHD is 0.9 to 2.6 per 1000 patient-years, which is 25- to 100-fold higher than for the general population. , , In a population-based series of CHD patients who underwent surgery between 1958 and 1996, 90% of all sudden deaths occurred in four main categories: aortic stenosis, aortic coarctation, dextro-TGA (d-TGA), and TOF. Similarly, a single-center cohort study of CHD patients with a reparative or palliative intervention before the age of 20 years reported the highest incidence of SCD in patients with TGA, univentricular hearts, aortic coarctation, and TOF. In a large multicenter case-control study that also included nonoperated patients, cyanotic Eisenmenger syndrome accounted for 19% of all SCD, followed by congenitally corrected and repaired TGA (19%), repaired TOF (16%), and left-sided outflow lesions.
Of interest, in a recent population-based study, including all patients with pediatric cardiac operations performed between 1953 and 2009, the reported incidence for sudden death decreased to zero among patients with ASD, VSD, TOF, and TGA who underwent surgery between 1990 and 2009. Nevertheless, a time-dependent incremental risk for VA and SCD has been observed, especially in patients with left heart obstructions and TOF, and longer follow-up is needed. Between 6% and 9% of patients who underwent TOF repair died suddenly after 21 to 35 years of follow-up (2%–3% per decade), accounting for up to 50% of all deaths in this group. ,
The majority of CHD-related SCD is presumably because of VAs. These can be either untolerated sustained monomorphic reentrant ventricular tachycardia (SMVT) related to ventricular scars from incision and patch closure of ventral septal defect (VSD), or monomorphic and polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) in the absence of surgical scars. Although data are lacking, the latter arrhythmia mechanisms may be similar to those observed in other cardiac diseases with pathologic hypertrophy, fibrosis, impairment of cardiac function, and, ultimately, heart failure. Impairment of right ventricular (RV) and left ventricular (LV) function is likely to result in altered ion channel and transporter function. Downregulation of K+ currents and action potential duration (APD) prolongation, which is a consistent finding in ventricular myocytes from subjects with cardiac dysfunction, promotes early afterdepolarizations. In addition, changes in Ca 2 +-handling proteins, which are also observed in heart failure, can cause diastolic Ca 2 + leak from the sarcoplasmic reticulum, resulting in delayed afterdepolarization and triggered activity. Advanced hypertrophy can be because of chronic pressure overload in left heart obstructions and unrepaired TOF. Ventricular dysfunction occurs if the RV serves as the systemic ventricle after an atrial switch operation for TGA or in congenitally corrected transposition of the great arteries (ccTGA). LV dysfunction may be because of long-standing cyanosis if TOF repair is performed at an older age or if chronic volume overload after palliative shunting has occurred. Furthermore, long-lasting volume overload owing to chronic pulmonary regurgitation after initial correction contributes to ventricular dysfunction. In unselected populations of adults with CHD, moderate to severe systemic ventricular dysfunction is a dominant predictor for SCD. , Nevertheless, two-thirds of patients who die suddenly or experience life-threatening VT, typically early to middle-aged adults, have preserved cardiac function before the first event. , Understanding of the different mechanisms of ventricular arrhythmogenesis in CHD is therefore crucial for both risk stratification and treatment. Current data on late morbidity and mortality are based on patients who underwent repair as adolescents. Early surgical intervention and changes in the surgical strategy, in particular for TOF and TGA, might not only influence early mortality but could also affect the incidence and the potential mechanism of arrhythmias, and perhaps late morbidity and mortality, in adult CHD patients in the future. Detailed descriptions of the most common forms of CHD related to VA are provided in the next sections.
TOF (see Chapter 115 ) affects approximately 7.5% of all children born with a CHD. It is characterized by subpulmonary stenosis, a subaortic ventricular septal defect, dextroposition of the aortic orifice, and RV hypertrophy, the latter being secondary to the volume and pressure overload produced by the ventricular septal defect and subpulmonary stenosis, respectively.
During normal development , the outlet portion of the heart needs to evolve from a single myocardial tube to a situation where the separated aorta and pulmonary trunk achieve their definitive positional relationship. This process requires proper septation of the outlet portion of the heart. Formation of the aortopulmonary septum (future outlet septum), orchestrated by neural crest cells, separates the common trunk into an aorta and pulmonary trunk. Asymmetrical, mainly subpulmonary myocardial contributions from the so-called second heart field result in marked lengthening of the subpulmonary myocardium and “push” the pulmonary trunk to its final position leftward and anterior to the aorta. After proper development, the RV outflow tract (RVOT) is characterized by the presence of a muscular subpulmonary infundibulum forming a circular muscular tube below the pulmonary valve. The posterior wall of the infundibulum, also known as the crista supraventricularis, is situated between the tricuspid valve and the pulmonary valve. The crista supraventricularis forms the summit of the ventriculo-infundibular fold (VIF), a fold of myocardium at the posterolateral wall of the RVOT, and extends toward the ventricular septum into the trabecula septomarginalis ( Fig. 116.1 ). The latter continues over the interventricular septum and contains the right bundle branch. The crista supraventricularis also encompasses the outlet septum (the muscular septum separating the aortic and pulmonary outlets), situated between the ventriculo-infundibular fold and trabecula septomarginalis, which is small and not recognizable as a separate structure in the normal heart. In TOF, however, there is anterior deviation of the outlet septum that, in contrast to normal, can be recognized as a separate structure and is a pathognomonic feature of TOF (see Fig. 116.1 ). The deviation of the outlet septum causes malalignment with the remainder of the ventricular septum, resulting in a subaortic, and in most cases perimembranous, ventricular septal defect. The deviation of the outlet septum also contributes to the subpulmonary stenosis. The amount of displacement and hypertrophy of the outlet septum determines the severity of the stenosis. The morphology of TOF encompasses a broad spectrum, ranging from very slight malformations and cyanosis to severe forms with pulmonary atresia.
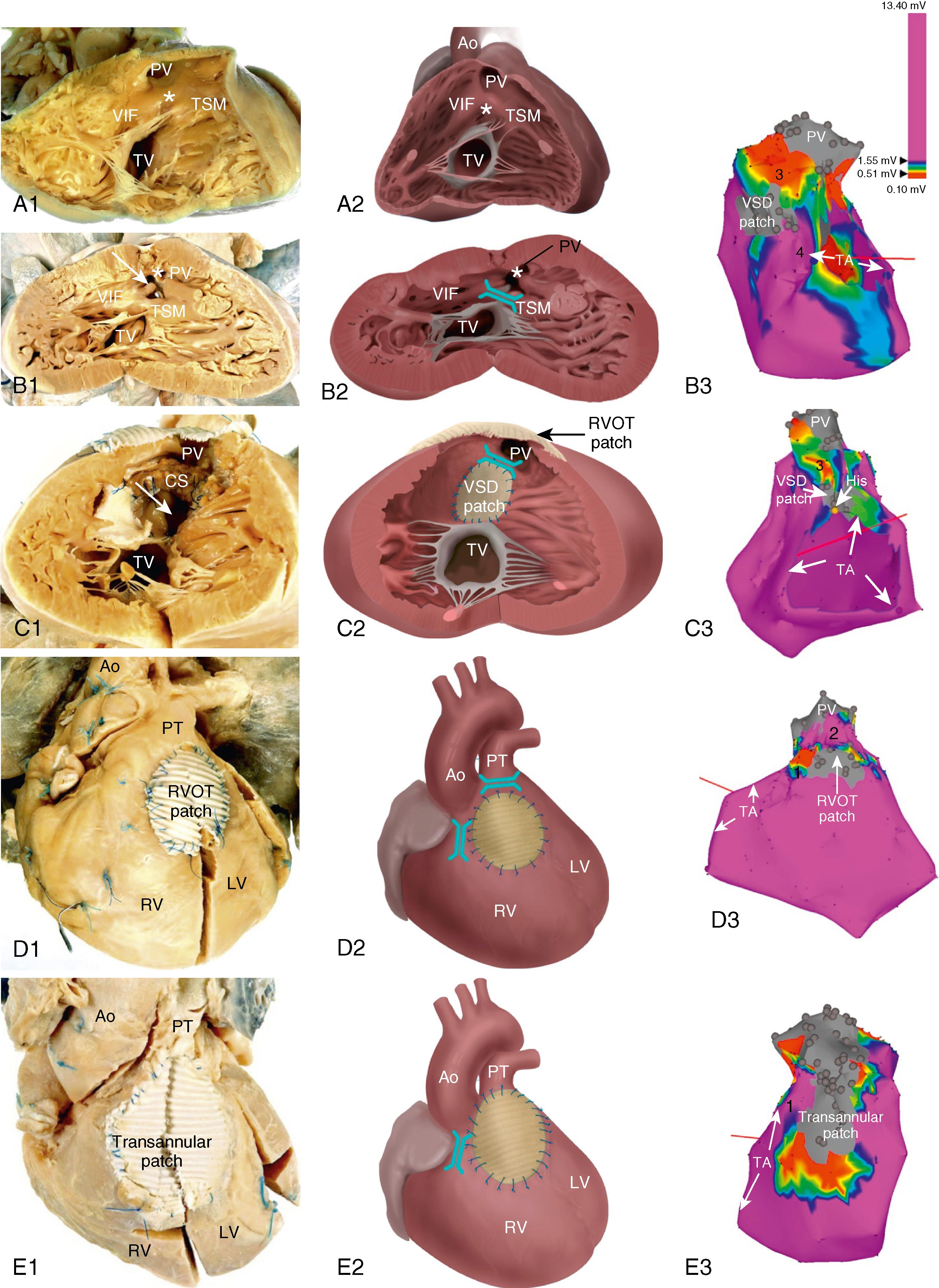
After the first intracardiac repair of TOF in 1954 by Lillehei in a 10-month-old boy, subsequent series of early surgical interventions reported a high perioperative mortality, leading to a two-stage repair with a palliative shunt to augment pulmonary blood flow, followed by total repair later in childhood.
Total repair includes (patch) closure of the perimembranous or muscular VSD and relief of the infundibular or valvular RVOT obstruction. This repair was initially performed through a vertical or transverse right ventriculotomy often combined with the use of an RVOT or a transannular patch to augment the restrictive RVOT or to relieve the stenosis of the pulmonary orifice. The malformation and type of repair are important determinants of potential reentrant tachycardia circuits that are the common cause of VT in repaired TOF. Areas of dense fibrosis owing to surgical incisions, but also patch material and the valve annuli, can form regions of conduction block that define reentry circuit borders and create intervening isthmuses of myocardial bundles that might contain the critical reentry circuit isthmus of a macroreentrant VT. Four anatomic isthmuses related to VT in repaired TOF have been identified : isthmus 1 bordered by the tricuspid annulus and the scar or patch in the anterior RVOT, isthmus 2 between the pulmonary annulus and the RV free wall incision or RVOT patch sparing the pulmonary valve annulus, isthmus 3 between the pulmonary annulus and the VSD patch or septal scar, and isthmus 4 between the VSD patch or septal scar and the tricuspid annulus in patients with muscular VSDs (see Fig. 116.1 ).
In two postmortem series of repaired TOF, isthmuses 1 and 3 were present in almost all specimens, whereas isthmuses 2 and 4 were observed in only 25% to 42% and 6% to 13%, respectively. , Of importance, isthmus 2 is after transatrial-transpulmonary correction but present in all patients after transventricular correction. Early correction, before 1 year of age, reduces transannular-patch size, which may influence isthmus 1 width later in life. Of interest, in specimens from patients aged at least 5 years at the time of death, isthmus 1 was significantly wider (3.9 ± 1.08 cm) and thicker (1.5 ± 0.29 cm) with less interstitial and replacement fibrosis than isthmus 3 (width 1.4 ± 0.77 cm, wall thickness 0.6 ± 0.25 cm). Not all anatomic isthmuses are related to VT. Specific characteristics of the anatomic isthmuses may determine further remodeling over time that creates the substrate for late VTs.
The right ventriculotomy and the frequent use of a transannular patch with consequent pulmonary regurgitation and chronic volume overload often resulted in RV dilation and dysfunction, which is one factor associated with VT and SCD in the long term ( Table 116.1 ). Consequently, a combined transatrial-transpulmonary approach has been introduced. Currently, patch augmentation of the RVOT is avoided or usually limited to the pulmonary annulus whenever possible. This approach positively affects RV function and can also prevent anatomic isthmuses 1 and 2.
| VT | SCD | VT + SCD | AE | |||||
|---|---|---|---|---|---|---|---|---|
| Risk Factor | Yes/No | N | Yes/No | N | Yes/No | N | Yes/No | N |
| Symptoms of (pre)syncope | Yes | 135 | Yes | 125 | ||||
| History of SMVT | Yes | 125 | ||||||
| History of atrial arrhythmias | Yes , | 873 465 |
||||||
| Age at total repair | Yes , , | 793 488 141 |
Yes , , | 163249 165 |
||||
| Presence of transannular patch | Yes | 793 | Yes | 793 | No , | 163 71 |
||
| Yes , | 490 165 |
|||||||
| First-degree AV block/ΔPR >2 ms/y | Yes | 176 | Yes | 176 | ||||
| QRS duration ≥180 ms, , , QRS duration | Yes , , , | 793 178 135 319 |
Yes , , | 793 178 125 |
Yes | 101 | Yes | 873 |
| QRS duration increase per year | Yes | 793 | Yes | 793 | ||||
| QRS fragmentation (severity) | No | 176 | Yes , | 794 465 |
||||
| Yes , | 794 465 |
|||||||
| QRS vector magnitude | Yes | 177 | ||||||
| QRS dispersion | Yes | 99 | ||||||
| QT duration | Yes | 99 | ||||||
| QT dispersion | Yes | 99 | Yes | 66 | ||||
| JT(c) dispersion | Yes | 99 | Yes | 101 | ||||
| PVC on ECG | Yes , | 207 488 |
||||||
| VA on Holter (PVC >30/min and/or nsVT) | Yes , | 36 210 |
No , | 793 86 |
Yes | 101 | Yes , | 68 873 |
| LV longitudinal strain on echo | Yes | 413 | ||||||
| LV reduced systolic function (CMR EF <45% ) | Yes | 125 | Yes , , , | 88 873 465 465 |
||||
| LV end-diastolic pressure (>12 mm Hg, >16 mm Hg ) | Yes , | 68 465 |
||||||
| RV reduced systolic function (CMR EF <30% ) | Yes | 319 | Yes | 154 | Yes , | 873 465 |
||
| RV LGE | Yes | 92 | ||||||
| No | 53 | |||||||
| RV/LV total scar area (LGE) | Yes | 103 | ||||||
| RV akinetic region length | Yes | 53 | ||||||
| RV dimensions (diameter, volume ) | Yes , | 135 319 |
Yes | 66 | Yes , , | 86 88 39 |
||
| RV end-systolic (diastolic > 16 mm Hg ) pressure | Yes , , | 207 488 141 |
Yes | 465 | ||||
| RV mass/volume ratio ≥0.3g/mL, RV mass | Yes | 319 | Yes | 873 | ||||
| RV ECV (%) | Yes | |||||||
| Moderate or severe PVR | Yes | 793 | Yes , | 793 125 |
||||
| Inducible for SMVT or SPVT | Yes | 252 | ||||||
Reentrant tachycardias are promoted by slow conduction. Interstitial fibrosis owing to long-standing cyanosis and pressure overload functionally prolongs the pathway for impulse propagation and can provide the substrate for slow conduction. In addition, cell-to-cell coupling can be diminished because of decreased gap junction density and altered connexin expression and distribution, as observed in clinical and experimental cardiomyopathies also contributing to slow conduction.
Myocardial histopathologic changes, in particular interstitial fibrosis of the muscular tissue of the crista supraventricularis, are more pronounced in patients who were operated on at an older age, specifically beyond the age of 4 years, and are thus subjected to long-standing cyanosis (Sa o 2 <80%) and higher end-diastolic RV pressure (>12 mm Hg).
The current clinical gold standard for detecting ventricular fibrosis is late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR). Myocardial RV LGE is present in almost all adult patients (age 32.2 ± 1 years) repaired at a median age of 5 years (interquartile range [IQR] 2–8) at the anterior RVOT (99%), the septal patch region (98%) and, frequently, at the RV insertion points (79%). Patients with more extensive fibrosis also involving the RV trabeculations and/or the LV tend to be older and to have had repair at a later age. A supramedian RV LGE score and LV LGE were related to adverse clinical effects, such as ventricular dysfunction; specifically, RV LGE was associated with atrial arrhythmias and VAs. In a series of 103 patients (age 28 ± 15 years), the total scar area from high-resolution LGE images was independently related to VA. In a younger cohort of patients (age 20 ± 12 years) with earlier repair at a median age of 2 years, LGE was less frequently detected in the RVOT (15%) and LGE in the LV and not associated with adverse events. Patients without LGE were repaired at a median age of 12 months, suggesting that earlier repair and refined surgical techniques may prevent fibrosis detectable by CMR.
QRS fragmentation (QRS-f) on standard 12-lead electrocardiograms (ECGs) may be considered a noninvasive surrogate for fibrosis. QRS-f severity, usually classified based on the number of leads with QRS-f (mild, ≤3 leads; moderate, 4 leads; and severe, ≥5 leads) correlated with the extent of LGE and was associated with all-cause mortality and VA in two cohorts of patients with repaired TOF (rTOF; median age, 27 years [IQR, 20–38 years]; and mean age 37 ± 14 years, respectively).
Histopathologic changes can contribute to the occurrence of complex ventricular ectopy, which has been considered a risk factor for fatal VAs. In particular, older age at repair has been associated with a higher grade of ventricular ectopy. , Only 11% of patients in whom repair was performed between the ages of 4 and 15 years showed complex ectopy on Holter monitoring 6 to 12 months after operation, compared with 39.4% of patients who underwent surgery beyond the age of 15 years. The age dependency of the occurrence of complex ventricular ectopy could also be seen in uncorrected patients. No significant ventricular ectopy (defined as Lown ≥2, <30 uniform premature ventricular complexes [PVCs]/hour) was observed in patients 0 to 7 years old. In contrast, 58% of patients 16 years or older had complex ectopy, with 21% having runs of nonsustained VT. Of interest, in corrected patients, the relationship between complex ventricular ectopy and time of repair persisted and was independent of the duration of follow-up or of the postoperative hemodynamic status. Although a higher grade of ectopy and nonsustained VT have been associated with VT inducibility, data are inconsistent regarding their association with SCD. , In a large multicenter cohort, asymptomatic complex ectopy and nonsustained VTs were not predictive for SCD. A small, retrospective, single center study analyzing Holter recordings reported only a weak association between nonsustained VT and sudden cardiac events, including aborted SCD and appropriate implantable cardioverter-defibrillator (ICD) therapy. Accordingly, treatment of asymptomatic complex ventricular ectopy is not recommended. In selected patients who received an ICD for primary prevention, nonsustained VT independently predicted ICD therapy, and in a cohort of 36 patients implanted for primary prevention, symptomatic but not asymptomatic, nonsustained VTs were the only factor associated with appropriate ICD shocks. Nevertheless, as in other nonischemic cardiomyopathies, ICD therapy may not be a reliable surrogate for SCD. Longer episodes of rapid, nonsustained VTs are more likely to cause symptoms and prompt inappropriate ICD activation if short detection times are programmed, with potential adverse effects.
Primary repair in infancy before the age of 18 months has been a common practice since the early 1990s and can be performed with low perioperative mortality. Avoiding long-standing prolonged hypoxemia and pressure overload can reduce the histopathologic changes and the substrate for slow conduction, complex ventricular ectopy, and fatal VA.
Despite early operation, progressive pulmonary regurgitation occurs in almost all patients after transannular patch repair and is an important reason for reintervention. Although often tolerated, pulmonary regurgitation ultimately leads to RV dilation and dysfunction, which can be further aggravated by residual RVOT obstruction. Moderate to severe pulmonary regurgitation, and abnormal RV hemodynamics in particular, and increased RV end-systolic pressure have been associated with VT and SCD (see Table 116.1 ).
In addition, a wide QRS (≥180 ms) and an increase in QRS duration (QRSd) have consistently been reported as risk factors for SMVT and SCD. In particular, RV dilation, but not restrictive RV physiology, has been associated with QRS prolongation, referred to as mechanoelectrical interaction.
Impairment of RV function and LV hemodynamics have important roles. Moderate to severe LV systolic dysfunction, defined as ejection fraction (EF) of less than 39% and 20%, respectively, was also more common in patients with TOF and (aborted) SCD. In addition, LV end diastolic pressure (LVEDP) of at least 12 mm Hg was a strong and independent predictor of appropriate ICD shocks in patients with TOF who received an ICD for primary prevention.
Although surgical pulmonary valve replacement (PVR) has been associated with a reduction in RV end-diastolic volume in selected patients, a consistent reduction in QRSd after surgery has not been demonstrated. , A QRSd greater than 180 ms 6 months after PVR or no reduction of the QRSd postoperatively were, however, strong predictors of adverse events defined as all-cause mortality, reoperation for pulmonary regurgitation, heart failure, or VT. In particular, patients with severely prolonged QRSd that did not change postoperatively had the highest incidence of adverse events. Prolonged QRSd may reflect global conduction delay in a dilated RV and may serve as one surrogate marker for the changes in mechanical forces that trigger changes on the cellular and subcellular level, also known as mechanoelectrical coupling associated with arrhythmogenesis. QRS prolongation can also be the result of right bundle branch damage, which can occur at different levels (proximal, distal, and terminal) during the initial repair or as a consequence of pressure or volume overload. QRS prolongation may, however, also reflect localized morphologic and functional changes of the RVOT beyond the conduction system. In patients with a QRS of at least 155 ms, there is a strong correlation between QRSd and echocardiographic delay in RVOT motion, which was outside the normal range in all patients with a QRSd greater than 165 ms.
QRSd also correlated with morphologic abnormalities, including the akinetic area length on CMR. In a single-center study that followed 154 TOF patients over a median of 5.6 years (IQR 4.6–7.0), the RVOT akinetic region length remained the only independent predictor for VA.
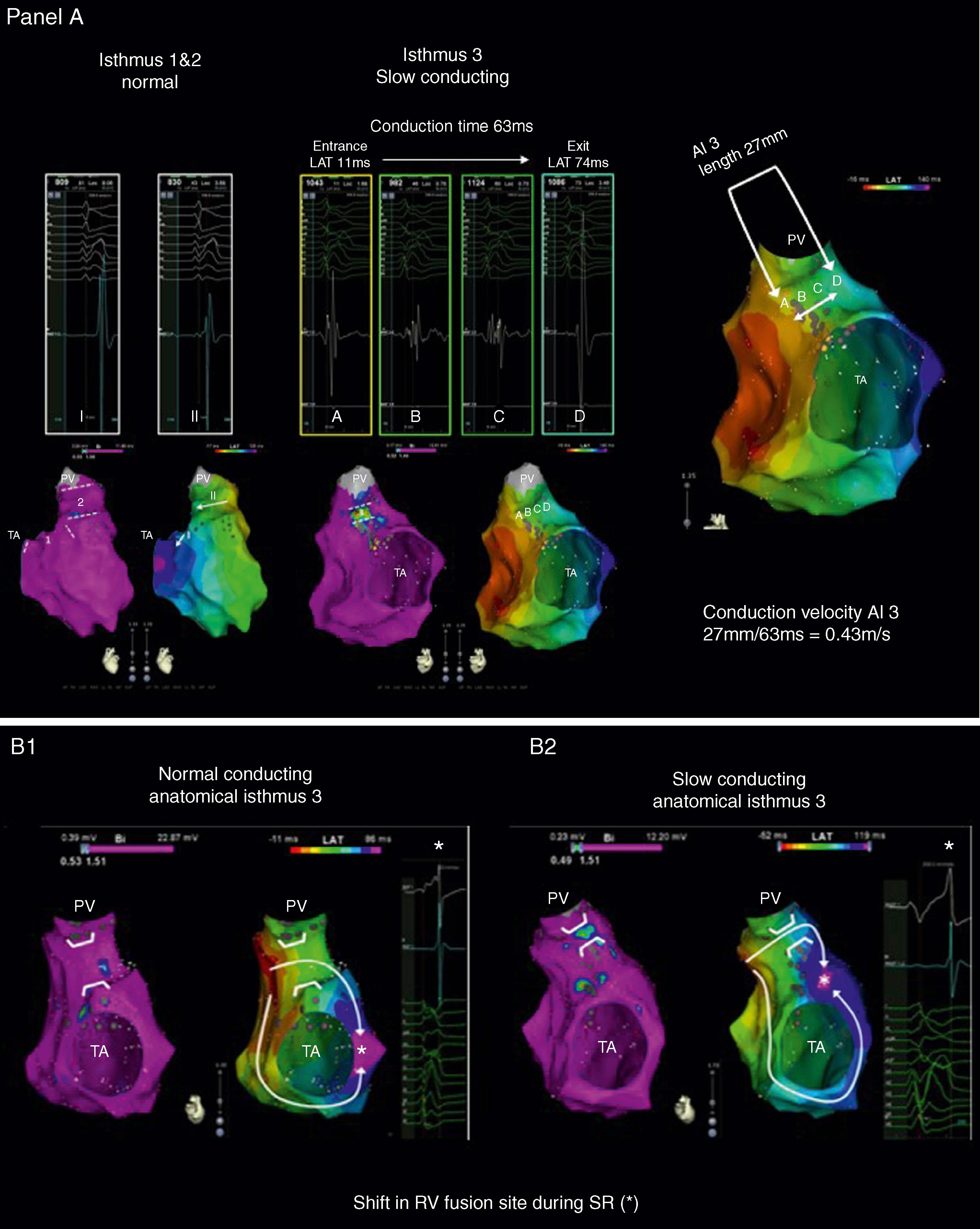
Of importance, localized conduction delay may have effects beyond mechanical dyssynchrony of the RVOT. Slow-conducting anatomic isthmuses (SCAIs) identified by electroanatomic mapping during sinus rhythm (calculated conduction velocity index <0.5 m/s) were related to 37 of 41 induced VTs in 26 TOF patients. In particular, slow conduction through anatomic isthmus 3 bordering at the infundibular septum may further delay activation of the lateral RVOT and basal RV in those with a preexisting terminal right bundle branch block (RBBB; Fig. 116.2 ). This finding may provide the important pathophysiologic link between a prolonged QRSd and VT.
Despite the remarkable reduction of RV volumes and hemodynamic improvement of RV function after PVR, simply replacing the valve might not eliminate the substrate for reentrant VTs; reentry was the underlying mechanism of all VTs that were associated with a QRSd greater than 180 ms in one series. After a late PVR performed in 98 patients 20 ± 9 years after TOF repair, VT occurred in 11, and death occurred in 6 patients after a mean follow-up of 2.8 ± 4.3 years. The 5- and 10-year measures of freedom from the composite outcome of death and VT were 80% and 41%, respectively, which were not different from those of a control group ( n = 77) matched for age, pulmonary regurgitation, RV dilation, and QRSd who did not undergo PVR. These data suggest that either PVR was performed too late and did not lead to favorable reverse remodeling despite a reduction in RV size, or that VTs are the result of a fixed substrate not influenced by hemodynamic improvements. Of interest, five of seven patients who underwent PVR and concomitant (not further specified) intraoperative cryoablation experienced VT during follow-up. In contrast, map-guided intraoperative ablation was more effective for controlling VT recurrences. Of 44 patients with documented monomorphic sustained VT (MSVT), 31 underwent cryoablation applied to the macroreentry site as assessed by combined endocardial and epicardial mapping. VT recurred in only three (10%) patients after ablation with 96% freedom of VT after 7.5 years.
Based on the observation that VTs often depend on four anatomically defined isthmuses, a surgical cryoablation lesion connecting the boundaries may be a reasonable alternative to mapping-guided ablation at the time of PVR. In 22 patients (history of VT in 4 and inducible VT at electrophysiology study [EPS] in 10), the superior aspect of the VSD and the pulmonary annulus (isthmus 3) were connected during PVR. In selected patients, an additional lesion connecting the ventriculotomy to the pulmonary (isthmus 2) or tricuspid annulus (isthmus 1) was performed. Only a single event occurred at 7 years after PVR. Preoperative programmed electrical stimulation (PES) may identify patients who will benefit from concomitant intraoperative surgical ablation. Based on the result of a pre-PVR PES, performed in 70 consecutive patients, 31 of 34 inducible patients (MSVT in 23, polymorphic VT [PMVT] in 8) underwent concomitant intraoperative cryoablation, connecting the RV incision/patch to the pulmonary annulus (isthmus 2) and the VSD to the pulmonary annulus (isthmus 3). In addition, a circumferential lesion below the pulmonary annulus (i.e., in the myocardial pulmonary infundibulum) was created. At postcryoablation PES, 14 of 31 patients remained inducible (MSVT in 11, PMVT in 3) and received ICDs. During a mean follow-up of 6.1 ± 3.2 years, VT occurred in 3 of the 14 patients with inducible VT (21%) compared with 2 of 53 patients (3.8%) with negative pre- or post-PES. In another single center cohort, 20 of 37 patients with RF ablation for VA had inducible VT at pre-PVR PES and underwent intraoperative surgical radiofrequency ablations of isthmus 1 and isthmus 3. At postoperative PES, 3 of 19 (16%) remained inducible and 10% had spontaneous VT during a median follow-up of 6.5 years.
Accordingly, for patients with inducible VT before surgery, after empirical surgical ablation without preoperative mapping and evaluation of acute procedural success, 16% to 48% of patients remained inducible for VT and 10% to 21% had spontaneous VT. Achieving bidirectional conduction block is an accepted endpoint for linear lesions and intraoperative ablation with endpoint confirmation is likely to improve outcome in patients with isthmus-dependent VTs. , , In addition, empirical ablation may have potentially proarrhythmogenic effects in those with normal conduction through a potential isthmus before surgery. A more thorough approach to preoperative electrophysiologic evaluation, including electroanatomic mapping to identify slow-conducting anatomic isthmuses, can potentially guide surgical ablation during revalving (See Fig. 116.2 ).
The potential loss of catheter access to slowly conducting anatomic isthmuses, precluding ablation, after revalving in rTOF and related defects is of concern. Accordingly, in patients with sustained VT who are undergoing surgical PVR or transcutaneous valve insertion, preoperative catheter mapping and transection of VT-related anatomic isthmuses before or during the intervention should be considered. Whether patients without documented VT benefit from preventive ablation before or during revalving requires further study.
PES is an important tool that provides strong evidence for the presence of substrate for reentrant VT when SMVT is inducible, although VT based on triggered activity may also be inducible. Accordingly, in one series, only 16% of patients who had documented sustained VT did not have VT inducible by PES. In another series, however, the clinical or presumed clinical VT was inducible in all patients if PES was also performed from a site close to the infundibular septum.
Consequently, PES has also been used to identify patients with TOF who are at risk for potential fatal ventricular arrhythmias. Inducibility of sustained VT is high in patients with presyncope and complex PVCs on Holter monitoring and is more likely in patients repaired at older age and when PES is performed late after surgery. , Importantly, inducibility strongly depends on the applied induction protocol; MSVT induction required three extrastimuli in 70% and isoproterenol in 11% of patients in two series. , Using a complete PES protocol (two RV sites, three cycle lengths [CLs], triples, and isoproterenol) the diagnostic value (sensitivity, 77.4%; specificity, 79.5%) and the prognostic significance (relative risk, 5.0 for subsequent clinical VT or SCD) of PES in TOF patients is comparable to that in the postinfarct population. Of interest, the highest event rate (SCD, VT, or both) was observed in patients inducible for sustained polymorphic VT (SPVT). Although derived from a small number of patients, induction of SPVT may not be an nonspecific finding and may reflect the presence of a different but potentially fatal arrhythmogenic substrate.
The majority of VAs documented in TOF patients are monomorphic VTs. The prevalence of SMVT was 14.2% in a recently conducted multicenter study involving 556 TOF patients and was markedly increased after the age of 45 years. In contrast, VF was documented in only 0.5% of the population. The incidence of VF in TOF patients may be underestimated because patients in the cohort are survivors. Nevertheless, in 121 TOF patients who received an ICD for primary or secondary prevention, 81.5% of all appropriately delivered therapy was for monomorphic VT. Of importance, VTs were fast with a median heart rate of 213 beats/min (range, 182–264 beats/min). There are no data available on the efficacy of antiarrhythmic drugs and only limited data on dosage and toxicity in different age groups. In general, antiarrhythmic drugs initiated to prevent recurrence of reentrant VT in structural heart disease have disappointing efficacy and their use is hampered by potentially serious side effects.
Table 116.2 provides an overview of studies reporting the feasibility of catheter ablation of VT in CHD, with the majority performed in TOF patients. In most of the earlier series and case reports, the targeted VTs were slow, hemodynamically tolerated, and therefore approachable by conventional mapping techniques, such as activation and entrainment mapping during ongoing VT. An intention to treat analysis was published by the group from the Boston Children’s Hospital. In this study, acute ablation failure was 50% because of noninducibility, hemodynamic instability, or anatomic reasons. More recently, published studies have used substrate mapping techniques to target all inducible and, in particular, fast and poorly tolerated VTs, which are common.
| Reference | CHD | Method | Patient (n) | Sus-VT (n) | VTCL Mean (ms) | Acute Success | Recurrence | |
|---|---|---|---|---|---|---|---|---|
| Burton, 1993 | TOF | PM | 2 | 2 | 270 | 2/2 | 0/2 | |
| Biblo, 1994 | TOF | AM | 1 | 2 | 430 | 1/1 | 0/1 | |
| Goldner, 1994 | TOF | PM | 1 | 1 | 240 | 1/1 | 0/1 | |
| Cinushi, 1995 | TOF | AM + LL | 1 | 2 | 420 | 2/2 | 0/1 | |
| Gonska, 1996 , c | TOF 7, VSD 1, TGA + VSD 1, PS 2 | AM | 11 | 11 | 377 | 9/11 | 2/11 | |
| Horton, 1997 | TOF | AM + LL | 2 | 2 | 430 | 2/2 | 0/2 | |
| Baral, 2004 | ccTGA + EA + TVR | AM (NC) + ENT | 1 | 1 | 380 | 1/1 | 0/1 | |
| Rostock, 2004 | TOF | AM + LL | 1 | 1 | 340 | 1/1 | 0/1 | |
| Morwood, 2004 | TOF 8, VSD 3, others 3 | — | 14 Patients 20 Procedures |
— | — | 10/20 | 4/10 | |
| Furushima, 2005 | TOF/DORV | AM + LL | 7 | 14 8 Targeted |
346 | 4/7 | 6/7 | |
| Kriebel, 2007 | TOF | SSM + LVA (NC) + LL | 10 | 13 | 269 | 8/10 | 2/8 | |
| Zeppenfeld, 2007 | TOF 9, AVSD 1, TGA + VSD 1 | SSM + EUS + LL + IMG (1/11, CT) | 11 | 15 | 276 | 11/11 | 1/11 | |
| Nair, 2011 | TGA + VSD + sPS | SSM + PM + IMG (CT) | 1 | 1 | 350 | 1/1 | 0/1 | |
| Piers, 2012 | TGA + sPS | SSM + AM + IMG (CT, CMR) | 1 | 1 | 340 | 1/1 | 0/1 | |
| Kapel, 2015 | TOF 28, TGA + sPS 1, TGA + VSD 1, VSD + sPS 1, PS 1 VSD + bAV 1, AVSD 1 | SSM + EUS + LL + IMG | 34 | 61 | 295 (242–346) a | 25/34 b | 0/25 c | |
| Ruckdeschel, 2016 | PS (PVR + RV incision) | SSM + AM + ENT | 1 | 2 | 343 | 1/1 | — | |
| Toyohara, 2016 | ccTGA + VSD (DSO) | AM + ENT | 1 | 1 | 310 | 1/1 | 0/1 | |
| Van Zyl, 2016 | TOF 10, TGA 4, VSD 2, PA 1, EA 1, cAS 1, TA 1 | SSM + AM + ENT | 21 | 33 | 300 (265–390) a | 17/21 | 3/21 | |
| Laredo, 2017 | TOF | SSM d + AM + ENT | 34 | — | 294 | 28/34 | 11/34 d | |
| Moore, 2018 | EA | SSM + PM + AM + ENT | 24 | 30 | 305 (268–400) a | 22/24 | 1/24 | |
| Yang, 2019 | TOF 29, DORV 2, EA 4, VSD 1, AS 1, TA 1, PS 1,TGA 1, others 2 | SSM + PM + AM + ENT | 48 | 77 | 299 (259–330) a | 36/48 | 10/48 | |
a Median, interquartile range.
b Acute success defined as noninducibility and transection of anatomic isthmus.
c One patient had ICD shock for VF.
d Electroanatomic mapping used in 13 of 34 (38%), 7 of 11 early repeat ablation without VT recurrence.
Using a noncontact mapping system consisting of a multielectrode balloon array that allows for the simultaneous acquisition of virtual unipolar electrograms (EGMs), the activation sequence of 13 fast or nonsustained VTs in 10 patients was obtained. Eleven of the 13 induced VTs were because of macroreentrant circuits, whereas two were because of microreentrant circuits. The anatomic location of the isthmus could be identified in all patients and was successfully targeted by a linear radiofrequency (RF) lesion in eight patients. In two patients, RF delivery was withheld because of the proximity of the His bundle. An alternative approach applies point-by-point electroanatomic voltage and activation mapping during stable sinus rhythm to obtain a three-dimensional (3D) reconstruction of all potential isthmuses by identifying the anatomic boundaries (see Figs. 116.1 and 116.2 ). Peak-to-peak bipolar EGM amplitudes can be displayed color-coded and projected on a 3D shell of the RV. EGMs greater than 1.5 mV are considered normal voltage. At sites with amplitudes less than 0.5 mV, high output pacing (10 mA, 2 ms) can be performed to identify unexcitable tissue, which is consistent with patch material or surgical scars. The critical reentry circuit isthmus of each induced VT can be determined by activation and entrainment mapping for tolerated VT or by pace mapping for poorly tolerate ones. If the critical isthmus is located within an anatomically defined isthmus, the “critical isthmus” can be transected by connecting the adjoining anatomic boundaries with linear RF lesions. A systematic approach ( Fig. 116.3 ) has been highly effective at treating all VTs, especially poorly tolerated ones, with promising long-term results. , Demonstration of conduction block after isthmus transection by radiofrequency catheter ablation (RFCA) or cryoablation provides a defined procedural endpoint similar to that for achieving block in the common cavotricuspid isthmus.
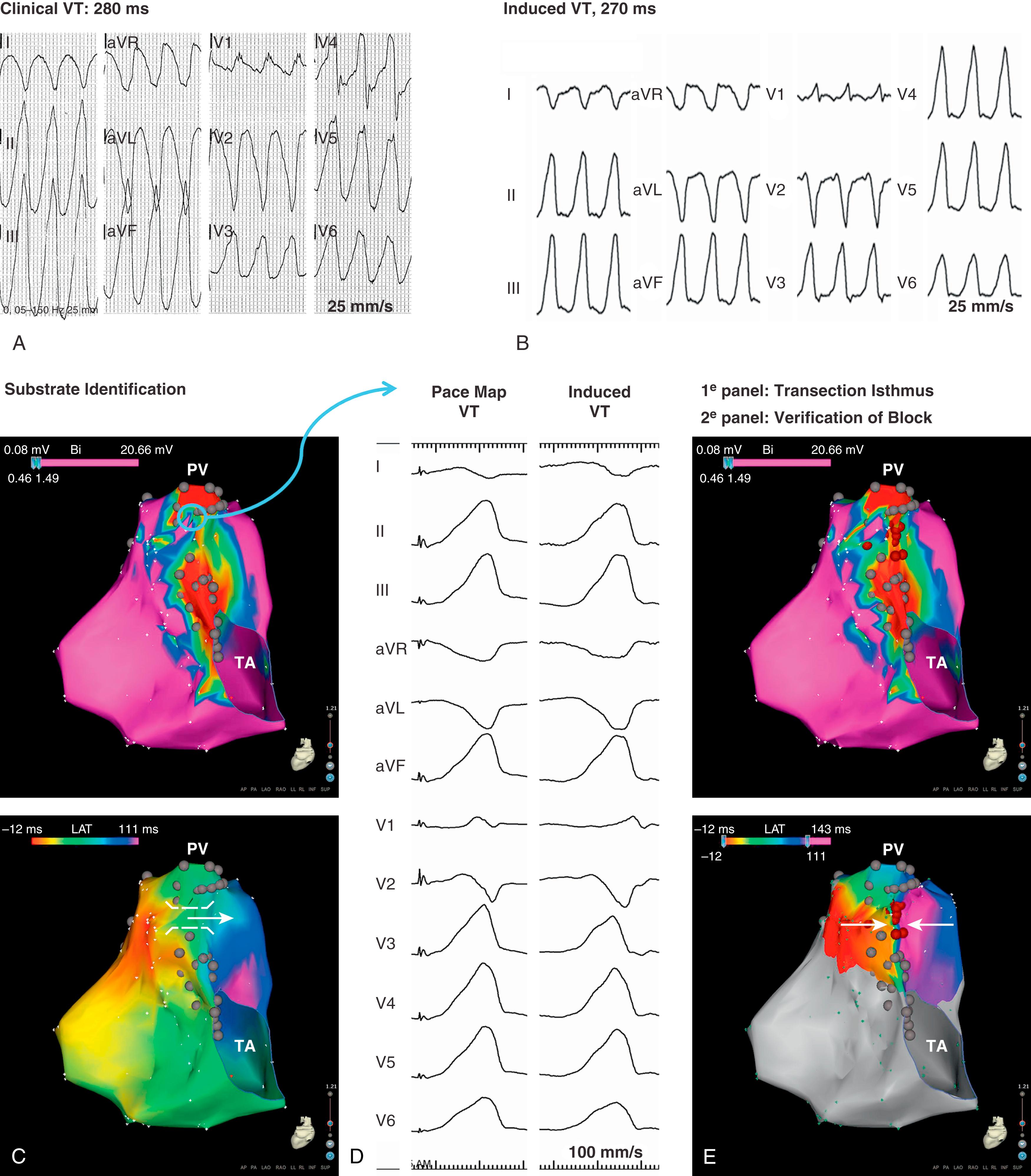
In a large series, reporting on VT ablation in 34 adults with CHD (82% TOF; median VT cycle length [VTCL], 295 ms; IQR, 242–346), complete procedural success was defined as noninducibility of any VT and transection of the critical anatomic isthmus. This end point was achieved in 25 adults (74%). None of the patients in whom complete procedural success was achieved had recurrence of monomorphic VT during the 46 ± 29 months of follow-up, whereas one patient with poor RV function experienced appropriate ICD discharge for VF. Similarly, in a cohort of 21 patients with repaired CHD (48% TOF, median VTCL 300 ms; IQR 265–390 ms), 14 had an electroanatomic isthmus-dependent reentrant VT, which was targeted by ablation. None of the 8 patients with confirmed isthmus block had VT recurrence during the 33 ±7 months of follow-up. These data suggest that macroreentrant VTs based on an anatomic substrate can be treated effectively with catheter ablation and that isthmus ablation with confirmed conduction block may be curative in patients with preserved cardiac function and no competing arrhythmia mechanism; however, other VA can occur, especially in patients with impaired RV function. Anatomic isthmuses are present in almost all TOF patients but not all are related to VT. Specific characteristics may be required for an isthmus to constitute the substrate for reentry VT. Detailed electroanatomic mapping during sinus rhythm (SR) performed in a cohort of 74 consecutive TOF patients with at least one risk factor for VA, including 13 with prior documented VT, demonstrated that narrow and slowly conducting anatomic isthmuses (calculated conduction velocity <0.5 m/s) were the substrate for all documented and induced VTs in patients with preserved cardiac function. An assessment of isthmus characteristics can be performed during SR in patients with a preexisting RBBB but may require pacing close to the anatomic isthmus in patients with a narrow QRS to unmask slow conduction. In these patients, rapid conduction through the branches of the right fascicle may result in fusion of activation wavefronts in isthmus 3. Considering the strong link between slow conducting anatomic isthmuses and macroreentrant VT in repaired TOF, inducibility of the clinical arrhythmia and hemodynamic tolerance is no longer a prerequisite for successful ablation. In those TOF patients with only isthmus 1 and 3 present, RBBB VTs are usually because of clockwise activation and left bundle branch block (LBBB) VTs because of counterclockwise activation of isthmus 3 ( Fig. 116.4 ). Involvement of both isthmuses in the VT circuit limits the reliability of the 12-lead VT ECG and pace-mapping for identifying the VT substrate. Isthmus 3, which is narrower and easier to access by ablation compared with isthmus 1, can always be targeted, regardless of the 12-lead VT-ECG.
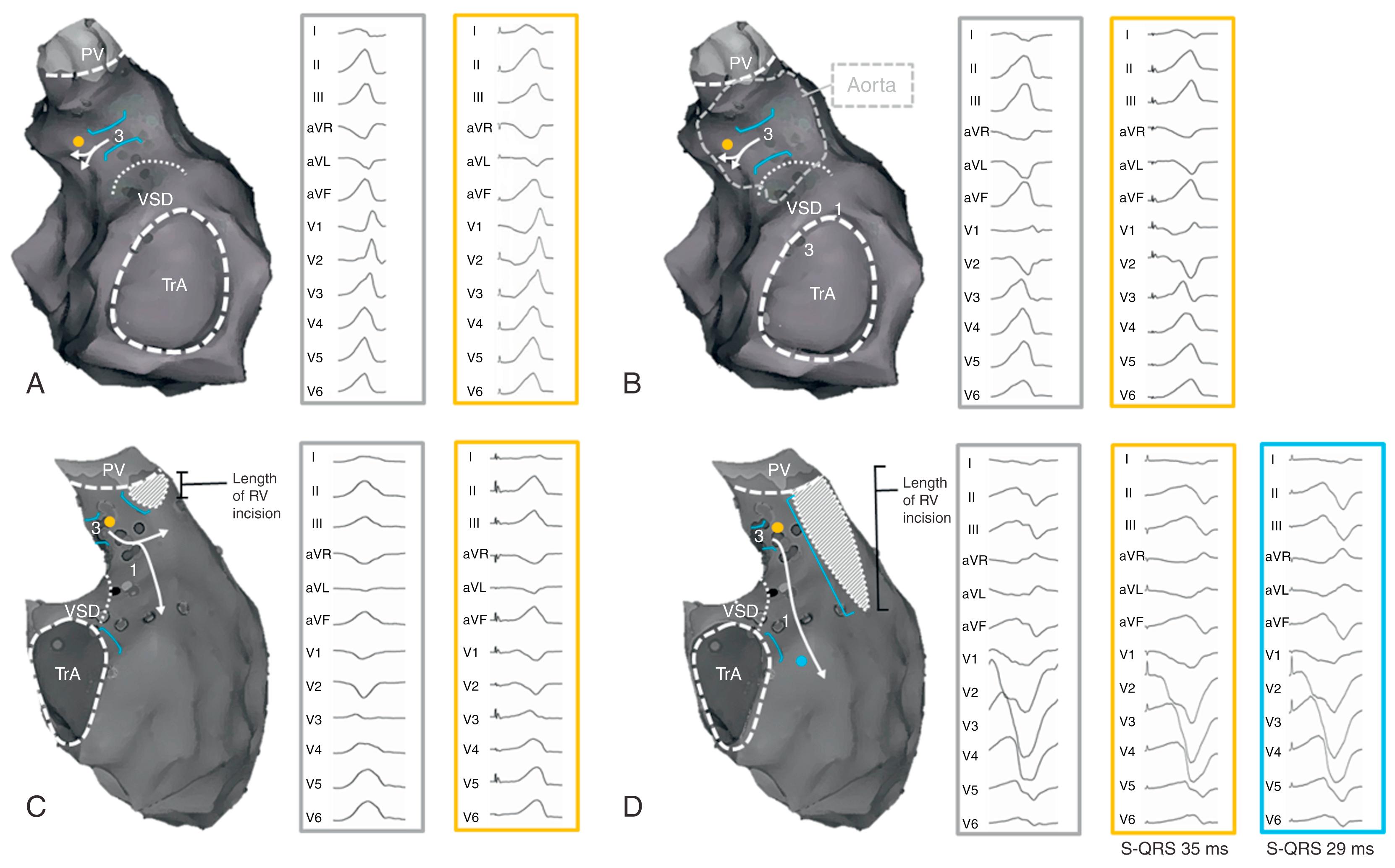
Direct identification of a substrate for VT in an individual patient is appealing because it could overcome the lack of adequate clinical arrhythmia predictors and allow for personalized risk stratification and tailored treatment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here