Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vagus nerve stimulation (VNS) delivered via the implantable Neurocybernetic Prosthesis (NCP) from LivaNova PLC (London, United Kingdon) is an established treatment option for patients with intractable epilepsy. It has also emerged as a novel adjunct in the management of patients with refractory depression and potentially other disorders. Since the first implantation in 1988 more than 80,000 patients worldwide have received VNS therapy. The NCP device delivers intermittent electrical stimulation to the left cervical vagus nerve trunk, which secondarily transmits rostral impulses to exert widespread effects on neuronal excitability throughout the central nervous system. This chapter summarizes the relevant considerations pertaining to VNS, and reviews pragmatic issues such as patient selection and surgical technique.
Fig. 82.1 depicts a schematic representation of VNS therapy. A pulse generator inserted in the subcutaneous tissues of the upper left chest delivers intermittent electrical stimulation to the cervical vagus nerve trunk via a bifurcated helical lead.
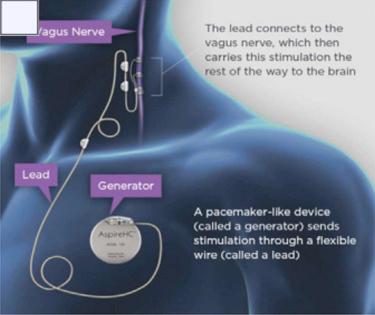
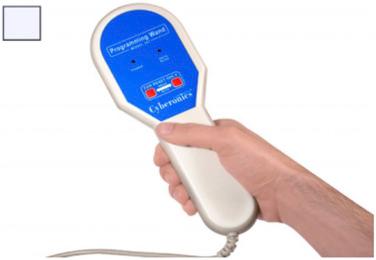
In addition to the pulse generator ( Fig. 82.2 ) and implantable lead ( Fig. 82.3 ), the NCP system includes a number of peripheral components. One is a telemetry wand that interrogates and programs the pulse generator noninvasively ( Fig. 82.4 ). This programming wand is powered by batteries and interfaced with a tablet or laptop. The system also includes a handheld magnet that patients may carry with them to alter the character of stimulation that the generator delivers.
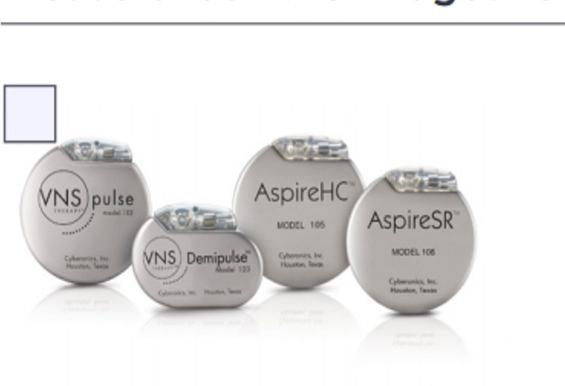
There are currently six available modes of VNS therapy generators with variable features, including single versus dual pin, volume, weight, and a cardiac-based seizure-detection algorithm that can detect ictal tachycardia and automatically trigger a defined autostimulation ( Table 82.1 ). Each generator type is powered by a single lithium battery encased in a hermetically sealed titanium module. The projected battery life of the generator varies with the stimulus parameters, but can be as long as 6–10 years under normal conditions.
| Generator Model | 102 Pulse | 102R Pulse Duo | 103 Demipulse | 104 Demipulse Duo | 105 AspireHC | 106 AspireSR |
|---|---|---|---|---|---|---|
| Lead compatibility | Single pin | Dual pin | Single pin | Dual pin | Single pin | Single pin |
| Available since | 2002 | 2003 | 2007 | 2007 | 2011 | 2015 |
| Thickness | 7 mm | 7 mm | 7 mm | 7 mm | 7 mm | 7 mm |
| Volume | 14 cc | 16 cc | 8 cc | 10 cc | 14 cc | 14 cc |
| Weight | 25 g | 27 g | 16 g | 18 g | 25 g | 25 g |
The generator contains an internal antenna that receives radiofrequency signals emitted from the telemetry wand and transfers them to a microprocessor that regulates the electrical output of the pulse generator. The generator delivers a charge-balanced waveform characterized by five programmable parameters: output current, signal frequency, pulse width, signal-on time, and signal-off time. These variables are titrated empirically in the outpatient setting, according to individual patient tolerance and seizure frequency. Altering the parameters of stimulation will have various consequences on VNS efficacy, side-effects, and battery life.
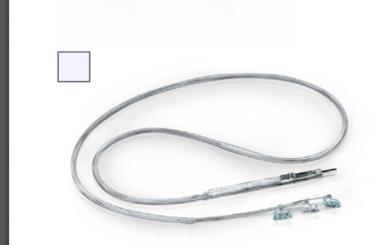
The bipolar lead is insulated by a silicone elastomer, and can thus be safely implanted in patients with latex allergies. One end of the lead contains a connector pin that inserts directly into the generator, while the opposite end contains an electrode array consisting of three discrete helical coils that wrap around the vagus nerve. The middle and distal coils represent the positive and negative electrodes, respectively, while the most proximal one serves as an integral anchoring tether that prevents excessive force from being transmitted to the electrodes when the patient turns his/her neck. The leads come in two sizes, measured by the internal diameter of each helix. Lead models are currently available in 2.0 and 3.0 mm helical inner-diameter sizes, but the majority of patients can be fitted with the 2 mm coil.
Each electrode helix contains three loops ( Fig. 82.3 ). Embedded inside the middle turn is a platinum ribbon coil that is welded to the lead wire. This shape permits the platinum ribbon to maintain optimum mechanical contact with the nerve. Suture tails extending from either end of the helix permit manipulation of the coils without injuring these platinum contacts. The electrode is intended to fit snugly around the nerve while avoiding compression, thus allowing the electrode to move with the nerve and minimizing abrasion from relative movement of the nerve against the electrode. Damage to the nerve is greatly reduced by the self-sizing open helical design, which permits body fluid interchange with the nerve. Thus compared with cuff electrodes, mechanical trauma and ischemia to the nerve are minimized. Histological examination of the vagus nerve following VNS has revealed no axonal loss, demyelination, lymphocytic infiltration, or other evidence of permanent damage resulting from electrical stimulation ( ).
The handheld magnet performs several functions. When briefly passed across the chest pocket where the generator resides, it manually triggers a train of stimulation superimposed upon the baseline output. Such on-demand stimulation can be initiated by the patient or a companion at the onset of an aura, in an effort to diminish or even abort an impending seizure. The parameters of this magnet-induced stimulation may differ from those of the preprogrammed activation. Alternatively, if the device appears to be malfunctioning or the patient wishes to terminate all stimulation for any other reason, the system can be indefinitely inactivated by applying the magnet over the generator site continuously. Finally, patients are instructed to test the function of their device periodically by performing magnet-induced activation and verifying that stimulation occurs. Most patients can perceive the stimulation as a slight tingling sensation in the throat.
The exact means by which VNS modulates seizure activity and its locus of action in the brain remain uncertain, although many well-documented potential mechanisms of action have been shown ( ). In 1938 it was reported that vagal nerve stimulation induced synchronized activity in the motor cortex of cats ( ). Subsequent work in by Zanchetti revealed electroencephalographic desynchronization in response to vagal nerve stimulation ranging from 2 to 300 Hz. As seizure activity also exhibits these specific synchronized patterns, it is hypothesized that appropriately timed stimulation of the vagus nerve may interfere with and thus prevent epileptiform activity. These afferent vagal tracts then project to multiple sites of potential seizure genesis throughout the brain, including the diencephalon, amygdala, hippocampus, insular cortex, cerebellum, and brainstem centers. The majority of these projections pass through the nucleus tractus solitarius (NTS), the entry level of peripheral vagal nerve afferents ( ). The NTS then projects sensory information to the forebrain via the parabrachial nucleus, and also has direct output to the amygdala and hypothalamus.
The NTS is hypothesized as a central mediator of VNS action, as afferent polysynaptic pathways project to widespread cortical regions, increasing activity in thalamo–cortical projection pathways and decreasing activity in the amygdala and hippocampus ( ). Studies employing positron emission tomography (PET) have further elucidated the cortical regions associated with VNS treatment by measuring regional cerebral blood flow (rCBF) during treatment. Rapid changes in rCBF are thought to reflect transsynaptic neurotransmission ( ). These studies suggest changes in several regions, including the ipsilateral anterior thalamus and cingulate gyrus, contralateral thalamus, and ipsilateral cerebellum, or bilateral activation of the hypothalami and insular cortices. PET scans demonstrating increases in thalamic blood flow during VNS therapy show positive correlation with improved seizure responsiveness ( ).
Several initial studies established the safety, efficacy, feasibility, and tolerability of VNS, as well as the durability of the NCP device ( ). VNS gained approval for the treatment of medically refractory epilepsy by the United States Food and Drug Administration (FDA) in 1997. Postmarketing experience has validated the earlier clinical trials, and in 1999 the Therapeutics and Technology subcommittee of the American Association of Neurology declared VNS “safe and effective,” based on a preponderance of class I evidence ( ). A metaanalysis of early studies was performed, covering 454 patients enrolled in five controlled multicenter clinical trials (two double-blind and three open-label studies) conducted in the United States ( ). For the study population as a whole, the median reduction in seizure frequency was 35% at 1 year, 43% at 2 years, and 44% at 3 years. Results from long-term follow-up of greater than 10 years have shown rates of seizure reduction to be 60.4% at 6 years, 75.5% at 8 years and 75.5% at 10 years. However, concurrent improvements in antiepileptic drugs regimens are likely to play a role in this outcome ( ). More recently, studies examining the effect of high stimulation (output current 103 mA, frequency 30 Hz, pulse width 500 μs, on time 30 s, off time 5 min) versus low stimulation (output current 0.25–0.5 mA, frequency 1 Hz, pulse width 130 μs, on time 30 s, off time 60–90 min) have been performed. A Cochrane Review of five trials covering 439 patients compared the efficacy of high- versus low-level stimulation on seizure control and examined the adverse effect profile. There was significantly better seizure control using the high-stimulation paradigm as compared to low stimulation, with no differences in reported rates of adverse effects ( ).
Although VNS is primarily utilized as a palliative option for patients with medically refractory epilepsy, a small population of patients will achieve seizure freedom. An analysis of the VNS Patient Outcome Registry and a systematic review was performed to determine the rates and predictors of seizure freedom with VNS. Short-term seizure freedom, defined as 0–4 months after implantation, was achieved in 5.1% of patients from the registry and 2.6% from the systematic review. Response to VNS defined as >50% reduction in seizure frequency was achieved in 49% of patients in the registry and 40% from the systematic review. Long-term seizure freedom, defined as 24–48 months after implantation, was observed in 8.2% of patients from the registry and 8% from the systematic review. Long-term response was seen in 63% of patients from the registry and 60% from the systematic review. Seizure freedom was most likely achieved in patients who developed epilepsy after age 12, those who had generalized seizures, and those with nonlesional epilepsy ( ).
A new VNS generator model, AspireSR, was approved by the FDA in 2015 and incorporates a cardiac-based seizure-detection algorithm to detect ictal tachycardia and trigger stimulation. Tachycardia associated with seizure onset occurs in approximately 80% of patients with epilepsy ( ). A multicenter industry-sponsored clinical trial examined outcomes in 16 patients who developed 66 seizures while under an epilepsy monitoring unit after implantation of the AspireSR generator. Seizure severity as scored by patients showed significant improves at 3 and 6 months, quality of life (QOL) improved at 12 months, and responder rates were comparable to noncardiac-based detection generators. The safety profile of implantation and complications was not significantly different from prior VNS trials ( ).
Presently, VNS is only approved by the FDA “as an adjunctive therapy in reducing the frequency of seizures in adults and adolescents over 12 years of age with partial onset seizures which are refractory to antiepileptic medications.” However, the NCP device has been successfully used in infants and youths. Subgroup analysis of the children and adolescents treated in one of the five multicenter trials suggests that they derived substantial benefit from VNS, achieving median reductions in seizure frequency and 50% responder rates at least as favorable as those in adults. Several early, small, uncontrolled trials exclusively studying pediatric patients established the efficacy of VNS in children ( ). A total of 60 pediatric patients were treated as part of the five prospective VNS studies conducted prior to FDA approval ( ). Children of 12–18 years old were included in the double-blinded controlled trials, while patients as young as 3½ years old were studied in the open-label compassionate-use protocol. After 3 months of stimulation the median reduction in seizure frequency among these 60 patients was 23%. Using a last visit carried forward analysis, this figure improved to 31% at 6 months, 37% at 12 months, and 44% at 18 months. At 12 months the 50% responder rate was 29%. These early trials did not find any seizure subtype or etiology more responsive to VNS. Adverse events were also similar to those in adults, and none necessitated termination of therapy ( ). Since then, studies with long-term follow-up have shown the durable efficacy and safety of VNS in children. A single center reported its experience with long-term (>5 years) follow-up in children with VNS implantation. It reported seizure reduction of >50% in 9.8% at 6 months, 24% by 24 months, 46.4% by 26 months, and 54% at 5 years. Overall 62.5% had a greater than 50% reduction in seizure frequency at the last follow-up, and 19.6% became seizure free ( ). A large multicenter retrospective analysis with extended outcomes of 24 h in 347 children from Europe demonstrated similar efficacy of VNS in children. The authors reported response rates of 32.5% at 6 months, 37.6% at 12 months, and 43.8% at 24 months of the predominant seizure type ( ). Additionally they observed a dose–response correlation (total VNS charges delivered per day) and seizure control which was not found in the previous reports ( ). The American Academy of Neurology concludes that VNS may be considered in children with medication-resistant epilepsy who are not surgical candidates or who have failed surgical intervention.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here