Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Reproducible patterns of cerebrovascular injury result from two basic pathologic processes that are occasionally encountered in surgical practice: (1) cerebral infarct resulting from blockage or stenosis of vessels that deprives the brain of oxygen and nutrients and (2) hemorrhage from diseased vessels that may then secondarily cause tissue destruction and hypoxia. A third major pattern results from global hypoxic/ischemic damage, such as occurs with cardiac arrest or hypotension. This type of injury is often referred to as hypoxic/ischemic encephalopathy clinically and is associated with damage to regions of selective vulnerability (e.g., watershed infarcts, laminar necrosis, selective neuronal necrosis, hippocampal neuronal necrosis in areas CA1 and CA4, cerebellar Purkinje cell necrosis). This third pattern is seen almost exclusively in the autopsy setting and is therefore not covered in great detail. Instead, this chapter will focus predominantly on the major pathologic changes and characteristic distributions of injury that occur in the clinical syndromes of stroke, brain attack, and cerebrovascular accident, particularly those lesions that may mimic a brain tumor or are biopsied for other reasons (e.g., rule out vasculitis). These clinical terms are often used synonymously and refer to an acute, nonepileptic, nonmigrainous, persistent alteration in neurologic status that results from sudden disruption of blood flow to the central nervous system (CNS). When considering all forms of cerebrovascular disease that result in stroke and stroke syndromes, these disorders are the second most common cause of death and a major cause of disability worldwide.
As inaccurate as the term “stroke” may be, it has been utilized in medicine to emphasize the acute onset, as in, the patient was suddenly “struck down” with illness. The first record is credited to Hippocrates, who described sudden paralysis of the right arm with loss of speech, although historical descriptions also date back to the ancient Egyptians. Hippocrates depicted sudden onset of numbness or anesthesia as “impending apoplexy,” derived from the Greek word meaning “struck with violence as if by a thunderbolt.” However, further understanding of strokes awaited the invention of the microscope in the 17th century, and another century passed before several investigators described the focal softening of the infarcted brain, the lack of an inflammatory etiology, and the associations with vascular anatomy. It was not until the work of Rudolf Virchow in the 19th century that the mechanisms of various strokes were more thoroughly and clearly elucidated. Arteriosclerosis was first described by Lobstein in 1829, although Virchow revived the term 20 years later and contributed further to its understanding, as well as the etiologic roles of thrombosis and embolism. In 1863, he also published a three-volume treatise on blood vessels, including vascular malformations, forming the basic premises for more current classification schemes. During the late 19th and 20th centuries, anatomists and neurologists described many of the distinct clinical syndromes associated with infarcts of specific regions in the brain and spinal cord and further clarified pathogenetic mechanisms, such as hypoxia, ischemia, and hypertensive hemorrhages.
The brain receives about 15% of total cardiac output and accounts for about 20% of the body's oxygen consumption. Neurons are absolutely dependent on a constant supply of oxygen for survival. Any disease process that reduces oxygen supply to all or some of the brain interferes with function and, when severe enough, can cause the death of brain cells (the most vulnerable being neurons). Anoxia refers to the absence of oxygen, and hypoxia describes reduced concentration of oxygen. The term “hypoxia” tends to be more widely used because it encompasses a range of reduced oxygen concentrations that may be encountered clinically. Ischemia is defined as absence of blood flow; oligemia connotes reduced flow. Hypoxia and ischemia almost always coexist, and these terms are often used together. A cerebral infarct is a circumscribed focus or area of brain tissue that dies as a result of localized hypoxia/ischemia due to cessation of blood flow.
The incidence of stroke increases with age from about 1/1000 between the ages of 45 and 55 to about 1/33 for those older than 85 years and is more common in men than women. In the United States, ischemic cerebral infarction accounts for about 80% of all strokes and is eight times more common than spontaneous brain hemorrhage. The main causes are atherosclerosis with thrombosis of large vessels or embolic occlusion of distal vessels (or both). Thus, the major risk factors for ischemic stroke are the same as those for atherosclerotic cardiovascular disease. These include diabetes, hypertension, smoking, positive family history, hyperlipidemia, and truncal obesity. Chronic hypertension aggravates atherosclerotic changes in both extracranial and intracranial blood vessels and leads to a more distal arterial distribution of atheromatous changes ( arteriolosclerosis or “small-vessel cerebrovascular disease” ).
Clinical characteristics of cerebral hypoxia/ischemia vary widely depending on severity and duration of the insult, the size and location of the lesion, whether brain involvement is widespread or focal, and the availability of collateral circulation. The clinical presentation of an infarct is sudden, and the spectrum of acute neurologic signs and symptoms depends on the amount of brain compromised, location of the lesion, duration of reduced flow, status of collateral circulation, and intrinsic vulnerability of brain cells. Occasionally, the clinical signs of infarction may present in a stuttering, or stepwise, progression as fractions of the ischemic tissue succumb. Infarcts resulting from embolization or intrinsic cerebrovascular disease occur in circumscribed vascular territories within the distributions of major cerebral arteries.
Most infarcts are believed to result from the disruption and embolization of platelet thrombi or friable plaque material with subsequent obstruction of intracranial arteries. The thrombus often originates from extracranial locations such as atherosclerotic disease at the bifurcation of the carotid artery or from the heart. Occasionally, emboli may arise from calcified, marantic, or infected heart valves. Transient ischemic attacks (TIAs) with brief reversible focal neurologic deficits often precede frank infarcts. Symptoms of a stroke depend on the location and extent of the lesion. The degree of reversibility and magnitude of deficit depend on the ability of the remaining brain tissue to compensate (which is referred to as plasticity ).
The venous side of the circulation may also undergo thrombosis and cause significant cerebral ischemia. A particularly striking example of this is thrombosis of the superior sagittal sinus, which can occur with some infections and various hypercoagulation states, including pregnancy and systemic malignancies. Here, venous outflow from superficial veins of the superior median aspect of the cerebral hemispheres is obstructed, resulting in marked congestion and hemorrhagic infarction in this region. Seizures are a common presenting complaint with venous infarctions.
The lesions under discussion here should be distinguished from so-called “watershed infarcts” that result from diffuse or global hypoxia and occur in arterial border zones representing end-artery regions of supply between the major cerebral blood vessels. Such lesions may result from cardiac arrest, shock, or severe hypotension. Global hypoxia/ischemia resulting in widespread cerebral necrosis may occur in survivors of acute cardiopulmonary arrest in which there was prolonged hypotension or hypoperfusion, drug overdose, birth injury, and profound shock.
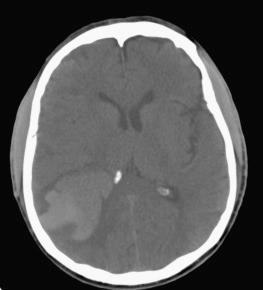
In classic presentations of cerebral infarct ( Fig. 26.1 ), the surgical neuropathologist is not involved, since the diagnosis is made purely on clinical and radiologic criteria. However, atypical presentations, such as young age, a lack of sudden onset, or unusual vascular distributions occasionally overlap with differential diagnostic considerations such as tumor or infection, prompting a biopsy ( Fig. 26.2 ). This occurs most often in the subacute phase when contrast enhancement and mass effects are increased (see next section). Additionally, biopsies are sometimes useful for identifying unusual underlying causes of ischemic damage, such as vasculitis, amyloid angiopathy, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and intravascular lymphoma.

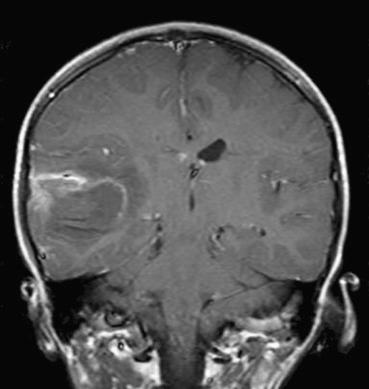
Patients who present with neurologic signs and symptoms of a stroke are typically evaluated by computed tomography (CT) in order to determine the extent of the lesion and the amount of mass effect, as well as to exclude the possibility of some other pathology, such as a hemorrhage. Most CT scans will be unremarkable during the first few hours after an ischemic cerebral infarct. Those with any findings often show only subtle evidence of tissue swelling, such as effacement of sulci or hypodensity with loss of anatomic definition and blurring of gray-white junctions.
Within 6 to 8 hours of the ictus, the infarcted area will become increasingly hypodense as focal brain swelling ensues due to cytotoxic edema (see Fig. 26.1A ). After 1 to 2 days, the lesion becomes easier to detect, often revealing a wedge-shaped region of hypodensity on CT and T1-weighted magnetic resonance imaging (MRI) within a classic vascular territory (e.g., middle cerebral artery). Increasing contrast enhancement (often in a gyral pattern) and mass effects will be observed within a week of the event and may persist as long as 2 months. Over months to years, this pattern gives way to tissue collapse and “encephalomalacia” or cystic degeneration, often with hydrocephalus ex vacuo on the side of the lesion. MRI is far more sensitive in detection of acute ischemic stroke, particularly diffusion-weighted imaging ( Fig. 26.1B ) in which changes can be seen within minutes of ischemia, but such scans are time consuming and may not be readily available. In atypical presentations, particularly during the subacute phase, infarcts may mimic other mass-forming lesions, such as neoplasms and infections (see Fig. 26.2 ).
Pathologic changes are not detectable on gross examination until 6 to 8 hours after an infarct. The first indication of abnormality is a subtle blurring of the gray-white junction and swelling of affected tissues. Within 1 to 2 days of infarction, there will be congestion with dusky discoloration of the gray matter and slight softening of the tissues. At this time, gross examination will reveal a well-circumscribed area of swelling, dusky discoloration, and hyperemia in the gray matter of a well-defined vascular territory ( Fig. 26.3A ). Large acute infarcts may be associated with significant mass effect and herniation. In such cases, a combination of a focal infarct in the specific vascular territory of the initial stroke and border zone lesions caused by compression may be present. If little or no reperfusion occurs in an area of ischemia, the infarct is called “bland” or “anemic.” However, if reperfusion occurs, as is the case for most embolic infarcts, the area of ischemia will appear hemorrhagic ( Fig. 26.3B ). The incidence of hemorrhagic transformation is strongly correlated to the duration of ischemia and volume of ischemic tissue. Hemorrhages may range from small or petechial and clinically symptomatic to lobar and devastating.
![Fig. 26.3, (A) Wedge-shaped region of hyperemia and softening in an internal carotid artery distribution (combined anterior cerebral artery [ACA] and middle cerebral artery [MCA]). (B) Hemorrhagic infarct. Petechial intracortical hemorrhages are seen as a secondary phenomenon in this hemorrhagic MCA territory infarct. Note the adjacent nonhemorrhagic, but necrotic, white matter. (C) Organizing cerebral infarct. Early cavitation and separation of the cortex from the underlying white matter in ACA infarcts. (D) Remote cerebral infarct. Cavitated (remote) infarct having undergone complete liquefaction necrosis in the right frontal lobe. Fig. 26.3, (A) Wedge-shaped region of hyperemia and softening in an internal carotid artery distribution (combined anterior cerebral artery [ACA] and middle cerebral artery [MCA]). (B) Hemorrhagic infarct. Petechial intracortical hemorrhages are seen as a secondary phenomenon in this hemorrhagic MCA territory infarct. Note the adjacent nonhemorrhagic, but necrotic, white matter. (C) Organizing cerebral infarct. Early cavitation and separation of the cortex from the underlying white matter in ACA infarcts. (D) Remote cerebral infarct. Cavitated (remote) infarct having undergone complete liquefaction necrosis in the right frontal lobe.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/VascularandIschemicDisorders/2_3s20B9780323449410000266.jpg)
With organization of the lesion, macrophages enter the tissue to remove necrotic material. This results in softening and disruption of the tissues and a circumscribed pattern of laminar necrosis in which the cortex appears detached from the underlying white matter ( Fig. 26.3C ). The subpial region of the cortex usually remains intact. With further elimination of the necrotic tissue, the brain tissue undergoes cavitation and the cerebral surface becomes depressed ( Fig. 26.3D ).
No apparent histopathologic changes are evident by routine light microscopy within 12 hours of the ischemic event. Low-magnification microscopic changes first emerge between 12 and 24 hours after significant ischemia and consist of circumscribed regions of pallor with variable degrees of vacuolization of the neuropil. At higher magnifications, neurons display cytoplasmic hypereosinophilia and dark shrunken nuclei, consistent with pyknosis ( Fig. 26.4A ). This neuronal reaction has been variously referred to as ischemic cell change, acute neuronal cell change, eosinophilic cell change, or, simply, “red dead” neurons. The last designation indicates that irreversible damage has occurred. Eosinophilic neurons may persist for several days following the acute event or even longer if blood flow is not reestablished to the region.
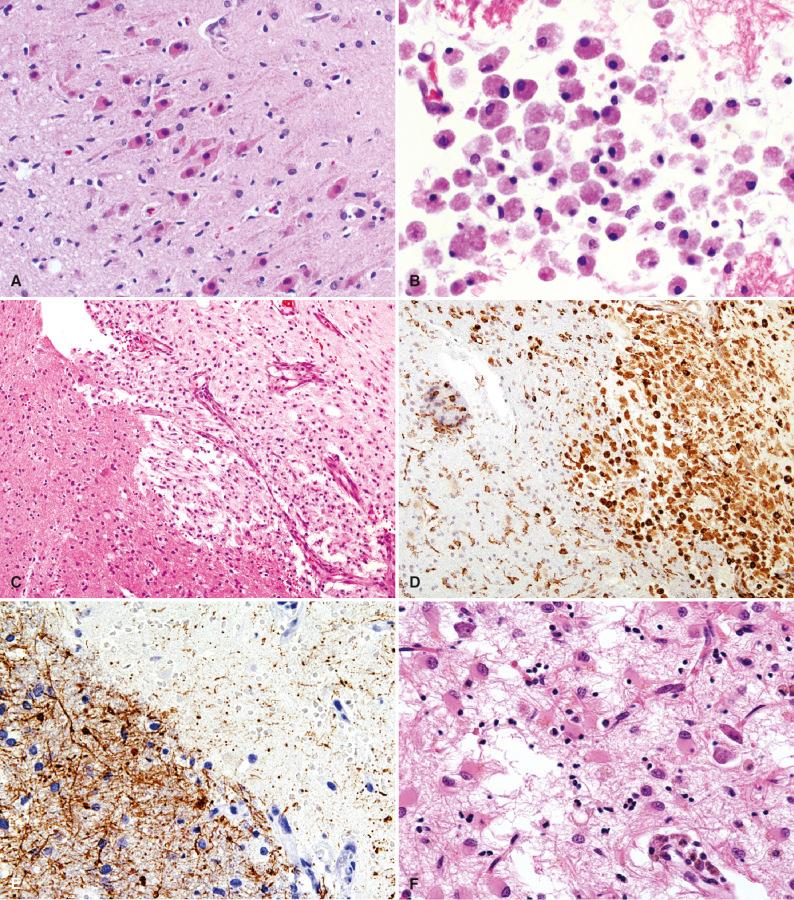
During the subacute or organizing stage of a cerebral infarct (5 to 30 days), microglia are activated and foamy macrophages infiltrate tissue and begin removing necrotic material ( Fig. 26.4B–E ). Removal of necrotic debris by macrophages ultimately results in cavitation of cortical layers 2 through 6, with sparing of the subpial layer and persistence of thread-like capillaries. Macrophages may persist in the resulting cavity for years after the original event. Capillary proliferation occurs during the first 2 weeks after infarction ( Fig. 26.4C ). Unlike demyelinating disorders, which show mostly loss of myelin, a severe axonopathy is also evident in infarcts at this stage, with marked loss of axons in the lesion and axonal spheroids at the edge ( Fig. 26.4E ). Astrocytic hyperplasia and hypertrophy are also apparent at the edges of the lesion 7 to 10 days after the insult ( Fig. 26.4F ).
In the chronic phase (weeks to years postinfarct), scattered macrophages with or without hemosiderin are found within organized areas of cavitation. Thin strands of residual glial tissue and blood vessels (“glial scar”) often traverse such areas, and scattered reactive astrocytes are seen at the edge. The formation of a cavity in the region of severe hypoxia/ischemia is referred to as “complete liquefactive necrosis,” and necrosis of the most vulnerable population of cells (the neurons) with sparing of astrocytes and other cellular elements of the neuropil is called “incomplete necrosis.”
The important concept of “selective vulnerability” maintains that brain cells in different anatomic sites vary considerably in their susceptibility to hypoxia. In general, neurons are most sensitive, followed by oligodendrocytes, astrocytes, and endothelial cells. Also, certain subtypes of neurons display a striking selective vulnerability to hypoxia. For example, the most sensitive neurons in the adult are the pyramidal cells in area CA1 of the hippocampus (“Sommer sector”) and the Purkinje cells of the cerebellum. In relatively mild hypoxic injury, only the most vulnerable neurons may be affected. With more severe global hypoxia/ischemia, laminar necrosis of neurons in layers 3, 5, and 6 of the cerebral cortex occurs, although there is considerable individual variability. For example, thalamic and basal ganglia neurons may be selectively involved in some individuals, rather than the typical patterns of watershed infarcts, laminar necrosis, and neuronal damage in the hippocampus and cerebellum.
Hemorrhagic transformation of an ischemic infarct may be difficult to distinguish from a primary brain hemorrhage. Occasionally, a glioma that diffusely infiltrates the cerebral cortex and subcortical white matter may give the radiographic appearance of an acute to subacute infarct (or vice versa; see Fig. 26.2 ). In most cases, these are clearly discernable on microscopic examination, although macrophages can display surprising nuclear atypia and mitotic activity. On frozen section in particular, they are notorious for their mimicry of high-grade glioma, whereas they are easily recognized as foamy macrophages on intraoperative smear. In its subacute phase, a macrophage-rich infarct may be difficult to distinguish from active demyelination (see Chapter 24 ), although the former is much more destructive (i.e., no relative preservation of axons). One must also rule out other destructive processes, such as necrotizing infections (e.g., toxoplasomosis, herpes encephalitis). Lastly, it is important to search carefully for clues to the cause of ischemia in clinically atypical presentations. These include the presence of thromboemboli, vasculitis, amyloid angiopathy, CADASIL, or intravascular lymphoma.
Ancillary diagnostic studies are not usually necessary in routine clinical practice since the characteristic histopathology of a cerebral infarct and its various stages of evolution are readily apparent on hematoxylin-eosin (H & E) staining. However, occasionally the surgical pathologist receives a specimen from a patient with an organizing cerebral infarct in whom the clinical or imaging workup suggested a neoplastic or infectious process. In this case, organizing necrosis in the brain can be distinguished from tumor by the identification of CD163 or CD68 -immunoreactive macrophages as the prominent component of a cellular-appearing lesion (see Fig. 26.4D ). In the differential with active inflammatory demyelinating lesion (“tumefactive” demyelination), Luxol fast blue/periodic acid–Schiff (LFB-PAS) staining shows loss of myelin and PAS-positive macrophages in both processes. However, axonal stains, such as the Bodian or Bielschowsky silver stains, or a neurofilament protein immunostain can be used to detect relative sparing of axons in the case of demyelination versus a loss of axons in organizing necrosis (see Fig. 26.4E ). Routine organism stains help to exclude an infectious cause if there are other features to suggest one (e.g., prominent inflammation, granulomas, possible viral inclusions).
Early diagnosis and improved management of complications have led to significant reduction in mortality from acute cerebral infarction. Although available only to a minority of patients, immediate thrombolytic therapy can substantially improve outcomes. Variable degrees of hemorrhagic transformation can occur with administration of thrombolytics, yet these events are often asymptomatic. Hemorrhage can reflect reperfusion, which is associated with improved outcomes. Symptomatic hemorrhage after thrombolytic administration occurs in a small percentage of patients. In addition, treatment with aspirin and other antiplatelet agents plays a significant role in preventive therapy. Treatment of diabetes and hypertension, lowering cholesterol, cessation of smoking, and other measures to control risk factors for stroke are important preventive measures.
Although the consequences of hypertension are legion, this section focuses on three areas of cerebrovascular disease in which hypertension is most closely causally related: (1) spontaneous intracerebral hemorrhage (ICH), (2) lacunar infarcts, and (3) hypertensive encephalopathy, which now includes the reversible posterior leukoencephalopathy syndrome (RPLS). Diseases included within the spectrum of “vascular dementias,” including Binswanger disease, are also discussed in Chapter 24 .
Hypertensive neuropathology is defined by the typical vascular and parenchymal complications of chronic high blood pressure. Chronicity is important to the definition because patients are often acutely “hypertensive” as part of the normal Cushing reflex response to brain hypoperfusion and this does not constitute true hypertension as a disease process. Thus, hypertensive cerebrovascular disease does not typically follow the many causes of increased intracrianal pressure (e.g., intracerebral hemorrhage) that induce a short-term increase in systemic blood pressure as the body attempts to preserve blood flow to the brain. Lacunar infarct is defined by a greatest dimension of less than 1 cm, usually within deep subcortical structures. By definition, intracerebral hemorrhage originates within the brain parenchyma itself (often deep subcortical structures), rather than external spaces, such as subarachnoid, subdural, and epidural hemorrhages.
Spontaneous intracranial hemorrhage affects thousands of patients annually in the United States and is believed to account for 10% to 20% of all strokes. Hypertension is the most important underlying cause of acute nontraumatic ICH. The incidence of ICH is around 28 per 100,000 in Caucasians, roughly 56 in African Americans, and even greater in Japanese individuals. Around 70% to 90% of patients with ICH have high blood pressure, and cerebral hemorrhage accounts for 15% of deaths in patients with chronic hypertension. The risk of spontaneous ICH increases dramatically after 55 years of age.
Lacunar infarcts are a common stroke subtype, accounting for 15% to 20% of all strokes, and have a strong relationship to hypertension. Incidence in Caucasians is about 17%, and roughly double that in African Americans and Hispanics, but distribution is nearly equal between the sexes. In Japan, lacunar infarcts account for nearly 40% of all strokes. Like most other stroke subtypes, risk generally increases with age.
Hypertensive encephalopathy is an older term that overlaps with the more recently introduced terms reversible posterior leukoencephalopathy syndrome (RPLS), reversible posterior leukoencephalopathy (RPLE), and posterior reversible encephalopathy syndrome (PRES). RPLS and PRES are the two most common terms used in the literature currently. Although RPLS can manifest without elevated blood pressure in certain clinical circumstances, the vast majority of cases occur in the setting of extreme hypertension and another risk factor, such as exposure to certain medications (e.g., FK506), pregnancy (as part of eclampsia), or drug abuse (e.g., cocaine).
Acute hypertensive hemorrhage typically occurs in distributions of the lenticulostriate branches of the middle cerebral artery and in pontine perforators of the basilar artery. Accordingly, hypertensive hemorrhages arise in the deep cerebral nuclei (60%; putamen, thalamus ), cerebrum ( lobar white matter 20%), cerebellum (13%), and pons (7%). Involvement of the deep nuclei may produce hemiparesis, hemisensory deficits, and visual field defects (putamen), or gaze abnormalities and vomiting (thalamus). Headache, vomiting, and truncal or limb ataxia may occur with cerebellar hemorrhage, and coma with quadriparesis may signal a pontine hemorrhage. Depressed level of consciousness due to acutely increased intracranial pressure is often present and is the most reliable clinical sign for differentiating hemorrhagic from ischemic lesions.
Lacunar infarcts often involve the same vessels that give rise to hypertensive hemorrhages, so the focal deficits are often similar, but lacunar infarcts rarely lead to a depressed level of consciousness. Classically, there are five lacunar syndromes: (1) pure motor or (2) pure sensory deficits (affecting face, arm, and leg equally), (3) a combination of these two, (4) ataxic hemiparesis, and (5) the dysarthria/clumsy-hand syndrome. Localization based on the clinical presentation is inexact, as there are multiple locations along the motor or sensory pathways that can produce the same syndrome. Accumulation of appropriately located lacunar infarcts can lead to a stepwise decline in cognition, often referred to as “multi-infarct dementia.”
RPLS is an acute neurologic syndrome characterized by diffuse cerebral dysfunction, severe headache, confusion, and visual changes precipitated by a sudden and severe increase in systemic blood pressure. It can occur in patients with kidney disease, eclampsia, disseminated vasculitis, catecholamine-secreting tumors like pheochromocytoma, or following discontinuation of antihypertensive therapy (i.e., “rebound effect”). More recently, cases of RPLS have been associated with antiangiogenic chemotherapeutic agents such as bevacizumab and cediranib. An association with sickle cell disease has also been reported. Untreated, the syndrome may progress to coma or fatal hemorrhage.
Massive hemorrhages involving the deep nuclei manifest as circumscribed areas of fresh and acutely clotted blood, causing tissue disruption, significant mass effect, and herniation ( Fig. 26.5A ). Blood may dissect medially into the ventricles but only rarely dissects outward through the cortex and into the subarachnoid space. In nonfatal cases, the region of hemorrhage undergoes cavitation and shows yellow-brown hemosiderin staining. Pontine ( Fig. 26.5B ) and cerebellar hemorrhages are associated with significant brainstem compression. Acute hemorrhages are hyperdense on CT (nearly as bright as bone) and become isodense to brain parenchyma with time. The appearance of hemorrhage on MRI is complex and evolves through stages of hypointensity, isointensity, and hyperintensity with time.
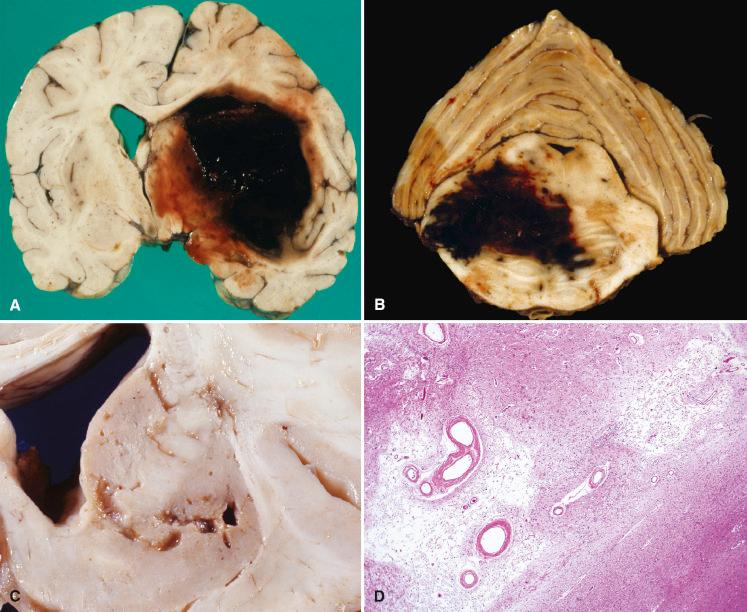
A lacunar infarct results from occlusion of a single perforating artery, and therefore they are predominantly located in the deep cerebral nuclei, especially in the putamen ( Fig. 26.5C ). They may also be found in the periventricular white matter. Careful autopsy studies of the brains of hypertensive individuals have demonstrated that perivascular spaces were frequently widened because of increased tortuosity of parenchymal arteries. Lacunar infarcts and enlarged perivascular spaces are usually readily differentiated with MRI or microscopic examination of surrounding tissue. Using either method, the tissue surrounding a lacunar infarct would show reactive changes and gliosis (bright signal on fluid-attenuated inversion recovery MRI), whereas the tissue surrounding a widened perivascular space would appear normal.
Autopsy studies of patients having suffered from RPLS have demonstrated a range of gross pathologic changes. Brain weights are generally within normal limits. As RPLS is a focal disorder that predominantly affects the posterior circulation, pathology may be confined to tissues supplied by branches of the vertebral or basilar arteries. Atypical distributions of RSLS may occur outside of the posterior circulation.
Multiple petechial hemorrhages are occasionally seen. Atherosclerotic changes of the vessels of the circle of Willis are often observed and may be severe. Because hypertension is a risk factor shared with many diseases, multiple pathologies may coexist, such as old ischemic or hemorrhagic infarcts. MRI demonstrates increased T2 signal in affected areas, and a variable degree of water restriction may be seen on diffusion-weighted imaging. Signal abnormalities are consistent with modest to moderate edema, below the threshold for gross swelling or herniation.
Lacunar infarcts derive their name from the fact that these small ischemic infarcts (see Fig. 26.5D ) organize by liquefaction necrosis and undergo cavitation, leaving behind a fluid-filled defect, or “lake.” Hemosiderin-laden macrophages may also be present in such lesions if the initial insult was hemorrhage.
Because of the strong association of diabetes and hypertension with accelerated atherosclerosis, atheromatous changes of cerebral blood vessels often extend more distally, involving smaller superficial and parenchymal arteries as well as large vessels at the base. Hyaline atherosclerosis and arteriolosclerosis consist of homogeneous, eosinophilic thickening of the walls of small arteries and arterioles, respectively, obscuring the underlying structural detail and narrowing vascular lumens ( Fig. 26.6A ). To some extent, this change is a consequence of age, but it is present to a much greater degree in patients with hypertension or diabetes and may occur with concentric mineralization of the media of small arteries, particularly common in the globus pallidus ( Fig. 26.6B ). Radiation injury (usually iatrogenic) is another less common cause.
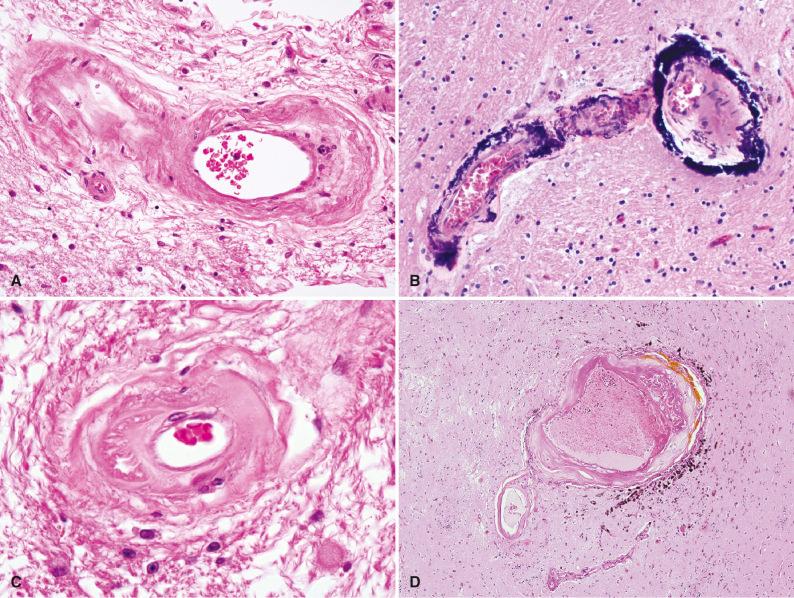
With acute or malignant hypertension, hyperplastic arteriolosclerosis develops, leading to concentric laminated thickening of the walls of arterioles with progressive narrowing of the lumen. With electron microscopy, these laminations have been shown to consist of smooth muscle cells with thickened, reduplicated basement membranes that lead to an appearance referred to as “onion-skinning” of the vessel wall.
Over time, chronic stress on vessel walls leads to degeneration of the tunica media in the form of fibrinoid necrosis (also called lipohyalinosis ). To some extent lesions of this type reflect leakage of plasma proteins across vascular endothelium and excessive extracellular matrix production by smooth muscle cells secondary to chronic hemodynamic or metabolic stress. Vessel walls appear thickened and hyalinized in a segmental distribution ( Fig. 26.6C ). The development of such a lesion includes the transient appearance of foamy or lipid-laden macrophages and eventually progresses to fibrosis and hyalinization.
The traditional view that local weakening of damaged blood vessels in hypertensive patients leads to focal microscopic aneurysmal dilatations at branch points (referred to as cerebral microaneurysms, or Charcot-Bouchard aneurysms ) has been debated for over a century. Such aneurysms are only rarely identified in routine autopsy material, and thus most descriptions in the literature are based on single or small numbers of cases ( Fig. 26.6D ).
The relationship between these microaneurysms and ICH has not been firmly established. A detailed pathologic study using light microscopy and high-resolution microradiography compared the brains of hypertensive patients (with and without ICH) and normal controls and failed to demonstrate any microaneurysms. However, sections though the twists and turns of tortuous blood vessels commonly seen in hypertensive patients often had the appearance of aneurysmal dilation in histologic sections. Although it remains possible that cerebral microaneurysms develop rapidly, immediately preceding fatal hemorrhage, and are obliterated in the process, most evidence suggests these lesions are rare and an unlikely source of ICH. The cause of massive deep nuclear hemorrhages associated with hypertension is most likely multifactorial, with vascular hyalinization and fibrinoid change causing decreased wall compliance and increased fragility. Evidence of microhemorrhage is common surrounding foci of small-vessel disease (see Fig. 26.6D ).
In the case of RPLS, milder forms do not come to the attention of pathologists, since the changes are typically reversible and likely due to poor autoregulation of the posterior circulation, resulting in cerebral edema. However, rare autopsy studies of acute malignant hypertension have demonstrated foci of fibrinoid vascular necrosis, with miliary microinfarcts and fibrin thrombi occluding the lumina of both necrotic and intact arterioles, most prominently in the brainstem. These vessels were often surrounded by a cuff of mononuclear cells. Extravascular deposits of fibrin were also commonly observed. In all cases, a mixture of segmental hyaline and hyperplastic arteriolosclerosis was demonstrated, with the degree proportional to the severity of hypertension. Chronic lesions showed gliosis with gemistocytic astrocytes and microglial nodules.
The vasculopathy of hypertension and the associated white matter changes and microinfarcts looks remarkably similar at first glance to the pathology of CADASIL (covered at the end of this chapter). However, hypertensive blood vessels lack the granular PAS-positive, electron-dense medial deposits of CADASIL. Therefore, histochemistry or electron microscopy may be helpful in the rare cases where this distinction is not already apparent based on other clinicopathologic features. Occasionally, it may be difficult to distinguish ICH from a hemorrhagic infarct. In cases of massive ICH, the typical histologic pattern is that of a large hematoma compressing or disrupting otherwise viable tissue. Ischemic changes are common in the tissue immediately surrounding the hematoma, but this differs from hemorrhagic infarcts, where the typical pattern is one of patchy (often petechial) hemorrhage within large zones of necrotic brain.
The genetics of hypertension are complex and multifactorial. No genetic markers are currently known for predicting which patients are at greatest risk of intracerebral complications.
The prognosis after ICH varies widely with the size of the hematoma. With small hemorrhages less than 10 mL in volume, patients most often make a good recovery. Massive ICHs with volumes greater than 60 mL have a 90% mortality rate. No definitive treatment exists for acute ICH, other than control of blood pressure. Medical control of hypertension and other risk factors are the mainstays of preventive therapy.
The prognosis after lacunar infarction is also extremely variable and dependent on the location. The small size of lacunes does not necessarily equate with a proportionally small deficit. Some infarctions, such as in the head of the caudate, may go unnoticed and be only incidentally discovered with imaging studies, whereas others of the same size involving the internal capsule may leave a patient hemiparetic. Preventive therapy is the same as that for hypertension.
The deficits caused by RPLS may, as the name implies, often be reversible if treated aggressively and early. Those cases related to acute malignant hypertension often respond to intravenous antihypertensives. Cases related to eclampsia respond only to delivery of the fetus; those related to certain drugs, such as chemotherapeutics, may respond to discontinuing the offending agent (see Chapter 21 ). In those cases that do not respond to treatment, the effects are often devastating or fatal.
Cerebral amyloid angiopathy (CAA) is a form of vasculopathy resulting from the deposition of Aβ-amyloid within the walls of meningeal and cortical blood vessels. By definition, it is distinct from the many systemic forms of amyloidosis. An older term is congophilic angiopathy, reflecting its common detection using the Congo red histochemical stain for amyloid, whereas a less common synonym is dysphoric angiopathy, sometimes utilized when the amyloid spills out into the adjacent parenchyma. The term Aβ-related angiitis (ABRA) has been applied to examples of CAA with associated vasculitis, giant cells, or granulomatous inflammation. Lobar hemorrhage is a form of ICH that tends to be more superficial than hypertensive hemorrhages and usually involves a single cerebral lobe, the occipital lobe being most common.
Emerging evidence suggests that CAA is not a distinct disease, but a pathologic process that may be the molecular link between Alzheimer disease (AD) and vascular dementia. Nonetheless, the distinctive phenotype of the most common variant warrants discussion as if it were a separate entity. The pathologic changes associated with CAA are very common and are found in patients spanning the full spectrum of cognitive decline. In a large retrospective autopsy study of 2060 elderly subjects, CAA of various degrees was detected in 73% of all and in 98% of confirmed cases of typical AD. This process also predisposes patients to both microscopic and large lobar hemorrhages, generally seen in patients 70 to 80 years of age. It is this clinicopathologic presentation to which the disease label “CAA” usually refers. This disease should be considered in any older adult who presents with spontaneous lobar ICH, especially if the patient is nonhypertensive and multiple microhemorrhages are present. Rarely, CAA may cause symptoms earlier than the fifth and sixth decades.
In contrast to the deep cerebral hemorrhages of chronic hypertension, hemorrhages associated with amyloid angiopathy are more peripheral, involving the cerebral cortex and adjacent structures. Clinical symptoms reflect the anatomic extent and location of the hemorrhage. Lobar hemorrhages can occur acutely as a massive acute stroke, or as recurrent hemorrhagic infarctions that take place over a period of years. CAA may involve any lobe of the brain, but has a particular predilection for the occipital lobe. As the disease progresses, patients become increasingly cognitively impaired. A small subset of CAA patients develop an intense inflammatory response (ABRA). Presentations overlap between those of typical CAA and those of primary angiitis of the CNS (PACNS; see next section). Patients are slightly younger than classic CAA patients and are more likely to present with mental status changes, seizures, and hallucinations. Occasionally, intralobar masses are difficult to distinguish from hemorrhagic neoplasms, and these are the cases most likely to undergo surgical resection. Others undergo “clot evacuation” in order to alleviate mass effects.
CT is the most useful imaging method in the setting of an acute hemorrhage ( Fig. 26.7 ). Recent advances in neuroimaging have expanded our knowledge of this process. Gradient-echo (GE) MRI is capable of detecting millimeter-sized foci of blood products in brain parenchyma, including hemosiderin, which remains at sites of previous bleeding, allowing hemorrhagic burden to be assessed over time. Blood products appear as black spots on GE MRI sequences and must be distinguished from vascular flow voids. One should also note that because of an artifact inherent to GE MRI, called the “blooming effect,” the hemorrhagic lesions appear larger than they really are.
With specific probes and positron emission tomography (PET), the distribution of β-amyloid can now be directly visualized. Many compounds, most notably Pittsburgh compound B (PiB) and the 2,6-disubstituted naphthalene derivative FDDNP, reliably predict β-amyloid deposition in human brain parenchyma. As this technology matures, clinical applications for the early detection of AD and evaluation of new antiamyloid therapies may be possible.
Gross pathology reveals a large acute lobar hemorrhage that is more peripheral in location than deep hypertensive hemorrhages. Often, multiple subcortical hemorrhages or hemorrhagic infarcts of different ages are also present. Cortical superficial siderosis, a distinct pattern of blood breakdown product deposition in the sulci of the cerebral hemispheres, has been increasingly associated with cerebral amyloid angiopathy and has been shown to predict recurrence of intracerebral hemorrhage in this disorder.
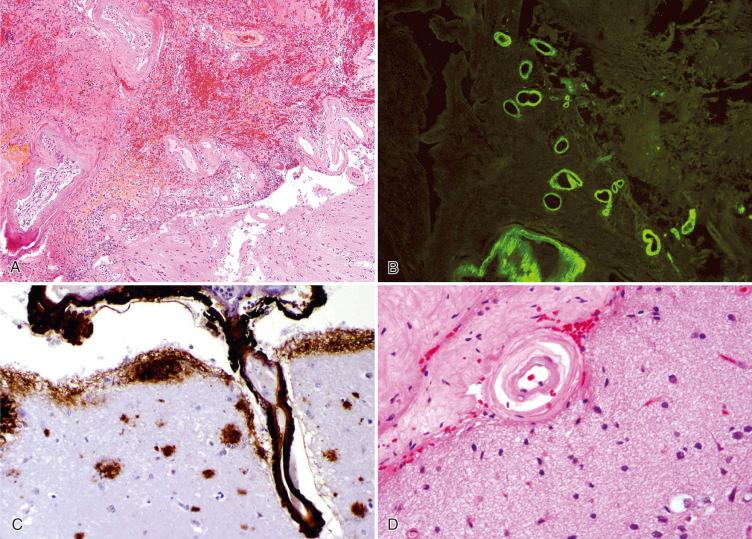
On routine H & E staining of lobar hemorrhage/infarct with associated CAA, the majority of the specimen often shows organizing hematoma, with thickened blood vessels in the adjacent brain parenchyma ( Fig. 26.8A ). The affected vessels of the leptomeninges and superficial cortex show mural thickening and effacement by acellular, homogeneous, eosinophilic material, which is highlighted with nonspecific histochemical stains for amyloid such as thioflavin S ( Fig. 26.8B ) and is strongly reactive for Aβ-amyloid peptide using immunohistochemistry ( Fig. 26.8C ). Capillaries and veins are less often involved, and blood vessels of the deep white matter are typically spared. Arterial and arteriolar amyloid accumulation initially appears in the basement membrane around smooth muscle cells at the peripheral aspect of the media and adventitia. There is progressive destruction of smooth muscle cells, but (generally) sparing of the endothelium until late stages of the disease. Affected vessels become rigid and fragile and in late stages assume a rounded or “double-barrel contour” ( Fig. 26.8D ). Some vessels ultimately undergo fibrinoid necrosis and rupture with hemorrhage. Vascular amyloid accumulation also likely impairs cerebral autoregulation. More recent studies have suggested that vascular accumulation of Aβ-amyloid may be due to failure of perivascular drainage, which may impair clearance of amyloid in CAA.
In cases of ABRA, the amyloid is associated with vasculitis ( Fig. 26.9 ), the inflammatory component variably consisting of lymphocytes, plasma cells, and epithelioid macrophages, often including multinucleated giant cells or fully formed granulomas, such as those seen in PACNS (see next section).
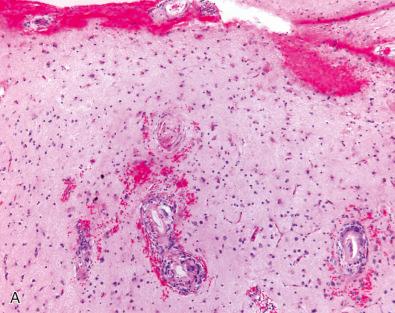
Hemorrhages associated with CAA may be accompanied by ischemic lesions that mimic a vasculitis. A high index of suspicion for the diagnosis of ABRA should be maintained for elderly patients with histology suggesting PACNS, especially if amorphous eosinophilic material is seen within vessel walls (see Fig. 26.9A ). Occasionally, white matter lesions may occur in a distribution suggesting a Binswanger-like leukoencephalopathy, although most cases are limited to the cortex. The small-vessel hyalinization overlaps with hypertensive vasculopathy (or diabetes), although this type of vasculopathy more often extends into deep subcortical structures. The detection of Aβ-amyloid peptide by immunohistochemistry confirms the diagnosis in most cases.
Aβ-amyloid deposition can be confirmed by the Congo red stain (see Fig. 26.9B ), typically associated with apple-green birefringence seen under polarized illumination; although this stain is tenuous in some laboratories and equivocal results are not uncommon. An unequivocally intense green fluorescence is usually seen with thioflavin S stain (see Fig. 26.8B ). However, since most sporadic and some familial cerebral amyloid angiopathies are due to abnormal accumulations of Aβ-amyloid, detection of this peptide by immunohistochemistry has greatly facilitated the histopathologic diagnosis. Immunostaining for Aβ-amyloid often also reveals neuritic and diffuse plaques in the cortex of individuals with CAA ( Figs. 26.8C and 26.9C ). In surgical cases, one must be careful not to overdiagnose AD based on this finding alone with a single sample of the cortex (especially in the absence of clinical dementia), although the possibility can be raised in a comment, so that appropriate clinical workup can subsequently be initiated and early therapy instituted if appropriate.
Amyloid is a pathologic protein in which abnormal folding produces an extensive β-pleated sheet secondary structure. In this conformation, protein polymers form highly insoluble 8- to 10-nm-diameter fibrils. The most common form of CAA is caused by the deposition of degradation products of amyloid precursor protein (APP), which is encoded by a gene located on chromosome 21. Rare inherited forms of CAA result from amyloid composed of cystatin C, transthyretin, gelsolin, and prion protein. APP may be cleaved by α-, β-, or γ-secretase, but under normal physiologic conditions, the action of α-secretase predominates, and the fragments produced are not amyloidogenic. Low levels of Aβ-amyloid, produced by the β- and γ-secretases, do not form deposits, as Aβ can be cleared from the brain by several mechanisms.
Most cases of CAA are apparently sporadic, but mutations in several genes have been implicated in the development of both CAA and AD, which likely accounts in part for the heterogeneity seen in these diseases. Mutations in the APP gene in Flemish and Dutch families have been associated with the most severe forms of CAA. Presenilin 1 and 2 gene mutations have been implicated in early-onset familial AD. All three of these gene mutations predispose cleavage of APP by β-secretase and increase the production of β-amyloid. The ApoE4 allele also plays an important role and may promote the deposition of Aβ-amyloid in vessel walls. This allele is also highly associated with vasculitis-associated pathology. A recent study demonstrated ApoE4 homozygosity in 10 of 13 (77%) ABRA versus 2 of 39 (5%) noninflammatory CAA patients.
Acute parenchymal brain hemorrhage may cause death due to mass effect and herniation. Excessive bleeding of amyloid-laden blood vessels may complicate surgery for removal of acutely clotted blood. Management of smaller hemorrhages is mainly symptomatic as no specific therapies are available at present. The diagnosis of ABRA is particularly important, since unlike ordinary CAA, immunosuppressive therapies are often part of the regimen, with some patients showing particularly dramatic responses.
Vasculitis, or inflammation of cerebral blood vessel walls, may occur in the context of a primary systemic disorder, such as giant-cell arteritis and polyarteritis nodosa, or may be localized to cerebral vasculature or to those vessels supplying peripheral nerves. Alternatively, inflammatory changes in nervous system blood vessels may represent secondary involvement by collagen vascular diseases, infections, tumors, and substance abuse (“secondary vasculitis”). Primary vasculitides may preferentially affect large (elastic), medium (muscular), or small (<0.5 mm in diameter) arteries. An idiopathic form of vasculitis limited to the brain and spinal cord is known as primary angiitis of the central nervous system (PACNS).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here