Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The author wishes to acknowledge the contribution of <ce:bold>John </ce:bold>A. Herring for his work in the previous edition version of this chapter.
The clavicle is one of the most frequently broken bones in children, , which is not surprising given that it is the only connection between the arm and trunk and consequently is subjected to all the forces exerted on the upper limb. Fortunately, almost all clavicle fractures in children heal uneventfully with minimal or no treatment. a
a References , , , , , , , , .
The clavicle is the first bone in the body to ossify, and it has the last physis in the body to close. Initially the clavicle ossifies via intramembranous bone formation. Later, secondary ossification centers develop at its medial and lateral ends. The medial epiphysis is the last physis in the body to close, often not until the third decade of life. , , , The abundant and mobile soft tissue overlying the clavicle makes open fractures unusual. , ,
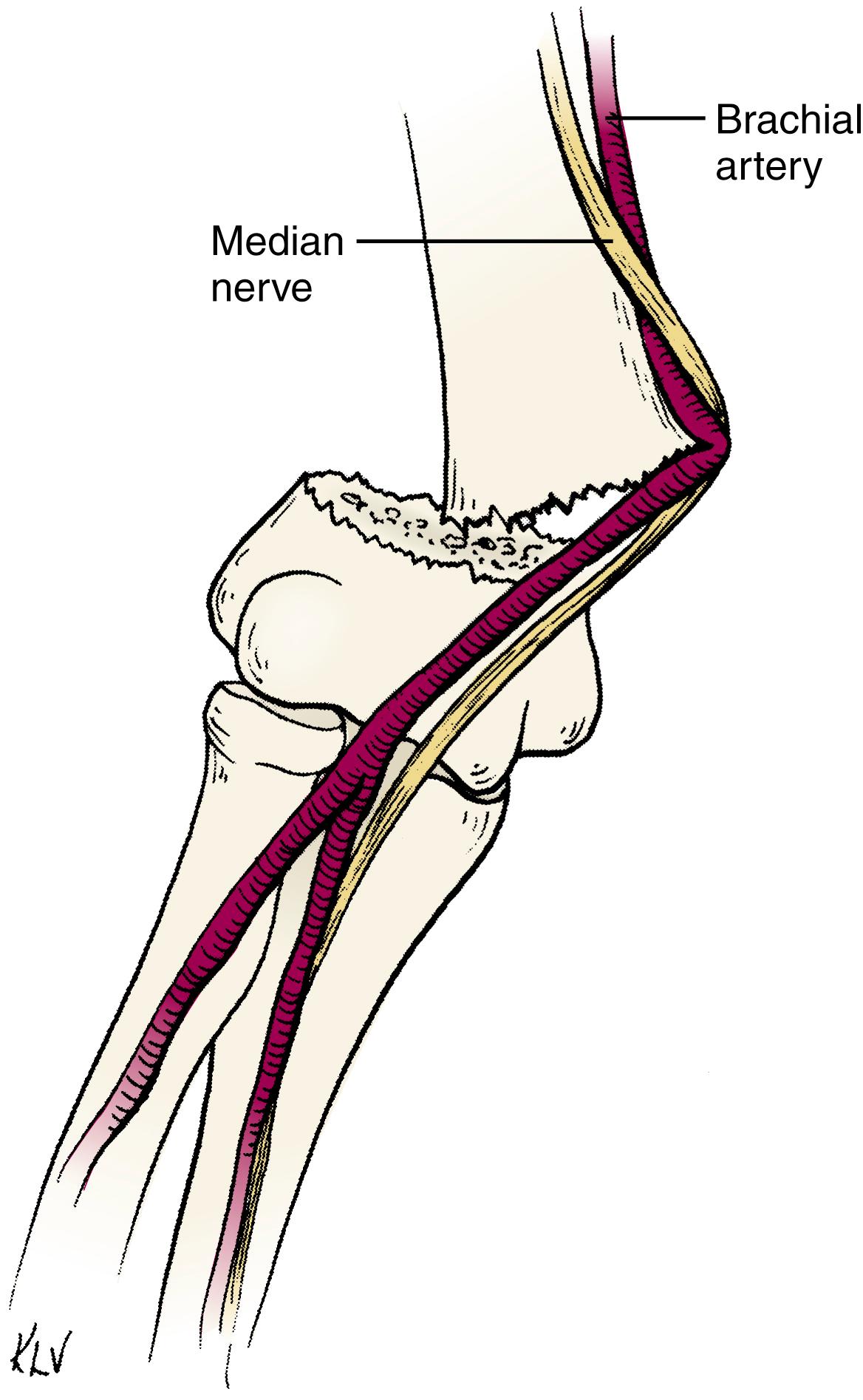
In the horizontal plane the clavicle has a double curve, convex forward in its medial two thirds and concave forward in its lateral third. Biomechanically, the point of juncture of the two curves is the weakest point. The superior surface of the clavicle is subcutaneous throughout its length. Along its inferior surface, the costoclavicular ligaments insert medially, the coracoclavicular ligaments (the conoid and trapezoid ligaments) insert laterally, and the subclavius muscle arises along the middle two thirds. , , The subclavian vessels and brachial plexus travel beneath the clavicle. In the middle third of the clavicle, the thin subclavius muscle and clavipectoral fascia are the only structures interposed between the clavicle and medial and lateral cords of the brachial plexus. Fortunately, when fractures of the midportion of the clavicle occur, the brachial plexus and subclavian vessels are protected by the thick periosteum, clavipectoral fascia, and subclavius muscle. , , ,
The physes present at the medial and lateral ends of the clavicle make true dislocation of the sternoclavicular or acromioclavicular joint a rare occurrence in children. Rather, injuries to either end of the clavicle are usually physeal separations. b
References , , , , , , , , , , , , , , .
As the medial physis does not close until between the 22nd and 25th years, most injuries to the medial clavicle in children and young adults are physeal separations, with the lateral metaphyseal fragment displaced anteriorly or posteriorly and the physeal sleeve left intact. The strong costoclavicular and sternoclavicular ligaments generally remain in continuity with the periosteal sleeve ( Fig. 29.1 ). , , , , It is important to remember the vital structures immediately posterior to the sternoclavicular joint. The innominate artery and vein, internal jugular vein, phrenic and vagus nerves, trachea, and esophagus all lie immediately posterior to the sternoclavicular joint and can be injured with posterior displacement of the clavicle (see Fig. 29.1 ). c
References , , , , , , , , , , , , , , , , , , , , , .

Injuries to the lateral clavicle are also more likely to be physeal fractures than true acromioclavicular separations. Laterally, the coracoclavicular ligaments (the conoid and trapezoid ligaments) generally remain in continuity with the periosteal sleeve and the small lateral epiphyseal fragment. d
References , , , , , , , .
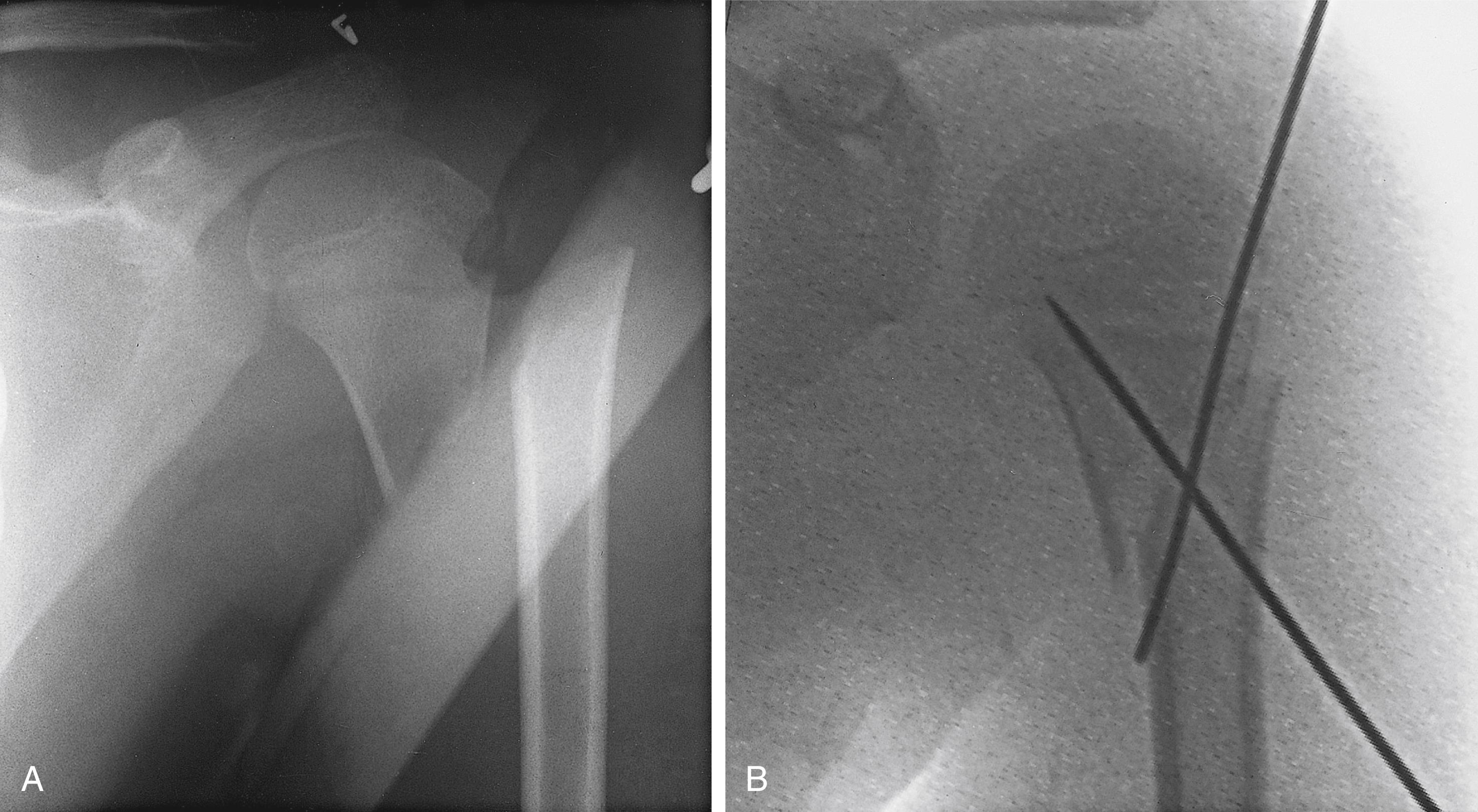
The medial metaphyseal fragment may be dramatically displaced, similar to a severe acromioclavicular separation ( Fig. 29.2 ). As these fractures heal, the intact periosteal sleeve may form a new metaphysis that results in a duplicated lateral clavicle ( Fig. 29.3 ). Growth disturbances are very rare, and 80% of the growth of the clavicle is complete by age 9 years in girls and age 12 in boys. Although uncommon, true dislocations of the sternoclavicular and acromioclavicular joints can and do occur in children. e
References , , , , , , .


In the newborn, clavicle fractures generally occur from compression of the shoulders during delivery. In children and adolescents, clavicle fractures are usually the result of a fall onto an outstretched extremity or the side of the shoulder. Child abuse is a rare cause. ,
A fractured clavicle in a newborn may be difficult to diagnose because the infant is often asymptomatic. , , In a radiographic survey of 300 newborns, 5 unsuspected clavicle fractures were discovered. Fractures during delivery usually involve the clavicle, which is most anterior in the birth canal, , but may also occur during cesarean delivery. The diagnosis is often made when the child has pseudoparalysis, or lack of active spontaneous movement of the limb. Occasionally, a birth fracture of the clavicle is accompanied by fracture of the upper humeral physis. Often this injury is not appreciated on the initial radiographs; however, on follow-up films, massive subperiosteal new bone formation will be seen and the condition may be mistaken for osteomyelitis. Fracture of the clavicle in a newborn may also be misdiagnosed as congenital muscular torticollis.
The differential diagnosis includes brachial plexus palsy and acute osteoarticular infection. It is important to remember that brachial plexus palsy and clavicle fractures may coexist. Although the clinical diagnosis of a fractured clavicle may be straightforward, assessing the status of the brachial plexus is frequently difficult. Neonatal reflexes such as the Moro and fencing reflexes may be helpful in demonstrating active upper extremity muscle function. The diagnosis of osteoarticular infection in a newborn may also be difficult to make. Often there are few systemic signs. Infection should be suspected in at-risk patients (e.g., those with indwelling catheters) or in the setting of radiographic lucencies in the metaphysis, diffuse swelling, or increasing pain. Historically, needle aspiration was used to make the diagnosis, f
References , , , , , .
but magnetic resonance imaging (MRI) is more frequently used currently.
In an infant or young child, clavicle fractures are often incomplete (greenstick) fractures. These greenstick fractures of the clavicle may escape notice until appearance of the developing callus. In these cases the fracture should not be mistaken for congenital pseudarthrosis of the clavicle, which is also painless. Radiographically, the distinction between congenital pseudarthrosis and acute fracture is straightforward. In congenital pseudarthrosis there is a wide zone of radiolucency, with smooth margins at the site of the defect and no evidence of callus formation. , , ,
Older children and adolescents usually have completely displaced fractures of the clavicle, which have a classic clinical appearance. The affected shoulder is lower than the opposite normal one and droops forward and inward. The child rests the involved arm against the body and supports it at the elbow with the opposite hand. The tension on the sternocleidomastoid muscle tilts the head toward the affected side and rotates the chin toward the opposite side ( Fig. 29.4 ). Any change in position of the upper limb or the cervical spine is painful. Local swelling, tenderness, and crepitation occur over the fracture site. In rare cases the spasm has been severe enough to result in atlantoaxial rotatory instability after a clavicular fracture.

Medial physeal separation, or pseudosubluxation, of the sternoclavicular joint may be displaced anteriorly or posteriorly. With anterior displacement of the metaphyseal fragment, the sternal end of the clavicle may be sharp and palpable immediately beneath the skin. The clavicular head of the sternocleidomastoid muscle is pulled anteriorly with the bone and is in spasm, which causes the patient’s head to tilt toward the affected side. g
References , , , , , , .
Posteromedial displacement is accompanied by local swelling, tenderness, and depression of the medial end of the clavicle. Severe posterior displacement can cause compression of the trachea and result in dyspnea or hoarseness. Posteriorly displaced fractures may also compress the subclavian vessels or brachial plexus and produce vascular insufficiency, with diminution or absence of distal pulses, paresthesias and paresis, or both. h
References , , , , , , .
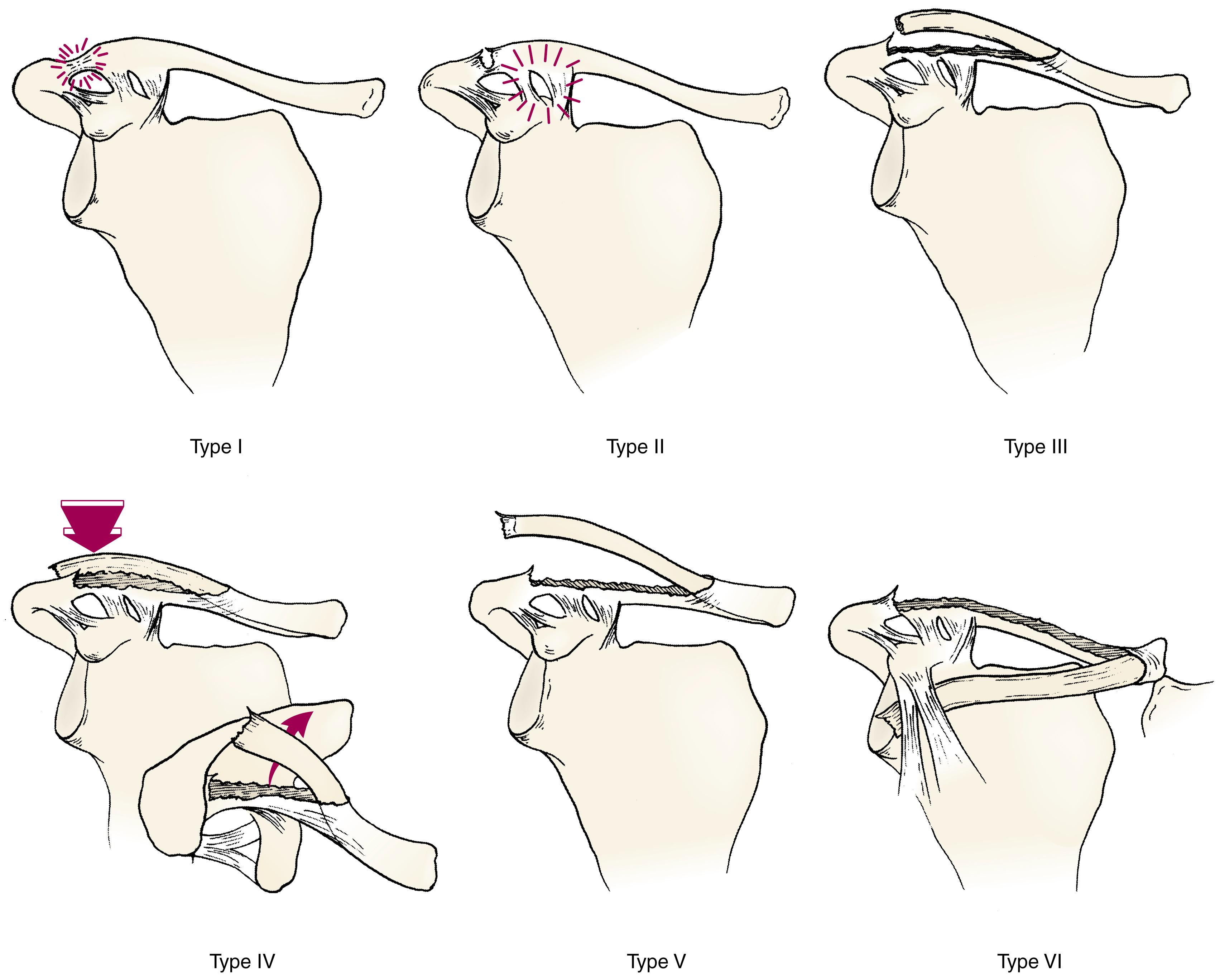
When there is separation of the lateral physis of the clavicle, the clinical findings will depend on the type of injury. Rockwood has modified the adult classification of acromioclavicular joint injuries to reflect the more common physeal fractures that occur in children ( Fig. 29.5 ). Types I and II injuries represent the classic mild acromioclavicular joint sprain. Patients with types III and V injuries have complete disruption of the acromioclavicular joint. As in type I and II injuries, there is pain with shoulder motion and point tenderness, but with more obvious deformity over the lateral clavicle. With type V injuries, the skin may be tented. The posterior displacement of type IV injuries may be difficult to appreciate unless the patient is examined from above. Patients who sustain the rare, inferiorly displaced type VI injury have a prominent acromion and severe limitation of motion. i
References , , , , , , , .
Fractures of the middle third of the clavicle will be easily identified on routine anteroposterior (AP) radiographs. Injuries to the medial end of the clavicle may be difficult to discern with simple AP radiographs. Rockwood has described the serendipity view to assess the medial end of the clavicle. This view is a 40-degree cephalic tilt, with both clavicles projected onto a chest radiograph cassette. Computed tomography (CT) can also be helpful in assessing the anatomy of the sternoclavicular region. , , Laterally, the anatomy of the acromioclavicular joint is often overpenetrated on a routine AP radiograph. A radiograph obtained with soft tissue technique and centered on the acromioclavicular joint will demonstrate pathology of the lateral clavicle. An AP radiograph obtained with a 20-degree cephalic tilt is also helpful for assessing the lateral clavicle. A stress view (AP radiograph of both clavicles obtained with the patient holding weights in each hand) can help distinguish between types I and II acromioclavicular joint injuries, although this is infrequently used in practice as the treatment for type I and II injuries is identical. , , An axillary lateral view may be required to demonstrate a type IV lateral physeal injury. ,
An asymptomatic clavicle fracture in a neonate or young infant may be treated with benign neglect. It will unite without external immobilization, and any malalignment will gradually correct with growth. Nurses and parents should be instructed to handle the infant gently and avoid direct pressure over the broken clavicle. , ,
When the fracture is painful and accompanied by pseudoparalysis, it may be necessary to swathe the arm for 1 or 2 weeks. A soft cotton pad is placed in the axilla, and the upper limb is loosely swathed across the chest with two or three turns of an elastic bandage. The parents are instructed in skin care and bathing. Within 7 to 14 days, the pain will subside, the fracture will be united clinically, and the swathe is removed. Parents should be warned about the palpable and often visible subcutaneous callus that will develop and later resolve. , ,
In children and adolescents, displaced fractures of the clavicle rarely require reduction. Malalignment and the bump of the callus will remodel and disappear within 6 to 9 months. Treatment consists of keeping the child comfortable with a figure-eight bandage or sling. Well-padded, premade, figure-eight clavicular supports are available commercially. The clavicular splints do not immobilize the fracture; their purpose is to provide patient comfort by holding the shoulders back. The fracture sling or harness is worn for 1 to 4 weeks until the pain subsides and the patient can resume normal use of the extremity. Some have suggested that pediatric clavicle fractures may not even require review by an orthopaedist.
Formal attempt at closed reduction of a displaced clavicle fracture that threatens skin integrity is no longer used in our institution. We consider this situation to be one of the rare instances that open reduction and internal fixation is indicated. While pen reduction of clavicle fractures in children has traditionally been considered to be rarely indicated, the Canadian Orthopaedic Trauma Society randomized control trial demonstrated improved patient outcomes, time to union, and decreased nonunions in displaced middle third clavicle fractures in adults treated with open reduction internal fixation (ORIF), and this has influenced increasing frequency of operative fixation in the pediatric population. , However, multiple studies have demonstrated that skeletally immature patients with clavicle malunions from nonoperative treatment do not have clinically meaningful loss of shoulder motion, do not have decreased contact or overhead sports participation, and do not have lower outcomes scores. , Generally, we consider open reduction and internal fixation of the clavicle if there is a neurovascular injury, open injury, posterior displacement with impingement of the underlying structures, and impending skin penetration by the fracture fragment. , Fixation options include a one-third tubular plate, a reconstruction plate, or a 2.7-mm plate, although anatomic plates are also used in older, larger adolescent patients. We do not use percutaneous pin fixation about the clavicle because of visceral problems associated with pin migration. j
References , , , , , , , , , .
Several reviews make a case for more frequent use of open reduction with internal fixation for older children with clavicle fractures. Vander Have reviewed 43 adolescent fractures, 25 nonoperatively treated and 17 having plate fixation for fractures displaced more than 2 cm. Shortening before treatment was 12.5 mm in the nonoperative group and 27.5 mm in the operative group. Union occurred earlier in the operative group (7.4 vs. 8.5 weeks) and return to activity was earlier in the operative group as well (12 vs. 16 weeks). Five patients in the nonoperative group had malunion, with 4 of the 5 electing corrective osteotomy. Mehlman reviewed 24 adolescent clavicle fractures treated with open reduction and internal fixation and found that 21 of 24 returned to unrestricted sports activities. Two patients reported scar sensitivity. Carry and associates surveyed Pediatric Orthopaedic Society of North America (POSNA) members ( n = 302) and found that most preferred non-operative treatment for all except the older adolescent with major displacement or angulation and for those with segmental fractures. Namdari and colleagues reviewed 14 adolescent cases treated with open reduction and internal fixation, 12 having had a trial of nonoperative treatment with increased displacement noted at 3 weeks. All healed and had high satisfaction on objective tests. However, the enthusiastic surgeon should be warned that complication rates after fixation of pediatric clavicle midshaft fractures have been reported in 21% to 86% of patients, with the majority requiring implant removal for prominence as well as anterior chest wall numbness, wound dehiscence or infection, skin breakage, fracture adjacent to the plate, refracture after plate removal, flexible nail deformation, flexible nail breakage, and nonunion. , ,
Because the physeal sleeve remains intact, a significant amount of remodeling can be expected with medial physeal injuries, and consequently conservative treatment is the rule. Patients with anterior displacement and those with posterior displacement without evidence of visceral injury to the mediastinal structures on CT scan can be managed symptomatically with a sling or figure-eight harness.
If there is a significant cosmetic deformity, we may attempt closed reduction, which frequently achieves stability. If the reduction is lost, we generally accept the deformity and anticipate significant remodeling. If there is posterior displacement with evidence of airway, esophageal, or neurovascular impingement, we will attempt closed reduction on an emergency basis in the operating room. If closed reduction fails, we proceed immediately to open reduction, preferably with the availability of a general trauma or thoracic surgeon. , , ,
Sutures are preferred for fixation because evaluation of the underlying structures by MRI may be impeded by metallic implants. The long-term outcome after reduction is excellent.
Anesthesia is achieved with conscious sedation techniques or hematoma block. The patient is placed supine, with a bolster between the scapulae. An assistant applies longitudinal traction to both upper extremities, and gentle posterior pressure is applied to the displaced medial metaphyseal fragment to obtain reduction. The displaced medial fragment may be grasped with a towel clip to help facilitate reduction. As noted, if reduction cannot be achieved or the reduction is unstable, we generally accept the deformity, with the knowledge that significant remodeling almost always occurs. , ,
If the metaphyseal fragment is displaced posteriorly with evidence of compression of the mediastinal structures, we first attempt closed reduction under general anesthesia. The patient is placed supine with a bolster between the shoulder blades. Longitudinal traction is applied to the arm, with the shoulder adducted. A posteriorly directed force is applied to the shoulder while the medial end of the clavicle is grasped with a towel clip in an effort to bring the metaphyseal fragment anteriorly. If closed reduction fails, we proceed to open reduction with repair of the sternoclavicular and costoclavicular ligaments. Patients with minimal posterior displacement can be managed symptomatically with a sling or harness. Closed reduction is contraindicated in late presenting posteriorly displaced fractures, as the fracture may be adherent to underlying vascular structures in the mediastinum.
Treatment depends on the degree of injury to the joint. All types I and II injuries and type III injuries in patients younger than 15 or 16 years can be managed symptomatically with a sling or harness until the patient can use the extremity comfortably. k
References , , , , , , , , .
Types IV, V, and VI injuries usually require open reduction. , , Frequently, fixation can be achieved by repairing the periosteal sleeve. Again, we avoid the use of percutaneous pins in the clavicle because of well-documented problems with migration. l
References , , , , , .
Neurovascular complications are extremely rare. They are usually the result of direct force or comminuted fracture. Laceration of the subclavian artery or vein can occur, although the thick periosteum generally protects the vessels from damage. The presence of a subclavian vessel laceration is suggested by the development of a large, rapidly increasing hematoma. Surgical intervention for repair of the torn vessel should take place immediately because the patient may die of extravasation. , , , Subclavian vein compression after a greenstick fracture of the clavicle with inferior bowing has been reported in a child. Venous congestion and swelling of the involved extremity suggest such a complication.
Nonunion in nonsurgically displaced adolescent clavicle fractures is uncommon and may be associated with prior fractures of the same clavicle. If nonunion develops, open reduction plus internal fixation with bone grafting has been shown to yield excellent results. , ,
Acute atlantoaxial rotatory displacement has been reported as a complication of clavicular fractures. The diagnosis may be missed if the orthopaedist inappropriately relates the torticollis to a clavicular fracture.
The scapula is a thin triangular bone attached to the clavicle by the acromioclavicular joint, coracoclavicular ligaments, and multiple muscular attachments. The flexibility of the attachment of the scapula to the torso and thick muscular envelope on its anterior and posterior surface make it resistant to fracture. When pediatric scapular injuries do occur, they are, as in adults, generally the result of high-energy trauma. ,
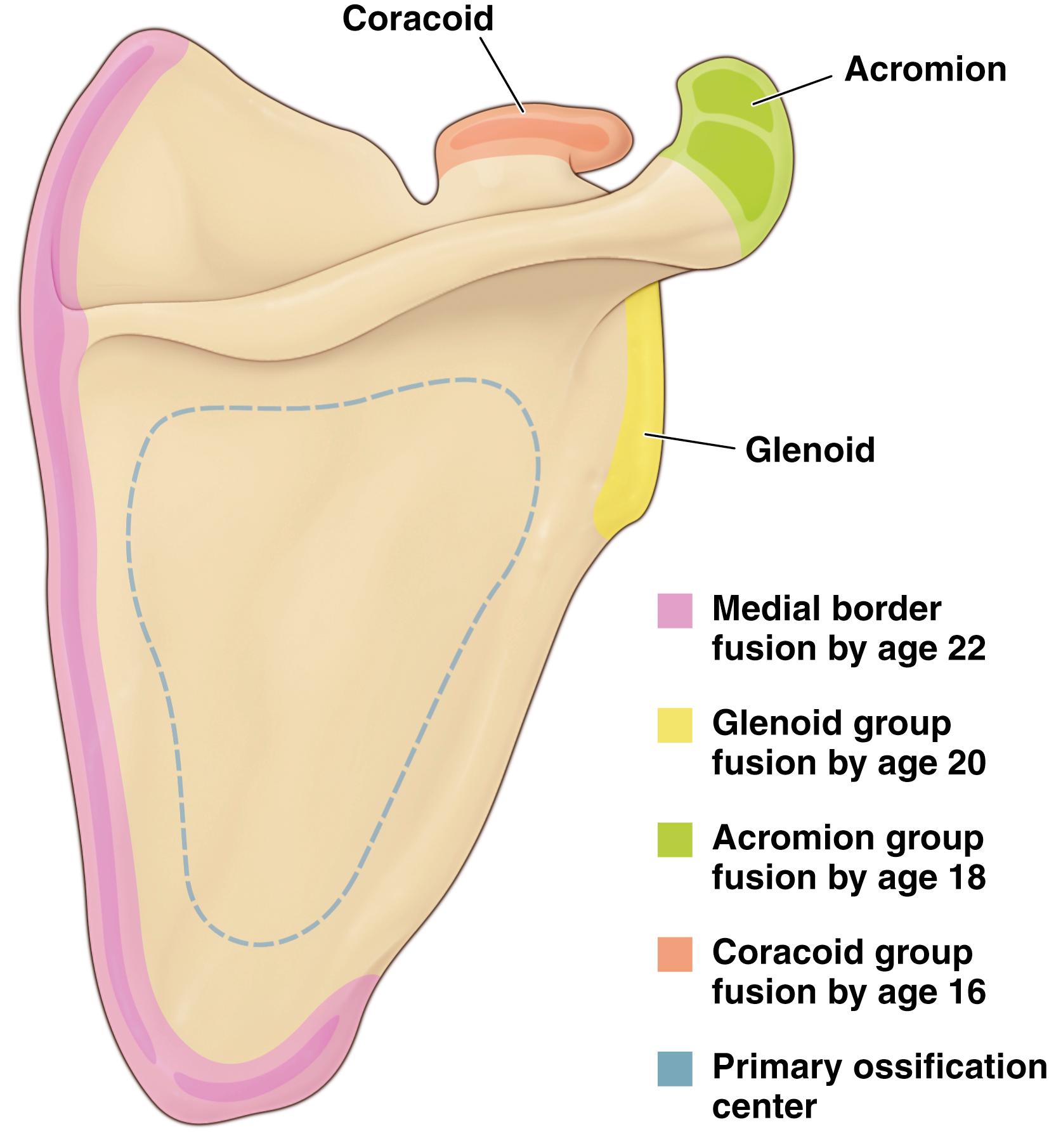
Scapular fractures may occur in the body, spine, neck, glenoid, acromion, or coracoid. The scapula contains at least eight secondary ossification centers—one at the inferior margin of the body, one along the vertebral border, one at the inferior glenoid, two for the acromion, two for the coracoid process, and a bipolar physis between the coracoid and body ( Fig 29.6 ). As in all physes, the zone of provisional calcification is a weak link, and avulsion fractures are likely to occur at these growth centers, particularly in adolescents. It is also important to be aware of these ossification centers so that they are not mistaken for injuries.
Fractures of the scapular body are often comminuted, with fracture lines running in multiple directions. The spine of the scapula may also be fractured with the body. (The infraspinous portion is more frequently fractured than the supraspinous portion.) The abundant muscular envelope and thick periosteum in children generally prevents significant displacement of scapular body fractures. , ,
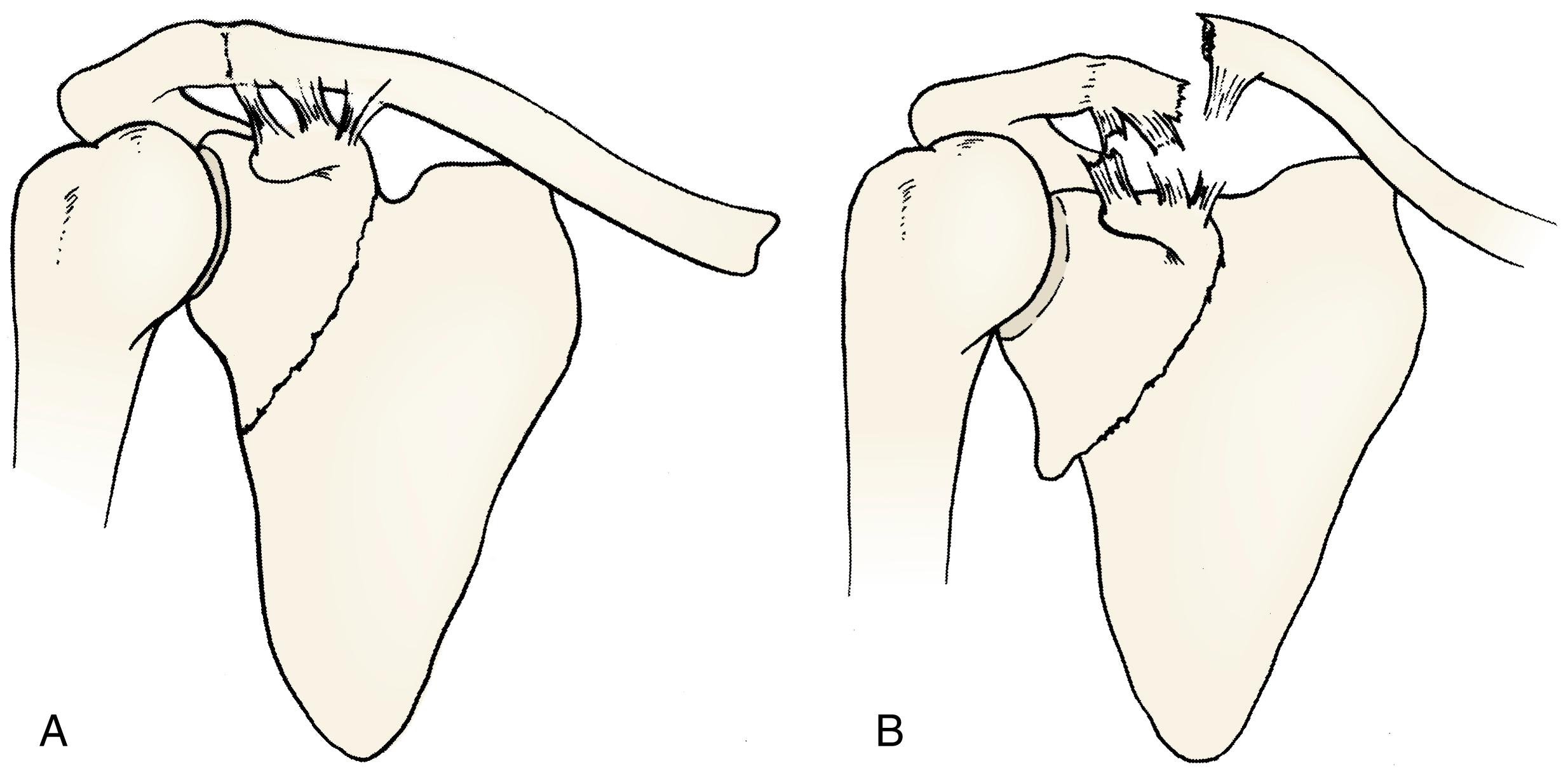
Fractures of the neck of the scapula usually begin in the suprascapular notch and run inferior laterally to the axillary border of the scapula. The capsular attachments of the glenohumeral joint and articular surface of the glenoid remain intact. If the coracoclavicular and acromioclavicular ligaments are intact, there is little if any displacement of the articular fragment; however, if these ligaments are torn or if the fracture line is lateral to the coracoid process, the articular fragment is displaced downward and inward by the weight of the limb ( Fig. 29.7 ).
Scapular fractures are usually the result of high-energy trauma, such as a crush injury in an automobile accident or a fall from a height. Fractures of the glenoid or acromion may result from direct trauma or force transmitted through the humeral head. In younger children, scapular fractures are frequently the result of child abuse. Fractures of the inferior rim of the glenoid may also result from eccentric contraction of the long head of the biceps. Similarly, fractures of the coracoid may be caused by direct injury or an eccentric contraction of the short head of the biceps and coracobrachialis muscles.
As in adults, the high energy required to produce pediatric scapular injuries may also result in significant injury to adjacent structures. Thus scapular fractures are frequently associated with rib or clavicle fractures, pulmonary contusions, pneumothorax, cervical and thoracic vertebral fractures, or fractures involving the humerus, and pediatric patients with high-energy scapula fractures have increased Injury Severity Scores compared to a cohort of high-energy trauma patients without scapula fractures.
The diagnosis of scapular fractures is frequently delayed or missed because of the significance of associated injuries. This difficulty is compounded by the fact that the scapula is projected obliquely on an AP chest radiograph, often the only radiograph of the scapula obtained in a polytraumatized patient. Thus to make a timely and accurate diagnosis, scapular fractures must be considered in any patient who sustains significant direct trauma to the upper thorax or proximal part of the upper extremity. m
References , , , , , .
To see the fracture, it is often necessary to obtain a true AP radiograph of the scapula ( Fig. 29.8 ). CT scans will also demonstrate the injury clearly. ,
Fortunately, the vast majority of scapular fractures can be managed conservatively. In general, management is directed toward patient comfort. Most patients do well with minimal immobilization in a sling or a sling and swath or shoulder immobilizer. Gentle range-of-motion (ROM) exercises can usually be started in the second week after injury, with progression to full use of the upper extremity, as tolerated.
Although few studies of the surgical management of scapular fractures have dealt with injuries in children and little can be definitively stated regarding operative indications, we believe that, similar to operative indications in adults, significantly displaced intraarticular fractures, as well as glenoid rim fractures associated with subluxation of the humeral head, require open reduction and internal fixation. n
References , , , , , .
Additionally, consideration should be given to operative stabilization of unstable fractures through the scapular neck, including ipsilateral fractures of the neck and clavicle and displaced fractures involving the scapular spine and neck. However, not all floating shoulder injuries require operative treatment, and reports of adults confirm that many do well with nonoperative treatment.
Complications from scapular fractures are rare. The most frequent problems encountered with scapular fractures are often related to associated injuries or a delay in diagnosis. , ,
Problems related to malunion or nonunion are uncommon. , , Untreated fractures of the glenoid can result in glenohumeral instability. Malunion of acromion fractures can result in symptomatic impingement. Coracoid fractures, however, have been reported to do well, even if they result in fibrous nonunion. o
References , , , , , , , , , .
As in adults, pediatric scapulothoracic dissociation is a rare injury that is usually the result of a massive traction injury to the upper extremity. , , It represents a traumatic forequarter amputation and is almost universally associated with major neurovascular injury. Radiographically, lateral displacement of the scapula is noted on an AP chest radiograph. Patients frequently have other life- or limb-threatening injuries, and recognition of the extent of damage to the upper extremity may be delayed, with devastating consequences. Death has been reported in 10% to 20% of adult patients. Patients almost universally have a poor result, with a functionless extremity. p
References , , , , , .
Sampson and colleagues noted that if the extremity is viable, attempts at vascular repair are not warranted and do not result in a functional extremity.
An os acromiale represents failure of the apophysis of the acromion to close. Although considered a normal variant that is present in almost 10% of shoulders, os acromiale is occasionally symptomatic. It has been shown to be associated with pathology of the rotator cuff in some cases. Symptomatic os acromiale has been successfully treated with internal fixation and bone grafting, as well as arthroscopic subacromial decompression of the unstable fragment. , ,
Fractures of the proximal humeral physis make up approximately 3% of all physeal injuries. They may occur in children of any age but are most common in adolescents. These fractures are almost exclusively Salter-Harris type I or II injuries and are most notable for their tremendous potential to remodel. This remodeling potential is a result of the universal motion of the glenohumeral joint (Wolfe’s law) and the fact that approximately 80% of the growth of the humerus comes from its proximal physis ( Fig. 29.9 ; see Chapter 26 ). q
References , , , , , , .

The proximal humeral epiphysis develops from three secondary ossification centers—one each for the humeral head, greater tuberosity, and lesser tuberosity. The secondary ossification center for the humeral head usually appears between the ages of 4 and 6 months, although it may be present before birth. These three ossification centers coalesce into a single large center at approximately 7 years of age ( Fig. 29.10 ).

The physis of the proximal humerus is concave inferiorly. Medially, it follows the line of the anatomic neck. Laterally, it extends distal to the inferior border of the greater tuberosity. The timing of closure of the proximal humeral physis is variable, with closure occurring as early as 14 years in some girls and as late as 22 years in males.
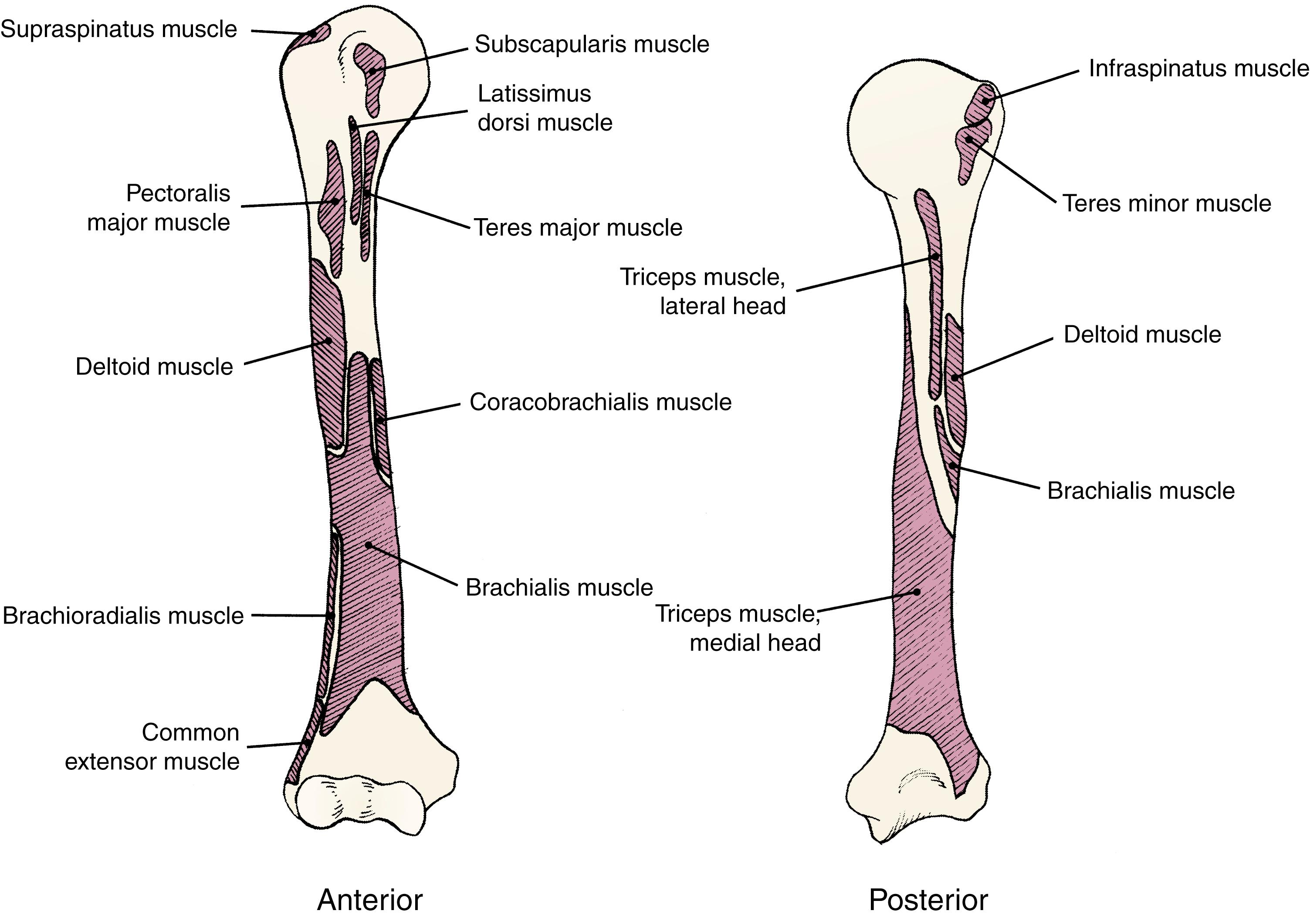
The supraspinatus, infraspinatus, and teres minor muscles insert onto the greater tuberosity, and the subscapularis inserts on the lesser tuberosity. At the metadiaphyseal junction, the pectoralis major tendon inserts onto the crest of the greater tuberosity, and the teres major attaches to the inferior crest of the lesser tuberosity. The latissimus dorsi arises from the floor of the intertubercular groove.
Dameron and Reibel performed a cadaveric study of the proximal humeri of 12 stillborn infants in an effort to explain the anatomic basis for the displacement of proximal humeral fractures. They found that it was difficult to displace the proximal metaphysis posteriorly but, with the arm extended and adducted, relatively easy to displace it anteriorly. They noted that the periosteum consistently tore just lateral to the biceps tendon and the stability of the fracture decreased as the periosteal stripping progressed. They attributed the preference for anterior displacement to the asymmetric dome of the proximal humeral physis, with its posteromedial apex, and to the stronger attachment of the periosteum to the posterior surface of the metaphysis. They noted that all 12 humeri fractured through the physis without an attached fragment of metaphyseal bone.
Fractures involving the proximal humeral physis can result from an indirect force extended through the humeral shaft, such as a fall on an outstretched hand, or from a direct blow to the lateral aspect of the shoulder. Neer and Horwitz attributed 59 of their 89 fractures of the proximal humerus to a direct force, usually applied to the posterolateral aspect of the shoulder. Neonates may sustain proximal humeral fractures as a result of birth trauma. Proximal humeral fractures in infants may be associated with child abuse.
Proximal humeral physeal fractures are generally classified according to the type of physeal injury, amount of displacement, or both. Generally, infants and small children with proximal humeral physeal injuries have Salter-Harris type I fractures, whereas older children and adolescents have Salter-Harris type II injuries. The universal motion of the glenohumeral joint makes the proximal fragment resistant to injury. Thus fractures extending through the proximal segment (Salter-Harris type III or IV injuries) or physeal fractures combined with dislocation of the glenohumeral joint are rare. However, these injuries have been described, and it is important to assess adequate radiographs carefully to ensure that no unusual occult injuries are present. r
References , , , , , , .
Neer and Horwitz used the amount of displacement to classify proximal humeral physeal fractures. In grade I injuries, there is less than 5 mm of displacement. Grade II injuries are displaced between 5 mm and one third the diameter of the humeral shaft. Grade III injuries are displaced between one and two thirds the diameter of the shaft, and grade IV fractures are displaced more than two-thirds the diameter of the humeral shaft. In grades III and IV displacement, there is always a varying degree of angulation.
Fracture of the proximal humeral physis should be the first diagnosis considered in injuries to the shoulder region in children between the ages of 9 and 15 years. If the fracture is displaced, the initial findings can be dramatic. The arm is often shortened and held in abduction and extension. The displaced distal fragment causes a prominence in the front of the axilla, near the coracoid process. Frequently, the anterior axillary fold is distorted, with a characteristic puckering of the skin caused by the distal fragment. The humeral head may be palpable in its normal position. With minimally displaced fractures, the physical findings may be limited to localized swelling and tenderness.
In displaced fractures, the epiphysis usually remains in the glenoid fossa but is abducted and externally rotated by the pull of the attached rotator cuff. The distal fragment is displaced anteromedially by the combined action of the pectoralis major, latissimus dorsi, and teres major muscles ( Fig. 29.11 ). The intact periosteum on the posteromedial aspect of the metaphysis prevents complete displacement and often makes closed reduction difficult. This intact periosteum also serves as a mold for the callus and later for the new bone produced by the physis (see Fig. 29.9 ). Occasionally, the fracture is impacted, with the upper end of the metaphysis driven into the epiphysis.

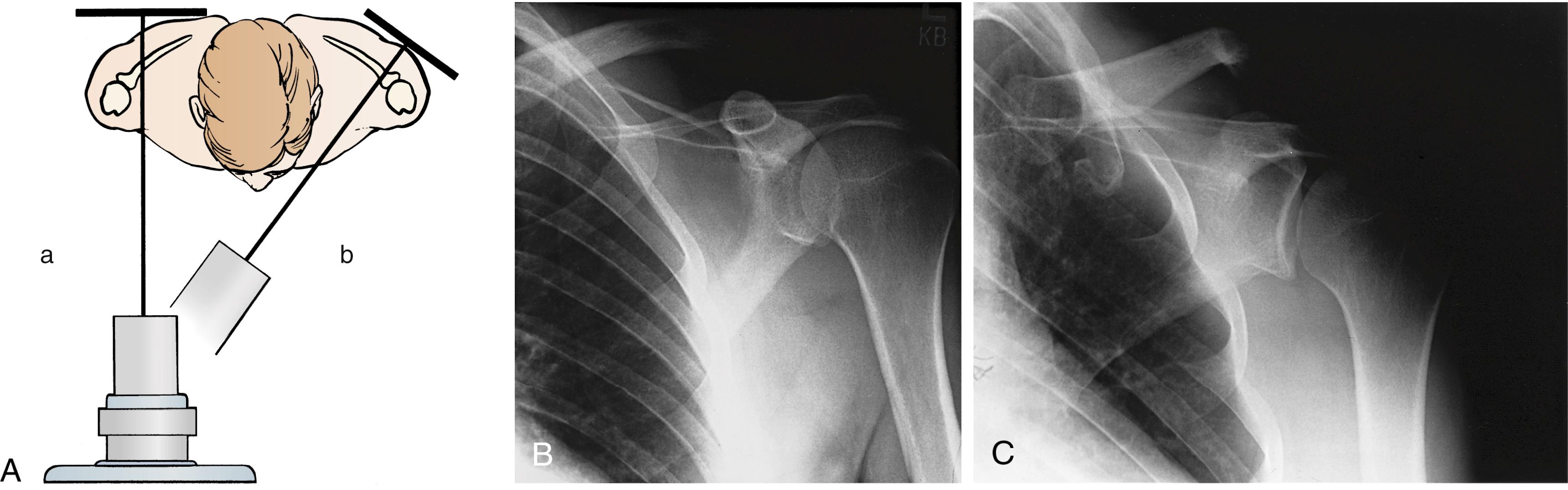
When assessing trauma about the shoulder, it is imperative to obtain two orthogonal radiographs to assess the glenohumeral joint adequately. Often this is difficult because the limb is painful and the patient and radiology technician are resistant to moving the extremity. It is incumbent on the treating surgeon to educate the radiology technician on the importance of obtaining a true AP view of the glenohumeral joint (rather than the torso; see Fig. 29.8 ) and positioning the arm in limited abduction to obtain an axillary lateral view of the proximal humerus. Alternatively, a Y-scapular view can be used to assess the status of the glenohumeral joint, although it is generally more difficult to obtain and interpret this radiograph than to obtain and interpret an axillary lateral view ( Fig. 29.12 ).

The differential diagnosis of a proximal humeral fracture in a neonate or infant includes septic arthritis, osteomyelitis, and brachial plexus palsy. Radiographs of the proximal humerus may be of little help in distinguishing among these entities because much of the anatomy is nonossified cartilage. Ultrasound has proved useful in these cases; it can easily demonstrate proximal humeral fractures and confirm reduction of the glenohumeral joint and the presence or absence of an intraarticular effusion. , ,
Historically, the vast majority of proximal humeral physeal fractures were treated nonoperatively, regardless of the age of the patient or degree of displacement. s
References , , , , , , , , , .
More recent reports demonstrate increasing popularity of surgical treatment, especially in older adolescents as well as Neer-Horwitz type III and IV fracture patterns.
Injuries with grades I and II displacement can be treated symptomatically without an attempt at reduction, regardless of the age of the patient. A simple arm sling or sling and swath or a hook and loop shoulder immobilizer should be worn until the pain subsides. Gentle pendulum exercises can be instituted in the second week, and most patients can resume some overhead activities within 4 to 6 weeks.
Indications for the treatment of more displaced proximal humeral physeal fractures (grades III and IV injuries) are controversial. Almost all researchers agree that displaced injuries in younger children (<6 years of age) can be treated symptomatically. t
References , , , , , , and .
Controversy exists about the management of displaced fractures in older patients. Some have advocated open reduction of severely displaced fractures in older children, noting that open reduction is justified on the basis of intraoperative findings of impediments to closed reduction, which include an infolded periosteum, interposed biceps tendon, deltoid muscle, and/or bone fragments. u
References , , , , , , .
Interestingly, in a review of 48 patients with displaced proximal humeral fractures (all grades III and IV), Beringer and colleagues reported complications in three of the nine operatively treated patients, whereas none developed in the 39 patients treated by closed reduction. Complications of operative treatment included fracture through a percutaneous pin site, symptomatic impingement requiring hardware removal, and osteomyelitis necessitating four operative debridement procedures. They further explored the functional results by comparing patients who maintained acceptable reduction with those in whom acceptable closed reduction could not be obtained or could not be maintained. No patient in either group had a functional deficit. Patients older than 15 years treated with closed treatment had no functional limitations and there were no significant differences between patients with an acceptable reduction and those with persistent malposition. However, they did note an increased prevalence of minor abnormalities in patients with persistent malposition, although these differences were not functionally or cosmetically significant.
A more recent study compared a small matched cohort of pediatric proximal humeral physeal fractures treated operatively and nonoperatively and found no difference in complications, rate of return to activity, or cosmetic satisfaction. QuickDASH scores tended to be slightly higher in those treated nonoperatively, but less desirable outcomes were more common in patients older than 12 years.
Despite these excellent results with conservative , , treatment, a number of reports have advocated surgical treatment. Hutchinson and co-workers reviewed 50 cases, most with grade IV injuries, treated with closed or open reduction and fixed with percutaneous pins or intramedullary nails. The final Neer grades were improved and angulation was reduced from 44.4 to 12.6 degrees. Pin tract infection and pin migration occurred in 40% of those treated with pins. The intramedullary nail cases had greater blood loss and longer operative times but no significant complications. Their general indications for treatment were grade IV displacement in patients 12 years of age or older. Fernandez and associates noted complications with intramedullary nails, including perforation through the humeral head, postoperative loss of reduction, and difficulties with nail removal.
Brachial plexus and major peripheral nerve palsies occasionally accompany proximal humerus fractures. Hwang and co-workers reported four patients with major nerve palsies, all of whom recovered slowly but had return of function between 5 and 9 months. All had neuropathic pain for at least 6 months after injury.
Our approach to the treatment of displaced proximal humeral physeal fractures parallels the recommendations of Beringer and colleagues. We attempt closed reduction under conscious sedation in the emergency department in all patients with grades III and IV displacement. Although these fractures generally reduce easily, the reduction is not always stable enough to be maintained ( Fig. 29.13 ). Thus, in younger patients, who have tremendous remodeling potential, we believe that the benefits of a stable closed reduction, primarily less pain and less immediate cosmetic deformity, must be weighed against the risks associated with conscious sedation in patients, regardless of age. The technique of closed reduction usually includes traction, abduction, forward flexion, and external rotation of the arm and forearm. Fluoroscopic guidance can be helpful during reduction, particularly if there is atypical displacement of the fracture. Once stable reduction has been achieved, the extremity is placed in a sling and swath or in a hanging arm cast for 2 to 3 weeks until the fracture fragments are sticky. At that point the immobilization can be discontinued and ROM exercises instituted.

In patients in whom reduction can be achieved but is lost once the traction or abduction is removed, and in patients in whom we cannot obtain adequate closed reduction, we generally accept the deformity in skeletally immature patients, and patients are managed symptomatically. The parents of these patients usually need a fair amount of reassurance that remodeling will provide an acceptable cosmetic and functional result.
Our indications for operative treatment of displaced proximal humeral fractures include patients with intraarticular fractures, open fracture, neurovascular injury, and those nearing skeletal maturity with Neer Horowitz grade IV fractures. A polytraumatized patient may have a proximal humeral fracture stabilized percutaneously because we believe that a stabilized extremity is easier to care for in an intensive care unit setting. Although rarer, avulsion fractures of the lesser tuberosity, which may be manifested as chronic shoulder pain without a definite injury, are another injury that could benefit from open reduction and internal fixation.
Intraarticular fractures require anatomic reduction, which can generally be performed through an anterior arthrotomy via a standard deltopectoral approach. Fixation can be achieved with a combination of screws and percutaneous pins. Every effort should be made to avoid crossing the physis with threaded fixation devices. Our goal for the operative treatment of nonarticular fractures is stabilization of the fracture to allow adequate management of concurrent injuries, whether they are neurovascular, soft tissue, or multiorgan injuries. We do not insist on anatomic reduction, and we usually stabilize the fracture with two percutaneous 0.062-inch Kirschner wires (K-wires; Fig. 29.14 ). We remove the K-wires after 2 to 3 weeks and limit motion of the extremity while they are in place in an attempt to minimize soft tissue complications. As with nonoperative treatment, ROM exercises are begun as soon as all percutaneous pins are removed and the patient is comfortable, generally in 2 to 3 weeks.
Complications of proximal humeral physeal fractures are rare. The complication reported most often is shortening of the humerus. This complication is rarely a functional or cosmetic concern and is noted more frequently in older children with more severely displaced fractures. Neer and Horwitz noted inequality of humeral length in 11% of patients with grade I or II displacement and approximately 33% of patients with grade III or IV displacement. No patient had shortening more than 3 cm, and inequality was seen only in patients older than 11 years at the time of injury. Baxter and Wiley noted shortening more than 1 cm in 9 of 30 patients. No patient had more than 2 cm of shortening, and none of their patients was clinically aware of the inequality. Unlike Neer and Horwitz, they noted shortening in patients younger than 11 years of age. Beringer and colleagues reported shortening more than 2 cm in 5 of 18 patients treated conservatively and available for review an average of 4 years after the injury. Again, none of these patients had a functional complaint.
Varus malalignment of the proximal humerus has also been reported as a complication of proximal humeral epiphyseal fractures. Like shortening, this complication is rarely a functional concern and is usually noted as an incidental finding at follow-up. There have been cases reported of severe varus combined with shortening that caused significant functional deficits. This complication is rare and probably represents an infantile fracture complicated by growth arrest.
Injuries to the brachial plexus and axillary nerve, as well as brachial artery disruption, valgus malalignment, and osteonecrosis of the humeral head, have been reported as rare or unusual complications of proximal humeral fractures.
Traumatic glenohumeral dislocation is an unusual injury in children; it usually occurs in older adolescents involved in contact sports.
It is important to distinguish traumatic dislocation from atraumatic or voluntary dislocation or subluxation because treatment of these conditions is vastly different.
The glenohumeral joint is one of the most mobile joints of the musculoskeletal system. Although its unique anatomic features give it almost universal motion, they do so at the expense of bony stability. Rather, shoulder stability is provided entirely by the muscles and ligaments that suspend the humerus from the glenoid. , ,
The muscles of the rotator cuff—supraspinatus, infraspinatus, teres minor, and subscapularis—provide dynamic stability to the shoulder, whereas the capsule and ligamentous complex provide static support. The shoulder capsule has about twice the surface area of the humeral head. The capsule extends from the glenoid neck and labrum to the anatomic neck of the humerus. Medially, the capsule extends distally past the physis and inserts on the proximal humeral metaphysis. The inner surface of the capsule is thickened into the anterior glenohumeral ligaments. The most important of these is the anteroinferior glenohumeral ligament, which is the most common site of pathology in anterior shoulder instability. v
References , , , , , , , .
With traumatic anterior dislocation of the humeral head, the inferior glenohumeral ligament and anterior labrum are usually traumatically disrupted. Although repair of this essential lesion was first described by Broca and Hartman, as well as Perthes, it was popularized by Bankart and is commonly termed a Bankart lesion (or Bankart repair ). When displaced anteriorly, the posterior aspect of the humeral head lies against the anterior glenoid, potentially producing a defect in the humeral head, the so-called Hill-Sachs lesion. With posterior dislocation, defects can be found on the anterior aspect of the humeral head ( Fig. 29.15 ). , ,

Traumatic shoulder dislocation usually occurs as a result of an indirect force. Anterior dislocations represent more than 90% of glenohumeral dislocations. Anterior dislocation usually occurs when a force is applied to an arm in an abducted, extended, and externally rotated position. Traumatic shoulder dislocations may also occur posteriorly or inferiorly. Posterior dislocations may be the result of a direct blow to the anterior aspect of the shoulder, an indirect force with the arm in flexion, adduction, and internal rotation, or a massive muscle contraction, as occurs with an electrical shock or seizure. w
References , , , , , , .
Inferior glenohumeral dislocation is also known as luxatio erecta. When seen in children or adolescents, luxatio erecta is almost always the result of a high-energy hyperabduction force. , ,
Traumatic dislocation of the glenohumeral joint generally results in a fixed dislocation that is usually acutely painful. With anterior dislocation, the arm is typically held in slight abduction and external rotation. Attempts to move the arm are often extremely painful because of the muscle spasm that occurs in an attempt to stabilize the joint. The humeral head is palpable anteriorly, and there is a defect inferior to the acromion. Occasionally, patients with recurrent anterior dislocations spontaneously reduce the dislocation, although care must be taken to distinguish these patients from those who voluntarily dislocate their shoulders, because the latter have a high incidence of psychological problems. It is important to distinguish a psychological voluntary dislocator from a patient who can voluntarily demonstrate the instability but whose primary problem is painful involuntary dislocation.
Historically, posterior dislocation of the glenohumeral joint has been a frequently missed diagnosis. Rowe and Zarins reported that 11 of 14 posterior shoulder dislocations were not recognized by the initial treating physician. However, careful physical examination of a patient with a posterior dislocation will reveal several characteristic findings. The arm is usually held in adduction and internal rotation and has limited and painful external rotation and abduction. Also, the shoulder will be flattened anteriorly and have a prominent coracoid process and posterior appearance.
Patients with luxatio erecta hold the arm maximally abducted adjacent to the head. The force of the injury may drive the humeral head through the soft tissues of the axilla and produce an open injury. x
References , , , , , .
The diagnosis of glenohumeral dislocation is often obvious on the basis of the physical examination alone and is simply confirmed radiographically. The high rate of missed diagnoses of posterior dislocations may be the result of the almost normal appearance of a posterior dislocation of the shoulder on an AP radiograph of the torso. This emphasizes the importance of high-quality orthogonal radiographs, as discussed earlier for fractures of the proximal humeral physis (see Figs. 29.8 and 29.12 ).
Every patient with a traumatic glenohumeral dislocation should undergo a complete neurovascular examination, including assessment of the radial, median, ulnar, musculoskeletal, and axillary nerves. The axillary nerve is the most commonly injured nerve with anterior dislocation. Often the pain associated with an acute shoulder dislocation makes assessment of deltoid muscle function difficult. Thus it is important to assess the sensory distribution of the axillary nerve in all patients with anterior shoulder dislocations ( Fig. 29.16 ).

Acute traumatic dislocation of the glenohumeral joint should be reduced as quickly and atraumatically as possible. There are numerous techniques for reduction, with descriptions dating to ancient times. , , We prefer closed reduction with the traction-countertraction technique performed under conscious sedation. A sheet is placed around the affected axilla to allow an assistant to apply countertraction. Once adequate sedation has been achieved, longitudinal traction is applied through the arm and forearm, with the arm abducted and elbow flexed. Gentle internal and external rotation will help disengage the humeral head. Eventually, the spastic muscles will be fatigued and reduction can be achieved. This technique is effective for anterior and posterior dislocations ( Fig. 29.17 ). Another technique that requires no assistant and is applicable to the training room setting is a modification of the technique described by Stimson. The patient is placed prone, with the affected extremity dangling over the edge of the table. With adequate relaxation and time, the shoulder will reduce. Reduction can be facilitated by adding weights to the wrist; the amount of weight depends on the size of the patient. We generally start with approximately 5 lb in an athletic adolescent ( Fig. 29.18 ).


Postreduction management consists of a careful repeat neurovascular examination, orthogonal radiographs, and sling immobilization. We generally treat patients sympto-matically after reduction, with a sling used for immobilization until upper extremity function can resume, usually in 2 to 3 weeks. Although children and adolescents with traumatic dislocation of the glenohumeral joint are at high risk for recurrence, little evidence has shown that prolonged postreduction immobilization alters the natural history of posttraumatic instability. Operative treatment is reserved for patients with open dislocations, unreducible dislocations, and intraarticular fractures.
The most common complication of traumatic dislocation of the shoulder is recurrent shoulder instability (see Sports chapter). Other rare but reported complications include fractures, neurovascular injuries and, rarely, osteonecrosis of the humeral head. y
References , , , , , , , .
Fractures of the glenoid or humeral head were discussed earlier. In general, intraarticular fractures require open reduction and internal fixation. z
References , , , , , , .
Fractures of the proximal metaphysis and shaft of the humerus are generally straightforward. Fractures of the proximal humeral metaphysis are more common in children than adolescents because adolescents are more likely to sustain physeal injuries. Humeral shaft fractures are the second most frequently occurring birth fracture. Fractures of the humeral shaft are less common in children than in adults but, as in adults, are frequently associated with radial nerve injury.
The humerus is cylindric proximally and becomes broad and flat in its distal metaphysis. The deltoid, biceps brachii, and brachialis muscles cover it anteriorly. The coracobrachialis muscle inserts beneath the upper half of the biceps brachii muscle. The pectoralis major inserts into the lateral lip of the bicipital groove. The posterior surface is covered by the deltoid and triceps muscles ( Fig. 29.19 ). On the lateral and medial aspects of the humerus, intermuscular septa divide the arm into anterior and posterior compartments. Anteriorly, the neurovascular bundle, which consists of the brachial vessels and median, musculocutaneous, and ulnar nerves, courses along the medial aspect of the humerus. The radial nerve lies in the posterior compartment in a shallow groove between the origins of the medial and lateral heads of the triceps. The radial nerve runs obliquely downward and laterally as it passes from the axilla to the anterolateral epicondylar region. aa
References , , , , , , , .
Fracture angulation depends on whether the fracture is proximal or distal to the insertion of the deltoid. When the fracture is distal to the deltoid insertion, the action of the supraspinatus, deltoid, and coracobrachialis muscles displaces the proximal fragment laterally and anteriorly, whereas the distal fragment is drawn upward by the biceps and brachialis muscles. If the fracture occurs proximally to the insertion of the deltoid but distally to that of the pectoralis major, the pull of the deltoid will displace the distal fragment laterally and upward, whereas the pectoralis major, latissimus dorsi, and teres major muscles will adduct and rotate the proximal fragment medially. Displacement of the fracture fragments is also influenced by gravity, the position in which the upper limb is held, and the forces causing the fracture. The distal fragment is usually internally rotated because the arm is held across the chest and the proximal fragment remains in midposition.
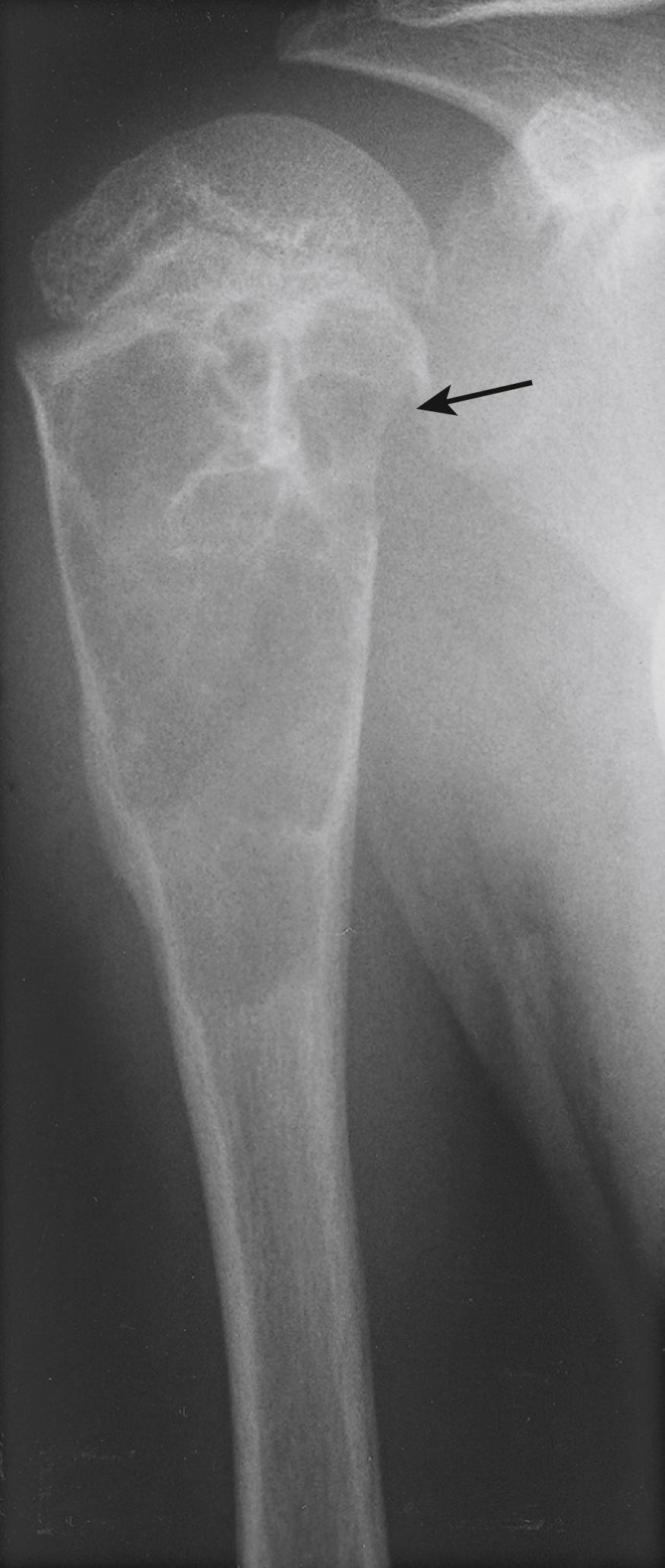
Fractures of the proximal humeral metaphysis are generally a result of a direct high-energy force. As such, they are frequently associated with multiple trauma. Fractures in this area that occur after minimal trauma should raise suspicion of a pathologic fracture because this is a common location for unicameral bone cysts and other benign lesions ( Fig. 29.20 ). Most fractures of the shaft of the humerus are also caused by a direct force, such as a fall on the side of the arm. Consequently, they are usually transverse or comminuted fractures and are frequently open injuries. An indirect force, such as a fall on an outstretched hand, can produce an oblique or spiral fracture of the humeral shaft. Forceful muscle contraction, such as when throwing a baseball, has also been reported to cause humeral shaft fractures, although such a history should raise the possibility of a pathologic fracture through a lesion such as a unicameral bone cyst or fibrous dysplasia ( Fig. 29.21 ). bb
References , , , , , , , , , , .

The obvious deformity, localized swelling, and pain caused by fractures of the proximal humeral metaphysis or humeral shaft make the clinical diagnosis straightforward. However, due diligence is required to detect associated neurovascular injury. The intimate relationship of the radial nerve to the humerus makes it especially vulnerable to injury. Radial nerve injury results in anesthesia over the dorsum of the hand between the first and second metacarpals and loss of motor strength of the wrist, finger, and thumb extensors, as well as the forearm supinators. The median and ulnar nerves are rarely injured. Vascular injury is also extremely rare. cc
References , , , , , , , , , .
In infants with obstetric fractures, the fracture is immobilized for a period of 1 to 3 weeks by bandaging the arm to the side of the thorax in a modified Velpeau bandage or a sling and swath. Parents should be instructed in skin care for the immobilized extremity and forewarned of the large palpable callus that will develop in 6 to 8 weeks. Efforts to control alignment are not necessary because the remodeling potential is great. Follow-up examination is required only for the assessment of brachial plexus function to ensure that a concomitant nerve palsy does not exist. Primitive reflexes such as the Moro reflex can be valuable for assessing upper extremity function in an infant. , ,
As with fractures involving the proximal humeral physis, the remodeling potential of proximal humeral metaphyseal fractures is great. Consequently, these fractures rarely require more than symptomatic treatment with sling immobilization. Occasionally, we manage polytraumatized patients or open fractures with operative fixation (see Fig. 29.14 ).
Fractures of the humeral shaft are generally best managed with closed techniques. Usually, we initially place these patients in a coaptation splint. After 2 to 3 weeks, patients can be managed in a sling, hanging arm cast, or fracture brace. It is not essential to obtain end-to-end anatomic alignment because overgrowth is common in humeral shaft fractures. Overriding of 1 to 1.5 cm can easily be accepted; however, angulation of more than 15 to 20 degrees in either plane is not desirable and as with any fracture, rotational remodeling potential is minimal. Consequently, rotational alignment should be maintained. Circumduction and pendulum exercises for the shoulder are demonstrated and begun as soon as pain allows, usually after 2 to 3 weeks. Again, we occasionally treat open injuries or polytraumatized patients with operative techniques. External fixation may be indicated for extensive soft tissue injuries, although internal fixation allows easier nursing care. We have found flexible nails to be an easy and effective means of managing humeral shaft fractures in polytraumatized patients ( Fig. 29.22 ).

Complications after fractures of the proximal metaphysis or shaft of the humerus are unusual. As with any fracture, open or vascular injuries can occur. These injuries should be managed individually with attention to the guidelines discussed in Chapter 26 .
Radial nerve injury, which is not uncommon in adults, is rare in children. Complete severance of the nerve in closed fractures is very unlikely, and nerve function generally recovers if the fracture is managed conservatively. Thus these patients should be managed with cast immobilization, with careful splinting of the wrist and hand in a functional position; passive exercises should be performed to maintain full ROM. If there is no evidence of functional recovery after 3 to 4 months, electromyographic studies or exploration of the nerve may be indicated. dd
References , , , , , , .
Nonunion of humeral shaft fractures is much less common in children and adolescents than in adults but it does occasionally occur. In general, we prefer to treat nonunion by open reduction and plate fixation.
Mercer Rang has said “Pity the young surgeon whose first case is a fracture around the elbow,” as an introduction to his chapter on elbow fractures, for good reason. Although common—fractures about the elbow account for 5% to 10% of all fractures in children —the unique anatomy of the elbow and the high potential for complications associated with elbow fractures make their treatment anxiety-producing for many orthopaedic surgeons. Fortunately, with an understanding of the anatomy and adherence to a few basic principles, treatment of such fractures can be straightforward.
It is best to address elbow fractures from an anatomic perspective because each specific fracture has its own challenges in diagnosis and treatment. One frequent source of problems in the management of pediatric elbow injuries is distinguishing fractures from the six normal secondary ossification centers. The six ossification centers develop in a systematic predictable fashion. The mnemonic CRITOE is helpful for remembering the progression of radiographic appearance of the ossification centers about the elbow in children— c apitellum, r adius, i nternal (or medial) epicondyle, t rochlea, o lecranon, and e xternal (or lateral) epicondyle. In general, the capitellum appears radiographically at approximately 2 years of age, and the remaining ossification centers appear sequentially every 2 years. It is important to remember that girls mature early and boys late, so the age at which these landmarks appear may vary—earlier for girls, later for boys; however, the sequence remains constant ( Fig. 29.23 ). Comparison radiographs of the contralateral uninjured elbow can also be helpful in evaluating whether there is a fracture or merely a normal ossification center.

The most common fractures about the elbow include fractures of the supracondylar humerus, transphyseal distal humerus, lateral humeral condyle, medial humeral epicondyle (often associated with elbow dislocation), radial head and neck, and olecranon. Fractures involving the capitellum, coronoid, medial condyle, and lateral epicondyle, as well as intracondylar or T-condylar fractures, occur but are rare. Each of these injuries are discussed in the context of their unique characteristics, which can assist in diagnosis and treatment.
Supracondylar fractures of the humerus are the most common type of elbow fracture in children and adolescents. They account for 50% to 70% of all elbow fractures and are seen most frequently in children between the ages of 3 and 10 years. The high incidence of residual deformity and the potential for neurovascular complications make supracondylar humeral fractures a serious injury. ee
References , , , , , , , .
The elbow joint is a complex articulation of three bones that allows motion in all three planes. The distal humerus has unique articulations with the radius and ulna that make this mobility possible. The radial-humeral articulation allows pronation and supination of the forearm, whereas the ulnohumeral articulation allows flexion and extension of the elbow. The separate articulating surfaces of the distal humerus are attached to the humeral shaft via medial and lateral columns. These two columns are separated by a thin area of bone that consists of the coronoid fossa anteriorly and olecranon fossa posteriorly. This thin area is the weak link in the distal humerus and is where supracondylar humeral fractures originate. When forced into hyperextension, the olecranon can act as a fulcrum through which an extension force can propagate a fracture across the medial and lateral columns ( Fig. 29.24 ). Similarly, a force applied posteriorly with the elbow in flexion can create a fracture originating at the level of the olecranon fossa ( Fig. 29.25 ). Thus whether the result of an extension or flexion force, fractures of the supracondylar humerus are usually transverse and at the level of the olecranon fossa. For reasons that are unclear, older patients often have fractures that are oblique rather than transverse. Oblique fractures are less stable than transverse fractures because rotation produces additional angulation ( Fig. 29.26 ).


Although the bony architecture of the distal humerus is responsible for the frequency of supracondylar humeral fractures, it is the soft tissue anatomy that has the potential to produce devastating long-term complications. Anteriorly, the brachial artery and median nerve traverse the antecubital fossa. Laterally, the radial nerve crosses from posterior to anterior just above the olecranon fossa. The ulnar nerve passes behind the medial epicondyle ( Fig. 29.27 ). In extension supracondylar fractures, the brachialis muscle usually shields the anterior neurovascular structures from injury. However, in severely displaced fractures, the proximal fragment may perforate the brachialis muscle and contuse, occlude, or lacerate the vessel or nerve. The vessels or median nerve may also become trapped and compressed between the fracture fragments.

Even without direct injury, a severely displaced fracture can cause neurovascular injury simply from the stretch or traction associated with displacement. Similarly, the radial nerve may be injured by severe anterolateral displacement of the proximal fragment. With flexion-type injuries (anterior displacement of the distal fragment), the ulnar nerve is at risk because it may become tented over the posterior margin of the proximal fragment. Neurovascular problems can also develop in minimally displaced fractures as a result of hematoma formation or swelling. Hematomas generally spread anteriorly across the antecubital fossa deep to the fascia and can potentially compress the neurovascular structures.
Supracondylar humeral fractures may be the result of an extension or flexion force on the distal humerus. Usually they are the result of a fall on an outstretched hand, which causes hyperextension of the elbow. , , These extension-type supracondylar humeral fractures account for 95% to 98% of all supracondylar fractures. With hyperextension injuries the distal fragment will be displaced posteriorly. Flexion-type supracondylar fractures are rare and occur in only 2% to 5% of cases. The mechanism of flexion supracondylar fractures is usually a direct blow on the posterior aspect of a flexed elbow that results in anterior displacement of the distal fragment. , ,
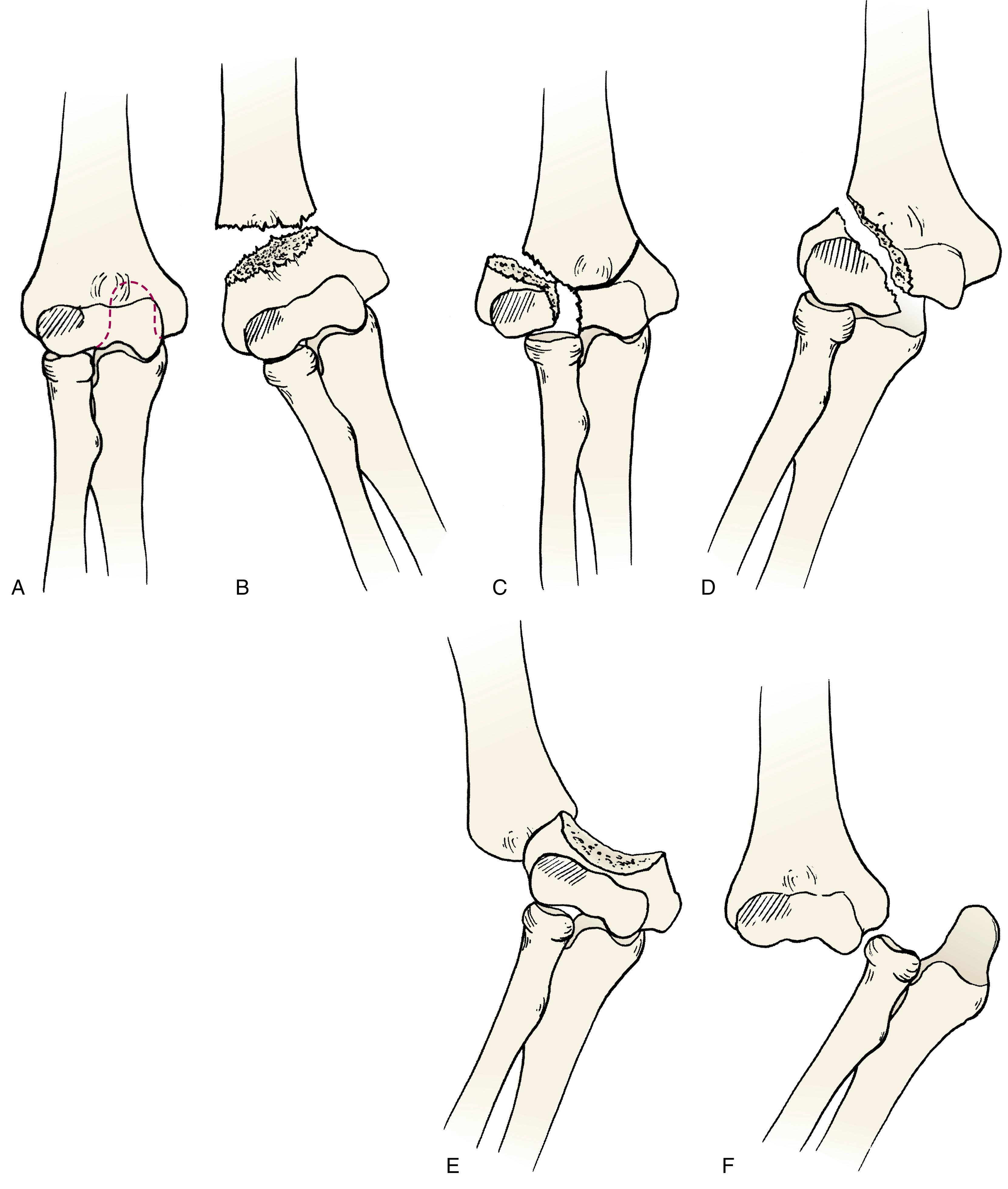
Supracondylar humeral fractures are usually initially classified as extension or flexion injuries and then according to the amount of radiographic displacement. This three-part classification system was first described by Gartland in 1959. It has been shown to be more reliable than most fracture classification systems. Type I fractures are nondisplaced or minimally displaced. Type II fractures have angulation of the distal fragment (posteriorly in extension injuries and anteriorly in flexion injuries), with one cortex remaining intact (the posterior in extension and the anterior in flexion). Type III injuries are completely displaced, with both cortices fractured ( Fig. 29.28 ). Leitch and colleagues added a type IV classification, describing a multi-directionally unstable fracture in both flexion and extension with complete loss of the periosteal hinge. Type IVs are often only diagnosed intraoperatively.

There have been several modifications of this scheme. Wilkins added subtypes A and B for type II fractures. Type IIa fractures are pure extension-type supracondylar humerus fractures, while type IIb fractures have malrotation or coronal malangulation. He also subdivided type III injuries according to the coronal plane displacement of the distal fragment ( Fig. 29.29 ). This modification is clinically helpful in identifying complications from the injury and problems with treatment. Posterolaterally displaced type III fractures, although less frequent and accounting for only 25% of extension supracondylar fractures, are more commonly associated with neurovascular injuries. Undoubtedly this is because the proximal fragment is displaced anteromedially in the direction of the neurovascular bundle ( Fig. 29.30 ). In extension supracondylar fractures, the coronal plane displacement of the distal fragment also helps predict the stability of the fracture at the time of reduction. In a classic study in monkeys, Abraham and colleagues demonstrated that the periosteal sleeve remains intact on the side to which the distal fragment is displaced. This periosteal sleeve helps stabilize the fracture when it is reduced. Pronation of the forearm tightens the medial sleeve to a greater extent than supination tightens the lateral sleeve; thus posterior medial fractures are usually more stable once reduced ( Fig. 29.31 ).


Mubarak and Davids subdivided type I fractures into IA and IB. Type IA injuries are truly nondisplaced fractures, with no comminution, collapse, or angulation. Type IB fractures are characterized by comminution or collapse of the medial column in the coronal plane and may have mild hyperextension in the sagittal plane ( Fig. 29.32 ). They expressed concern that if unreduced, these minimally displaced type IB fractures could lead to a cosmetically unacceptable result, particularly in children with a neutral or varus preinjury carrying angle.
Supracondylar fractures may be inherently obvious or almost impossible to diagnose. The clinical findings in severely displaced fractures are generally so obvious that the most difficult part of the diagnosis is remembering to perform a thorough examination to assess for other injuries, as well as possible neurologic injury. This is particularly important given that neurologic injury is present in 10% to 15% of cases , , and ipsilateral fractures occur in 5% (usually the distal radius).
A complete and thorough assessment of the neurologic function of the hand is often difficult in a very young child with an acute elbow fracture. However, if a gentle and deliberate effort is made, most children by the age of 3 or 4 years will cooperate with a two-point sensory and directed motor examination. Examining the contralateral uninjured hand is helpful to establish baseline cooperation in the preschool child. For uncooperative children, it is important to forewarn the parents that when a thorough examination is possible, there is a 10% to 15% chance that a neurologic injury will be discovered. Fortunately, these injuries almost always do well. ff
References , , , , , , , , .
Although a complete neurologic examination is not always possible, it is always possible to assess the vascular status of patients with displaced supracondylar humeral fractures. It is also of paramount importance to be vigilant for clinical signs of a developing compartment syndrome. The most reliable signs of compartment syndrome in children are increasing analgesia needs and increasing anxiety. Pain with passive extension of the fingers may also be present. Unfortunately, by the time that the classic symptoms of pallor, paresthesia, and paralysis develop, there has typically been irreversible damage to the neuromuscular tissue. After stabilization of the fracture (whether splint stabilization in the emergency department or reduction and pinning in the operating room), only oral analgesics should be ordered, and the physician should be notified if the patient is requiring more pain medication.
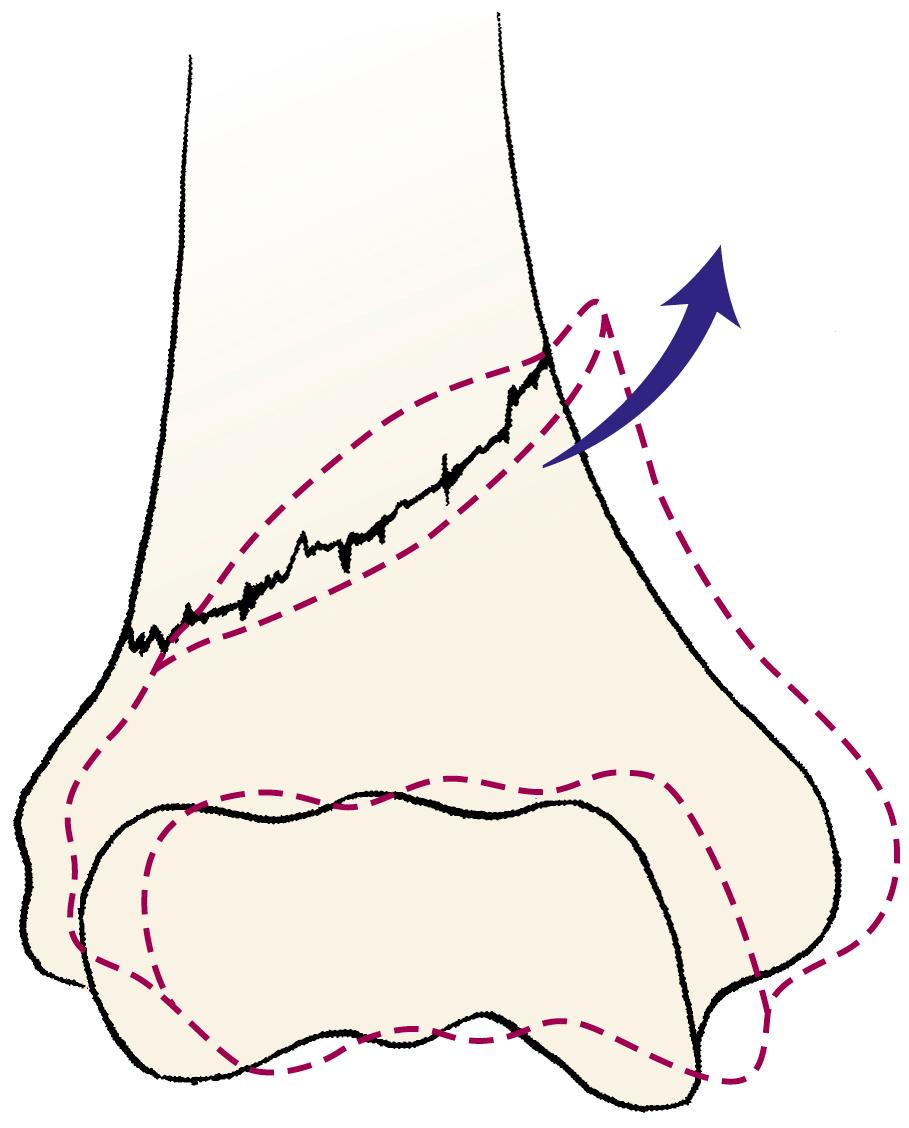
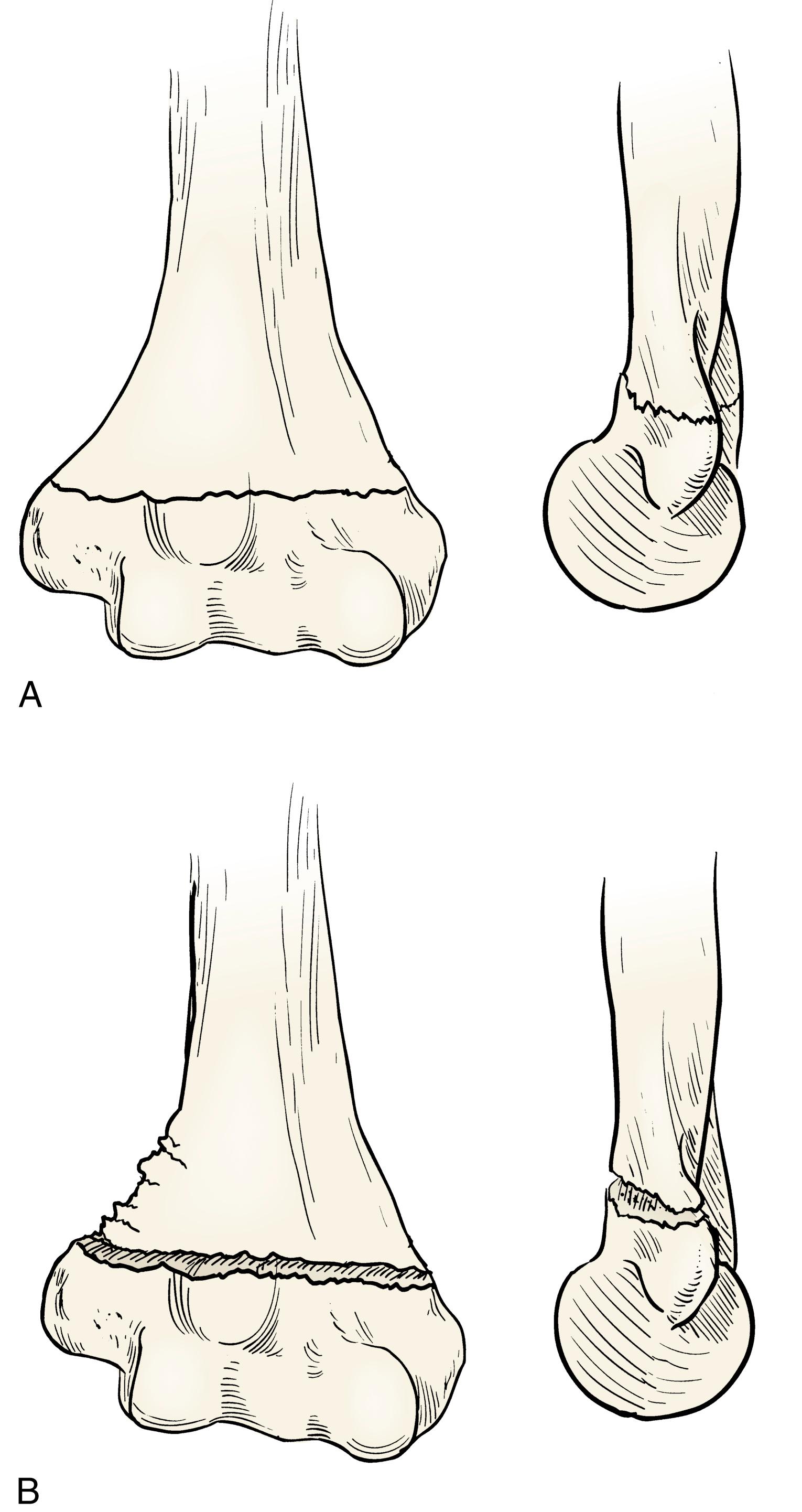
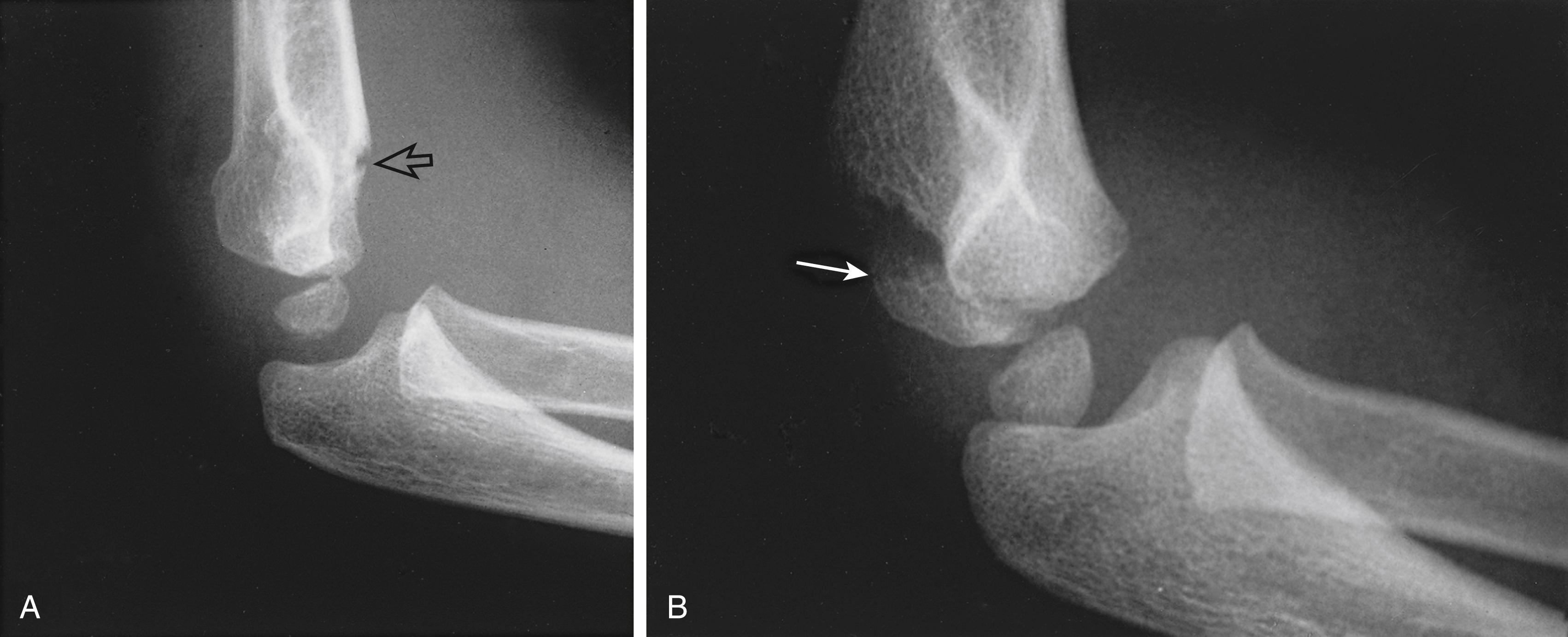
The differential diagnosis of severely displaced supracondylar humeral fractures includes elbow dislocations and all conditions that mimic them, such as transphyseal distal humeral fractures in children younger than 2 years and unstable lateral condylar fractures ( Fig. 29.33 ). Unstable lateral condylar fractures can be differentiated from supracondylar fractures most readily on the lateral radiograph. Supracondylar fractures usually originate at the olecranon fossa and are transverse or, less commonly, short oblique. Lateral condylar fractures originate more distally, often with only a small metaphyseal fragment visible on the lateral radiograph (Thurston-Holland sign; Fig. 29.34 ).
The diagnosis of a minimally displaced supracondylar humeral fracture may be difficult to make clinically. Careful clinical examination will reveal tenderness medially and laterally over the supracondylar ridges, whereas with lateral condylar fractures the tenderness is lateral and with medial epicondylar fractures it is medial. In radial neck fractures the tenderness is over the radial neck posterolaterally. However, a small child with a painful elbow does not always cooperate with such a careful examination. In these cases the definitive diagnosis may not be evident until the cast is removed several weeks later ( Fig. 29.35 ).

When the fracture cannot be seen clearly on radiographs, it is important to obtain a thorough history to ensure that there was indeed a witnessed fall and that the symptoms began immediately after the injury because patients with musculoskeletal infection often have a swollen, painful elbow and a history of trauma. If the elbow pain did not begin immediately after a witnessed traumatic event, consideration should be given to the assessment of laboratory indices (e.g., complete blood cell count, differential, erythrocyte sedimentation rate, and C-reactive protein level) to ensure that the symptoms are not a result of occult infection.
The diagnosis of a supracondylar humeral fracture is confirmed radiographically. Obtaining good-quality radiographs is complicated by the fact that the elbow is painful and difficult to move. Because of rotational displacement, it may be impossible to obtain true orthogonal views of severely displaced fractures. However, with proper instruction to the radiographer, true AP and lateral radiographs of fractures with moderate or minimal displacement can be obtained. Obtaining a true AP view of the elbow requires full elbow extension and is therefore seldom possible. Consequently, we obtain an AP view of the distal humerus, which can be achieved with any degree of elbow extension ( Fig. 29.36 ). The importance of obtaining a true lateral radiograph of the distal humerus cannot be overstated because treatment decisions are made from assessment of the lateral radiograph. If a nondisplaced or minimally displaced fracture is suspected but the AP and lateral views do not show a fracture, oblique views may be useful.
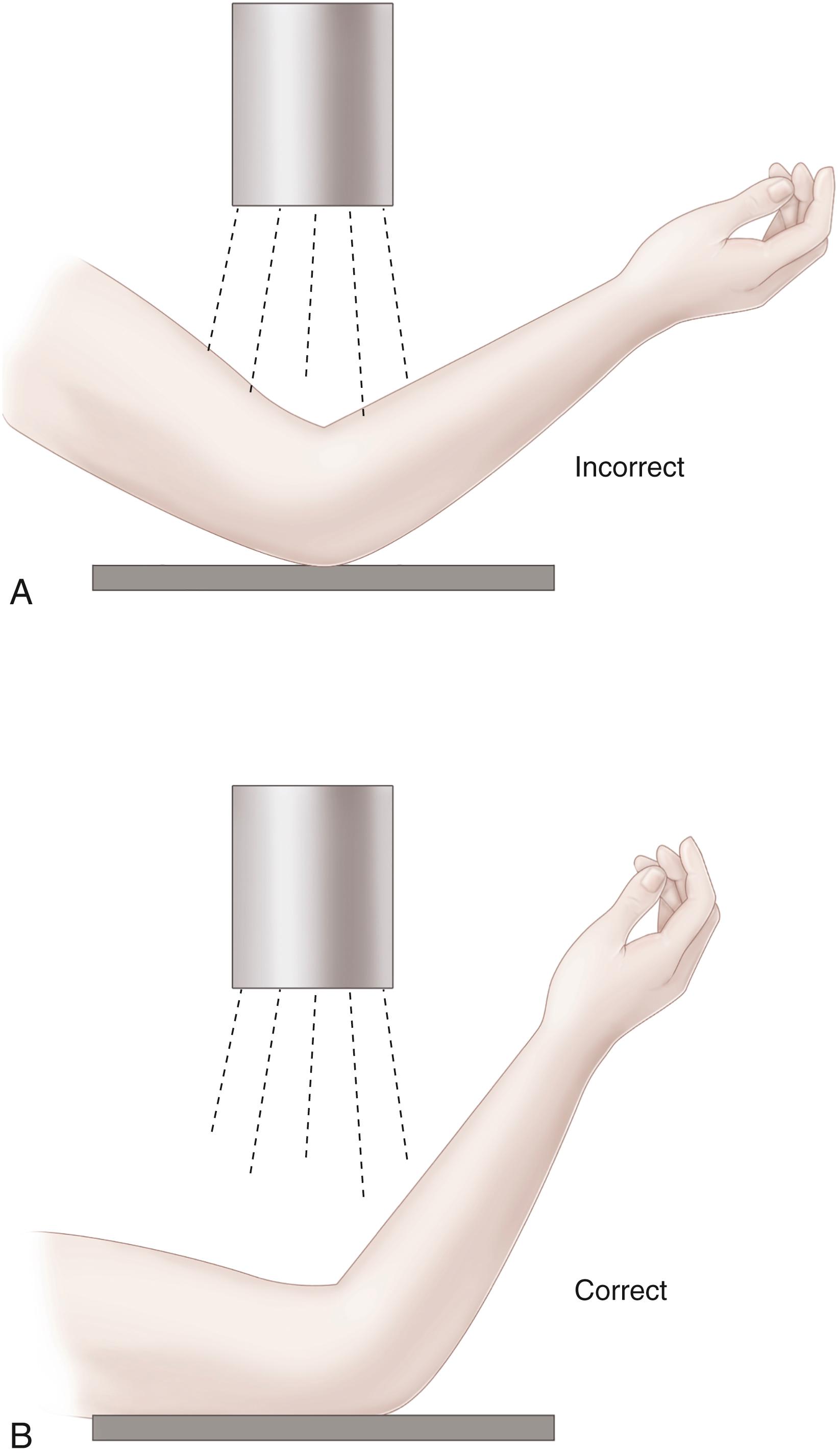
Several radiographic parameters are helpful for managing patients with supracondylar humeral fractures. One is Baumann’s angle, determined from an AP radiograph of the distal humerus. It is the angle between the physeal line of the lateral condyle of the humerus and a line drawn perpendicular to the long axis of the humeral shaft. ( Fig. 29.37 ). Studies have shown that although the normal Baumann’s angle varies from 8 to 28 degrees, depending on the patient, there is little side-to-side variance in any one individual. However, relatively small changes in elbow position, rotation, or flexion may alter Baumann’s angle significantly. gg
References , , , , , , , .
Obtaining a comparison view to calculate Baumann’s angle on the uninjured extremity may be a useful adjuvant in the decision making process for minimally displaced fractures. The AP radiograph should also be assessed for comminution of the medial or lateral columns, and for translation. Occasionally a completely displaced fracture will look relatively well aligned on the lateral radiograph but will show translation on the AP film. This translation cannot occur without complete disruption of the anterior and posterior cortices. Therefore, if present, it always represents an unstable fracture ( Fig. 29.38 ).
A posterior fat pad sign on the lateral radiograph may be the only radiographic abnormality acutely; this radiographic finding should alert the physician to the presence of an effusion within the elbow. The anterior fat pad is a triangular radiolucency anterior to the distal humeral diaphysis; it is seen clearly and, in the presence of elbow effusion, it is displaced anteriorly. The posterior fat pad is not normally visible when the elbow is flexed at right angles; however, if an effusion is present, it will also be visible posteriorly ( Fig. 29.35 , Fig. 29.39 ). Skaggs and Mirzayan reported that 76% of patients with an elevated posterior fat pad sign and no other acute radiographic abnormalities had radiographic evidence of an occult fracture at 3 weeks follow-up, with the majority sustaining supracondylar humerus fractures. In our practice, a child with a history of trauma, tenderness about the elbow, and a radiographic finding of an isolated fat pad sign, is treated in a long arm cast with repeat radiographs out of the cast at 3 weeks. Periosteal reaction and callus will often make the fracture obvious at that time.

There are several additional radiographic parameters to assess on the lateral radiograph ( Fig. 29.40 ). First, the distal humerus should project as a teardrop or hourglass. The distal part of the teardrop or hourglass is formed by the ossific center of the capitellum (see Fig. 29.40A ). It should appear as an almost perfect circle. An imperfect circle or obscured teardrop or hourglass implies an oblique orientation of the distal portion of the humerus from an inadequate radiographic technique or fracture displacement. Second, the angle formed by the long axis of the humerus and the long axis of the capitellum should be approximately 40 degrees (see Fig. 29.40B ). In supracondylar fractures with posterior tilting of the distal fragment (seen with extension fractures), the humerocapitellar angle will diminish, whereas with anterior tilting of the distal fragment (seen with the less common flexion injuries), it will increase. Third, the anterior humeral line—a line drawn through the anterior cortex of the distal humerus—should pass through the middle third of the ossific nucleus of the capitellum (see Fig. 29.40C ). However, in children younger than 4 years, the line may pass more anteriorly through the capitellar ossific nucleus than in older children. With extension supracondylar fractures the anterior humeral line will pass anterior to the capitellum. Finally, the coronoid line, a line projected superiorly along the anterior border of the coronoid process, should just touch the anterior border of the lateral condyle of the humerus (see Fig. 29.40D ). However, with extension supracondylar fractures, the coronoid line will pass anterior to the anterior border of the lateral condyle.

To quote Mercer Rang again, the goal of treatment of supracondylar humeral fractures is to “avoid catastrophes” (vascular compromise, compartment syndrome) and “minimize embarrassments” (cubitus varus, iatrogenic nerve palsies). With this goal in mind, treatment of supracondylar humeral fractures can be divided into a discussion of their management in the emergency department, care of nondisplaced fractures, and treatment of displaced fractures.
It is important that the child and limb receive proper care while awaiting definitive treatment. Unless the patient has an ischemic hand or tented skin, the limb should be immobilized as it lies with a simple splint. If possible, radiographs should be obtained before splinting, or radiolucent splint material should be used. If the distal extremity is initially ischemic, an attempt to align the fracture fragments better should be made immediately in the emergency department. This can be accomplished by extending the elbow, correcting any coronal plane deformity, and reducing the fracture by bringing the proximal fragment posteriorly and the distal fragment anteriorly ( Fig. 29.41 ). Often, this simple maneuver immediately restores circulation to the hand. In extension-type fractures, hyperflexion of the elbow should be avoided because it may cause further damage to the neurovascular structures. The distal circulation should always be checked before and after the splint is applied. Sensation, motor function, and skin integrity should also be carefully checked and recorded. Patients with open fractures should receive intravenous antibiotics and appropriate tetanus prophylaxis (see discussion of open fractures in Chapter 26 ). All patients should be kept from having any food or drink by mouth (NPO) until a definitive treatment plan has been outlined.

Treatment of nondisplaced fractures is straightforward and noncontroversial. It consists of long-arm cast immobilization for 3 weeks. We often initially treat the patient in the emergency department with a posterior splint. For truly nondisplaced fractures there is no theoretic advantage to pronation or supination of the forearm. We generally immobilize nondisplaced fractures with the forearm in neutral position. The patient returns 5 to 10 days after injury for removal of the splint. Radiographs are repeated to ensure that no displacement has occurred, and the patient is placed in a long-arm cast for an additional 2 to 3 weeks, at which time immobilization is discontinued, but the patient is still restricted from sports and physical education class for another 4 weeks. After cast removal, the parents are forewarned that normal use of the arm may not resume for 1 to 2 weeks, and that some pain and stiffness should be expected for the first 2 months. Parents should be reassured that mild loss of motion for the first 2 months out of the cast is normal and will resolve on its own.
There are a few potential pitfalls in the management of nondisplaced supracondylar humeral fractures that merit further discussion. An occult infection or nursemaid’s elbow may be misdiagnosed as a nondisplaced supracondylar humeral fracture. A thorough history will suggest the correct diagnosis. At times, undisplaced fractures cause soft tissue swelling acutely and may even result in compartment syndrome, especially when the injured arm stays in a dependent position. We are careful to not immobilize the arm in more than 90 degrees of flexion and often use a posterior splint rather than a cast. If a cast is applied, it is generously split. The parents must be educated on the importance of edema control and watching for signs of increased swelling and pressure. Too often patients are discharged from the emergency department with instructions to elevate the arm and use a sling. Unfortunately, the sling keeps the extremity in a dependent position and promotes swelling. Time should be taken in the emergency department to explain to the parents (and the nurses giving discharge instructions) that the extremity should be elevated with the fingers above the elbow and the elbow above the heart for the first 48 hours after the injury. The sling is for comfort after the swelling has subsided. Parents should be instructed to return immediately to the emergency department if it appears that the splint or cast is becoming too tight or the pain seems to be increasing inappropriately.
Several treatment options are available for the management of displaced fractures (types II and III). By definition, all these fractures require reduction. Usually, even for severe type III injuries, reduction can be accomplished in a closed fashion. Options exist in regard to the method of maintaining the reduction until the fracture has healed, including cast immobilization, traction, and percutaneous pin fixation. If adequate closed reduction cannot be achieved, open reduction should be performed; this is almost universally followed by pin fixation.
Under general anesthesia, the child is positioned at the edge of the operating table, with the arm over a radiolucent table to allow assessment of the reduction with an image intensifier ( Fig. 29.42 ). Some surgeons elect to use the image intensifier itself as the table. An assistant grasps the proximal humerus firmly to allow traction to be placed on the distal fragment. If there is puckering of the anterior soft tissues over the proximal metaphyseal fragment, known as the “brachialis sign,” the entrapped muscle and soft tissue most be extricated with the “milking maneuver” where the biceps and brachialis muscle bellies are milked in a distal direction to release the muscles from the metaphyseal fragment. Once the soft tissues are extricated, longitudinal traction should be applied with a steady continuous force, with the elbow in full extension. Once adequate traction has been applied, the coronal plane deformity (translation and varus-valgus) is corrected while traction is maintained (see Fig. 29.42 B). Continuing to maintain traction with the dominant hand, the surgeon uses the fingers of the nondominant hand to apply a posteriorly directed force to the proximal fragment. The thumb of that hand is then placed on the olecranon to apply an anterior force to the distal fragment while the fingers continue to pull the proximal fragment posteriorly (see Fig. 29.42C ). Concurrently, the dominant hand flexes the elbow and pronates the forearm for posterior medially displaced fractures and supinates the forearm for posterior lateral fractures. While the elbow is being flexed, the surgeon’s dominant hand can continue to exert a distracting force on the distal fragment. The patient’s hand should be touching the shoulder. With the elbow hyperflexed, the reduction is then assessed on AP and lateral views. The lateral is obtained by externally rotating the shoulder while keeping the elbow hyperflexed. If hyperflexion causes malreduction in the coronal plane, pressure from the nondominant fingers and thumb on both the distal fragment and opposing metaphyseal spike may be needed to maintain coronal alignment during the flexion maneuver. With very unstable fractures the surgeon may need to rotate the image intensifier to avoid displacing the fracture.
In general, Gartland type IIa fractures without coronal malalignment can be treated with either cast immobilization or pin fixation after reduction, depending on surgeon preference. Reduction and immediate casting may be performed in the emergency department with fluoroscopy and sedation if needed. Parikh and colleagues reported a success rate of 72% in maintaining alignment in emergency department closed reduction and casting. However, varus or valgus malalignment cannot be controlled in a cast and mandates pin fixation after reduction to maintain alignment.
Gartland type III fractures are almost always stabilized with percutaneous pin fixation after reduction.
There are several caveats to achieving successful closed reduction that need mention. The first is that every effort should be made to avoid vigorous manipulations and remanipulations because they only damage soft tissue and elicit more swelling. The second is the management of extremely unstable fractures, which are often posterolaterally displaced. Maintenance of reduction is difficult because supination is not as effective at tightening the intact lateral soft tissue hinge as pronation is at stabilizing posteromedially displaced fractures (see Fig. 29.31 ). During reduction, as the elbow is placed into hyperflexion, these fractures occasionally displace into valgus. When this occurs, a varus force must be applied with the nondominant hand as the elbow is flexed, and flexion is stopped at 90 degrees. The reduction is confirmed (the image intensifier generally needs to be rotated for a lateral view) and stabilized with percutaneous pinning (see Fig. 29.42D and E).
Closed reduction is obtained with longitudinal traction and the elbow in extension; the distal fragment is reduced with a posteriorly directed force ( Fig. 29.43 ). Any coronal plane deformity is then corrected. Once adequate reduction has been confirmed, it is maintained with percutaneous pinning. Severely displaced flexion-type injuries and those with ulnar nerve injuries are more likely to require open reduction than the more common extension-type fractures.

Open reduction of supracondylar humerus fractures is needed when closed reduction fails to adequately reduce the fracture. Other indications for open reduction include operative debridement in open fractures, compartment syndrome, and neurovascular injuries that require open exploration and potential repair of injured structures. The decision that a closed reduction is unacceptable and an open reduction is indicated must be made on an individual basis. We accept mild angulation in the sagittal plane as long as the anterior humeral line intersects the capitellum. Mild amounts of translation in the coronal plane and sagittal plane are also acceptable, as is a minimal amount of valgus angulation in the coronal plane. However, varus angulation in the coronal plane, particularly if associated with a small amount of hyperextension in the sagittal plane or a contralateral carrying angle that is neutral or varus, is likely to yield a cosmetically poor result that will not remodel ( Fig. 29.44 ) and predisposes to development of posterolateral rotatory instability and ulnar nerve symptoms as an adult. , , If significant varus deformity exists after the best attempt at closed reduction, we proceed to open reduction.
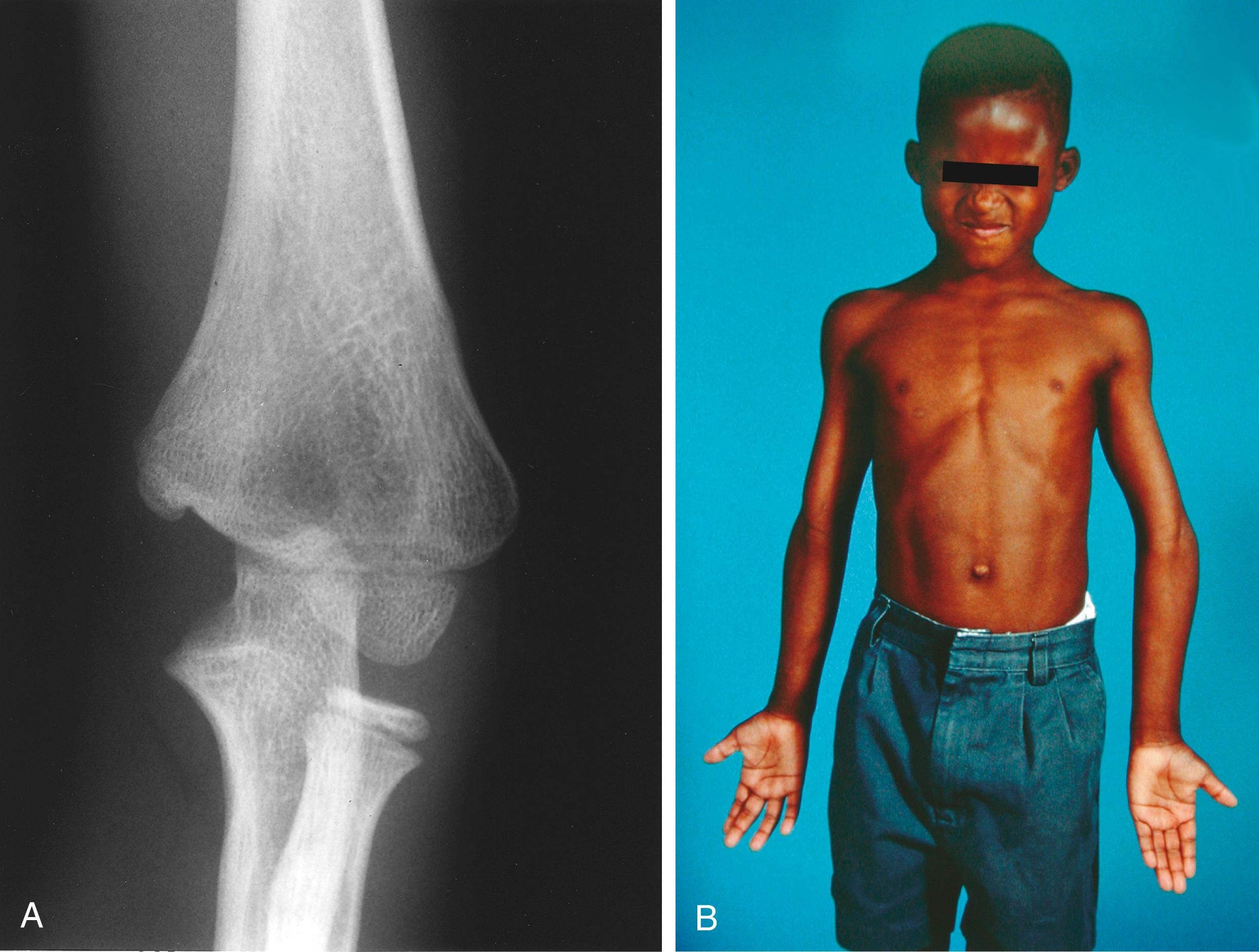
The surgical approach can be anterior, medial, or lateral, and is based on the location of the metaphyseal spike. The posterior approach has increased risk of postoperative stiffness, the potential for disruption of the blood supply, and is rarely, if ever, used at our institution. Approaching the fracture from the metaphyseal spike allows direct visualization of the structures impeding reduction. Skin incisions should be planned to avoid compromised skin that may cause necrosis or problems with wound healing. The anterior approach has the most utility as it allows for exploration of the neurovascular bundle in extension-type fractures. A “lazy S” incision in the flexion crease of the antecubital fossa is made, with the proximal limb extending medially and the distal limb extending laterally to allow for increased exposure if needed.( Fig. 29.45 ) Blunt dissection is used to complete the deep exposure and identify the proximal metaphyseal fragment. Many times, the metaphyseal spike has performed the dissection already. If the approach is used to extract interposed structures from the fracture site, the dissection should be lateral to the biceps tendon to avoid iatrogenic injury to the neurovascular bundle. However, the neurovascular bundle can be displaced in a nonanatomic position in severely displaced supracondylar humerus fractures, so care during dissection is of paramount importance. Once the metaphysis is exposed, the interposed structures can be extricated and the fracture reduced under direct visualization. This maneuver is similar to the maneuver for a closed reduction of an extension supracondylar. If difficulty is encountered with obtained length, a small Hohmann retractor or Freer elevator can be used as a lever on the distal fragment while balancing the instrument shaft on the proximal metaphysis fulcrum. The reduction and maintenance of reduction are often difficult once the fracture has been opened due to the complete lack of stability from the periosteal stripping. Many times, the reduction is only stable at 90 degrees of flexion with the surgeon’s fingers manually holding the reduction while an assistant percutaneously drives the K-wire pins across the fracture site.

The medial approach is used for posterolaterally displaced fractures as well as flexion type fractures that cannot be reduced due to entrapment of the ulnar nerve or other soft tissue. Once the skin incision is made, blunt dissection is used to identify the ulnar nerve, which is mobilized and protected. The brachialis muscle is then reflected anteriorly to expose the proximal metaphyseal fragment.
Percutaneous pin fixation yields the most predictable results with the fewest complications and is the preferred technique for immobilization of displaced supracondylar humeral fractures. hh
References , , , , , , , , , , , , , , , , , , , .
The technique for percutaneous pinning involves the placement of two or three 0.62-inch smooth K-wires (smaller K-wires may be used in patients younger than 2 years) distally to proximally in a crossed or parallel fashion. While the use of a crossed pin or parallel pin technique has been the subject of considerable debate, it is now accepted that lateral-entry parallel pins are the safest and most appropriate stabilization of supracondylar humerus fractures although a medial-entry pin is acceptable when needed to obtain sufficient stability of the fracture ( http://www.orthoguidelines.org/go/auc/auc.cfm?auc_id=224922 ). Once closed reduction has been achieved, the extremity is held in the reduced position by the surgeon’s nondominant hand or by an assistant. For successful all-lateral pin construct several objectives must be achieved: (1) Two or more pins must engage both fracture fragments; (2) all pins must achieve bicortical fixation; (3) pins must have maximal pin separation at the fracture site, ideally with one in the medial column and one in the lateral column ( Fig. 29.46 ); and (4) use of a third lateral-entry pin if more stability is required.

If a medial pin is used, care must be taken to ensure that the ulnar nerve is not injured. As 28% of children have subluxating ulnar nerves, and 10% have dislocating nerves, the elbow should be held at less than 80 degrees of flexion when placing a medial pin, and after pinning, the elbow should not be flexed past 90 degrees to avoid tethering of the ulnar nerve ( Fig. 29.47 ). The starting position for a medial pin is the inferiormost aspect of the medial epicondyle ( Fig. 29.48 ). The pin should be started as far anteriorly as possible. The surgeon can often palpate the ulnar nerve subcutaneously, just proximal to the medial epicondyle. He or she should milk the soft tissue posteriorly, with the thumb placed immediately posterior to the medial epicondyle to protect the ulnar nerve with the elbow held in gentle extension ( Fig. 29.49 ). If the elbow is extremely swollen, a small incision can be made and blunt dissection can ensure that there are no interposing structures between the pin and medial epicondyle.


Placement of K-wires percutaneously through the narrow distal humerus requires some finesse. As in all percutaneous procedures in orthopaedics, it is facilitated by knowing the anatomy and by reducing the task into two separate, two-dimensional problems. Appropriate pin placement is made easier by first lining up the pin driver in the AP plane, locking this angle in, and then lining up the pin driver in the lateral plane without changing the angle in the AP plane. Positioning the pin driver and subsequently the pin sequentially in only these two orthogonal planes simplifies a conceptually difficult task.
Once the fracture has been stabilized with at least two pins, the elbow is extended, and the reduction and pin placement are confirmed on orthogonal radiographic views. If the reduction and pin placement are acceptable, the pins are bent, cut (it is best to leave a few centimeters of pin out of the skin to facilitate removal), and covered with sterile felt to decrease skin motion around the pin. The arm is immobilized in 60 to 85 degrees of flexion in a posterior splint or widely split or bivalved cast. If a medial pin is used, care should be taken to ensure that the elbow is not flexed past 80 degrees to avoid tethering of the ulnar nerve over the medial pin. While the majority of type II supracondylar humerus fractures can be safely treated as outpatient surgery, most type III fractures should be observed in the hospital for swelling and pain control after surgery. The family should be educated on cast care and elevation. The child usually returns in 7 to 10 days for examination, and in-plaster radiographs are generally taken to check for maintenance of reduction. At 3 weeks the radiographs are repeated, the pins are removed, and the immobilization is discontinued. Although several recent studies have called into question the utility of postoperative radiographics, it is still our practice to obtain radiographs at 1 week and 3 weeks. The parents are instructed to expect gradual ROM and to avoid forced manipulation. Final follow-up is at 7 to 10 weeks post-operatively to evaluate alignment and ROM and to allow return to activities.
As with all treatment methods, there are potential complications with percutaneous pinning, including pin tract inflammation or infection, iatrogenic ulnar nerve injury, and loss of reduction. Pin tract inflammation or infection occurs in 2% to 3% of patients in most large series of supracondylar humeral fractures treated by pin fixation. Fortunately these infections usually respond to removal of the pin and a short course of oral antibiotics, although osteomyelitis can develop. Ulnar nerve injury from a medially placed percutaneous pin is another potential complication, although several randomized prospective series have not demonstrated a statistically significant difference in complication rates between lateral entry compared to medial and lateral entry pin fixation. , , If an ulnar nerve deficit is noted postoperatively and a medial pin is present, we recommend removal of the medial pin and observation. Fortunately, in most cases, the ulnar nerve makes a complete recovery. ii
References , , , , , , , .
Loss of reduction can occur after closed reduction and percutaneous pinning of supracondylar humeral fractures ( Fig. 29.50 ). This complication is generally the result of inadequate surgical technique and can be minimized by close attention to detail to ensure that the pins are maximally separated at the fracture and have adequate purchase in the proximal fragment.

The advantages of cast immobilization are that a cast is easy to apply, readily available, and familiar to most orthopaedists. Casting does not require sophisticated equipment, there is little chance of iatrogenic infection or growth arrest, and it can yield good results. However, most displaced supracondylar fractures are stable only if immobilized in more than 90 degrees of flexion. Casting an injured elbow in hyperflexion may lead to further swelling, increased compartment pressure, and possibly the development of Volkmann’s ischemic contracture (compartment syndrome) in the setting of a rigid circumferential dressing.
In our institution, cast immobilization as the only form of treatment is utilized in type Ia fractures and some select type IIa fractures without coronal malalignment. Attention to detail is needed when casting type IIa fractures after a closed reduction is performed. First, the cast should be carefully applied to avoid compression in the antecubital fossa. Second, the parents must understand the importance and technique of edema control. Finally, repeat radiographs are obtained with the arm in the cast in 7 to 10 days to ensure that alignment is maintained. If significant swelling has resolved and the cast is loose, the cast may need to be reapplied to maintain reduction. Again, the cast is maintained for 3 to 4 weeks after the reduction, and the parents are warned to expect a period of pain and stiffness after cast removal.
While numerous traction techniques have been historically described, including overhead or lateral traction with skin or skeletal traction applied with an olecranon pin or screw, we do not use traction in the management of supracondylar humeral fractures. It is mentioned here only for historical completeness. It may have a role in the rare fracture that cannot be managed routinely because of extenuating circumstances.
There is debate regarding the necessity of closed reduction and pinning for all displaced supracondylar fractures, particularly minimally displaced type IB or II fractures. A number of studies have reported good results with closed reduction and casting of extension supracondylar fractures. , ,
Although we recognize that some minimally displaced fractures may be managed successfully without pin fixation, there are several potential hazards with cast management of minimally displaced supracondylar fractures. Type IB or IIB fractures with medial column collapse or comminution may be more unstable than appreciated on initial radiographs ( Fig. 29.51 ). If treated by simple immobilization, these occultly unstable fractures are likely to displace into varus and hyperextension, leading to malunion and a cosmetically unacceptable result. Second, even if the fracture is stable, collapse of the medial column may produce enough varus and hyperextension to produce a poor result if the fracture is not reduced.

There are also two potential problems with closed reduction and cast management of type II fractures. The first is loss of reduction, and the second is increased swelling and the potential development of compartment syndrome secondary to immobilization with the elbow in flexion. The difficulty with cast management of minimally displaced fractures was demonstrated in the studies of Parikh and colleagues and Hadlow and colleagues. Both series reported successful treatment with closed reduction and casting in 72% to 77% of patients with type II extension-type. However, 23% of Hadlow’s cohort required a second procedure, due to unacceptable alignment, and 20% of Parikh’s group required secondary reduction and pinning, with an additional two patients having a poor result after losing reduction but failing to have any intervention. Obviously, the problem is correctly identifying which fractures are at risk for malunion. To our knowledge, no reliable predictors of malunion exist, and many studies have reported superior results with percutaneous pinning of displaced supracondylar fractures. , , Therefore we prefer closed reduction and pinning for all types IB and IIB supracondylar humeral fractures and will occasionally attempt closed reduction and cast treatment for very minimally extended IIA fractures. Although this aggressive management may lead to a few unnecessary pinnings, we believe that it also results in the fewest complications.
Although it is agreed that pin fixation yields the best results for type III fractures, there is some controversy regarding the timing of treatment. Historically, type III fractures were regarded as an orthopaedic emergency that had to be treated immediately. However, good results have been reported when type III fractures were treated on an urgent rather than emergency basis. Those who advocate delayed treatment cite the advantages of an adequate NPO status and more efficient operative setting.
Provided that the skin is intact and not tented, the swelling is minimal, and the neurovascular examination is normal, we will allow an 8- to 10-hour delay to avoid operating on these fractures in the middle of the night. Type III injuries that are treated in delayed fashion are splinted in minimal flexion, with care taken to ensure that the proximal fragment is not displacing the skin, and patients are admitted for elevation and observation until definitive treatment. Patients in whom the skin is compromised, the swelling is severe, or the neurovascular examination is abnormal are treated by closed reduction and pinning on an emergency basis. A multicenter review found 11 patients who developed compartment syndromes of the forearm. Severe swelling on presentation was present in all, all had palpable pulses, and delay of treatment averaged 22 hours. When severe soft tissue injury is noted, urgent treatment seems indicated, especially since soft tissue injury is associated with neurovascular compromise as well as a decline in a previously normal neurologic or vascular exam.
Pin placement configuration unfortunately still continues to be debated. Although several biomechanical studies have shown that crossed pins are the most stable configuration, a number of reports have shown good clinical results with parallel lateral pin fixation. jj
References , , , , , , , , , , , , , , , , , , , , .
In a randomized trial, Kocher and co-workers found no difference in outcome comparing crossed pins with lateral pins, with no ulnar nerve injuries in either group. In follow-up, five of eight surgeons at the originating institution had changed from crossed to lateral pin fixation. Although more stable, the crossed pin technique requires the placement of a medial pin, which may injure the ulnar nerve. kk
References , , , , , , , , , , .
A meta-analysis showed a 4.3-fold increased incidence of ulnar nerve injury with cross pinning. Slobogean and co-workers calculated a number needed to harm relative to ulnar nerve injury and proposed an iatrogenic ulnar nerve injury for every 28 patients treated with cross pinning. Skaggs and colleagues, in a review of 369 supracondylar fractures, reported that the incidence of ulnar nerve injury could be decreased from 15% to 2% by placing two lateral pins, followed by the selective use of medial pins only for fractures that remain unstable after placement of the lateral pins.
In our institution, we use two lateral-entry pins in type II fractures and three lateral-entry pins in type III fractures. However, we do not hesitate to place a medial pin using the careful technique described earlier if the fracture demonstrates continued instability after three lateral-entry pins or if the fracture is a very proximal fracture or obliquely oriented pattern that precludes two divergent lateral-entry pins.
Although iatrogenic ulnar nerve injury almost always recovers, there are case reports of permanent injury. Thus we believe that an ulnar nerve palsy associated with a medial pin requires immediate treatment. Initially we ensure that the elbow is immobilized in a semi-extended position. Often the ulnar nerve is not directly injured by the K-wire but is stretched around the medial pin when the elbow is in a flexed position (see Fig. 29.47 ). If the elbow is adequately extended or if extension does not alleviate the ulnar nerve symptoms, we remove the medial pin immediately.
Controversy exists regarding the best management of a pulseless pink hand. The elbow’s abundant collateral circulation allows the distal extremity to remain viable, despite complete disruption of the brachial artery ( Fig. 29.52 ). Past recommendations for management of a viable but pulseless hand range from observation to arteriography to immediate surgical exploration. ll
References , , , , , , , , , , , , , , , , , , , , .

Several groups have shown that the hand can remain viable and a radial pulse can even return after ligation of the brachial artery. , , Scannell and colleagues reported 20 patients with pink pulseless hand after a supracondylar fracture; at follow-up, 5 patients had occluded brachial arteries and 1 patient had a stenotic brachial artery; despite this, patients had a palpable radial pulse, normal growth of the arm, and good/excellent outcomes although one occluded patient had cold intolerance. Nevertheless, some recommend aggressive surgical attempts to restore a normal pulse because of concern that conservative management of a pulseless viable extremity could lead to progressive ischemia as a result of thrombus formation or future problems with cold intolerance, exercise claudication, or growth discrepancy. mm
References , , , , , , , .
Our approach to a viable hand with abnormal pulses is close observation postoperatively using the Doppler ultrasound to evaluate distal radial pulses. Our institution reported on 54 pink pulseless hands after supracondylar fracture; 26 patients had restoration of palpable radial pulses after closed reduction and pin fixation, and 20 patients had restoration of a dopplerable but nonpalpable radial pulse. Four patients underwent an immediate vascular procedure due to lack of dopplerable pulses after pinning. However, one patient who had restoration of dopplerable pulses after reduction and pinning was observed to have a cool, pale hand at 9 hours post-operatively and was emergently brought to surgery for a saphenous vein graft due to a thrombosed brachial artery. This highlights the necessity of close hospital observation and serial examinations for 24 to 48 hours following closed reduction and pinning of these fractures.
Appropriate management of a patient who is initially evaluated 1 to 2 weeks after injury and found to have a nonreduced or unacceptably reduced fracture is often difficult to determine. Some surgeons advocate watchful waiting because attempts at manipulation once early callus begins to form may not improve the reduction and could risk increasing stiffness. This argument is strengthened by the knowledge that functional limitations are rare after malunion. Others favor a more aggressive approach and attempt closed or open reduction of these fractures. Unfortunately, there is little in the literature to guide the decision-making process. Alburger and colleagues have shown that a 3- to 5-day delay before closed reduction and pinning is not deleterious. Lal and Bhan reported good results in 20 children treated by open reduction 11 to 17 days after injury. Vahvaven and Aalto performed routine remanipulation at 2 weeks for all redisplaced fractures, without adverse sequelae.
We have had success with remanipulation of supracondylar fractures after delays of 2 to 3 weeks ( Fig. 29.53 ). Management of these injuries must be determined on an individual basis and must take into account factors such as the patient’s age, condition of the soft tissue, amount of residual deformity, and degree of radiographic healing. It is important that treatment decisions regarding these malreductions be made based on good information. Unfortunately, obtaining an adequate examination and radiographs in a young patient a few weeks after a displaced supracondylar fracture can be extremely difficult and may require examination under anesthesia. Although functional limitations are uncommon in the pediatric population with malunion of supracondylar humeral fractures, these injuries have little potential to remodel. Even a small improvement in alignment may represent the difference between a cosmetically acceptable result and one that is unacceptable. In addition, malunited supracondylar humerus fractures may lead to elbow instability as an adult. If an attempt is made to improve the alignment of a supracondylar fracture in delayed fashion, a perfect anatomic reduction may not be an achievable goal. In such cases, we usually accept an adequate nonanatomic reduction rather than proceed to open reduction.
The complications of supracondylar humeral fractures can be categorized as early or late. Early complications include vascular injury, peripheral nerve palsies, and Volkmann’s ischemia (compartment syndrome). Late complications include malunion, stiffness, and myositis ossificans. Although attention to detail at the time of initial treatment may limit the long-term sequelae of early complications and minimize late complications, the severity of the injury and nature of the anatomy make problems from supracondylar fractures unavoidable.
Management of the pink, pulseless hand with a supracondylar humerus fracture was discussed earlier. The incidence of vascular compromise in type III extension supracondylar fractures has been reported to be between 2% and 38%. nn
References , , , , , , , , , , .
However, the reported incidence varies with the definition of vascular compromise inasmuch as this term has been used to describe a wide variety of patients, including those with a diminished pulse, those without a pulse, and those with an ischemic limb. Vascular injury may be induced directly or indirectly. Direct injury by the fracture may result in complete transection of the brachial artery, an intimal tear, or compression between the fracture fragments or over the anteriorly displaced fragment. Indirect injury is usually the result of compression. Compression can produce temporary ischemia that is reversible with reduction, reversible spasm, or permanent sequelae, such as intimal tears, aneurysms, or thrombosis. If the level of vascular injury, whether produced directly or indirectly, is distal to the inferior ulnar collateral artery, the rich collateral circulation about the elbow will generally provide adequate blood supply to the forearm and hand (see Fig. 29.52 ).
Management of acute vascular injury associated with supracondylar fractures of the humerus is controversial and must be individualized. The initial treatment consists of a thorough assessment of the skin and neurologic status, as well as evaluation for other injuries. If the hand is obviously ischemic, the arm should be immediately manipulated into a semi-extended position. Often this instantly restores circulation to the hand (see Fig. 29.41 ). If improving the alignment fails to provide distal circulation, the child should be immediately taken to the operating room for closed reduction and pinning.
We do not believe that arteriography or other studies are warranted before an operative attempt at closed reduction for two reasons. First, reduction of the fracture frequently restores the circulation. Second, even if the limb remains ischemic after reduction, the location of the arterial pathology is known. Thus an arteriogram provides little information that will alter the clinical management but can significantly prolong the ischemic time. Similarly, we do not generally obtain preoperative vascular or microsurgical consultation because the ischemia frequently resolves with reduction. If the limb remains ischemic, exposure of the brachial vessels can be performed while awaiting the arrival of a vascular surgeon or microsurgeon. If on exploration the artery is found to be trapped within the fracture fragments, the pins can be removed, the artery liberated, the fracture repinned, and circulation of the limb reassessed. Spasm and intimal lesions of the brachial artery may require arteriography for complete assessment, which can usually be performed intraoperatively with little difficulty using standard fluoroscopy. Spasm may be relieved with a stellate ganglion block or local application of papaverine, or resection and reverse interpositional vein grafting may be required. These decisions are generally made in conjunction with a vascular surgeon or microsurgeon. It is important to remember to perform a fasciotomy if there has been significant ischemic time or there is any concern about elevated compartment pressure.
Peripheral nerve injury occurs in approximately 10% to 15% of supracondylar humeral fractures. oo
References , , , , , , , , , , , , , .
There has been a growing consensus that the anterior interosseous nerve is the nerve that is usually injured with extension-type supracondylar fractures, although the median, radial, and ulnar nerves all may be damaged. pp
References , , , , , .
Anterior interosseous nerve palsy is probably underreported because it is not associated with sensory loss. Median nerve injury has been reported more commonly with posterolaterally displaced fractures and radial nerve injury with posteromedial displacement. Although ulnar nerve injury may occur as a consequence of the fracture, the ulnar nerve is more frequently injured iatrogenically from a medial pin. qq
References , , , , , , , , .
Perhaps the single most important and often the most difficult aspect of managing peripheral nerve injuries associated with supracondylar humeral fractures is the challenge of reaching an accurate and timely diagnosis. Unfortunately, it is often impossible to perform an adequate neurologic examination in a young child with a supracondylar humeral fracture in the emergency department. Thus it is imperative to counsel the parents that as time progresses, there is a chance that a nerve injury will be discovered. Fortunately, the parents can be reassured that almost all such injuries will spontaneously improve. Because the majority of motor nerve palsies can be expected to recover spontaneously within 6 months, little treatment is required other than close monitoring for recovery and perhaps splinting or ROM exercises, or both, to ensure that a fixed contracture does not develop. Although most peripheral nerve injuries recover fully, there have been numerous reports of those that do not. rr
References , , , , , , , , , , , , , .
Thus, if within 6 months there is no advancing Tinel’s sign or demonstrable return of motor function, consideration should be given to electrodiagnostic testing and exploration. If a peripheral nerve is found to be transected, appropriate reanastomosis with grafting or tendon transfers should be undertaken.
In 1881, Richard von Volkmann described ischemic paralysis and contracture of the muscles of the forearm and hand and, less frequently, the leg after the application of taut bandages in the treatment of injuries occurring in the region of the elbow and knee. He suggested that the pathologic changes primarily resulted from obstruction of arterial blood flow, which if unrelieved would result in death of the muscles. Fortunately, with improved management of elbow fractures in children, the incidence of Volkmann’s ischemic contracture after supracondylar humeral fractures has decreased. Patients with floating elbows may be at increased risk for compartment syndrome and should be monitored appropriately. This potentially devastating complication may be better described as a consequence of a high-energy injury and may develop despite appropriate care.
The pathophysiology, diagnosis, and management of compartment syndrome are discussed in Chapter 26 . A supracondylar fracture associated with a compartment syndrome is generally best managed by emergency closed reduction and pinning followed by fasciotomies. After decompression of a compartment syndrome, proper splinting and active and passive ROM exercises for the extremity are essential to maintain joint mobility until function returns.
Management of an established Volkmann’s contracture consists of two stages. In the first stage, all necrotic and fibrosed muscle is debrided via an extensile approach from the elbow to wrist; utmost care is taken to identify and protect the median and ulnar nerves as well as the radial and ulnar arteries. The tendon ends are likewise carefully preserved. Range of motion of the fingers and wrist is started once the wounds are healed at 2 to 3 weeks. Once full passive range of motion is established and maintained, the 2nd stage consists of harvesting a free gracilis muscle transfer with an accompanying proximal skin paddle. The proximal gracilis is sutured to the medial epicondyle, and artery, vein, and nerve anastomoses are performed using the anterior interosseous nerve and a branch of the brachial artery as a donor. The flexor digitorum profundii and flexor pollicis longus tendon ends are sutured to the gracilis muscle at its resting tension.
Cubitus varus and cubitus valgus are the most common complications of supracondylar humeral fractures. The reported incidence ranges from 0% to 50%. ss
References , , , , , , , , , , , , , , , , , , , , , , .
In general, posteromedially displaced fractures tend to develop varus angulation, and posterolaterally displaced fractures tend to develop valgus deviation. Cubitus varus deformity is more commonly noted to be a problem than cubitus valgus, probably because posteromedial fractures are more common. However, varus deformity may be more frequently reported simply because it is more cosmetically noticeable. Although some have suggested that angular deformity is a result of growth imbalance, the consensus opinion is that cubitus varus and valgus are the result of malunion ( Fig. 29.54 ). tt
References , , , , , , , , , , .
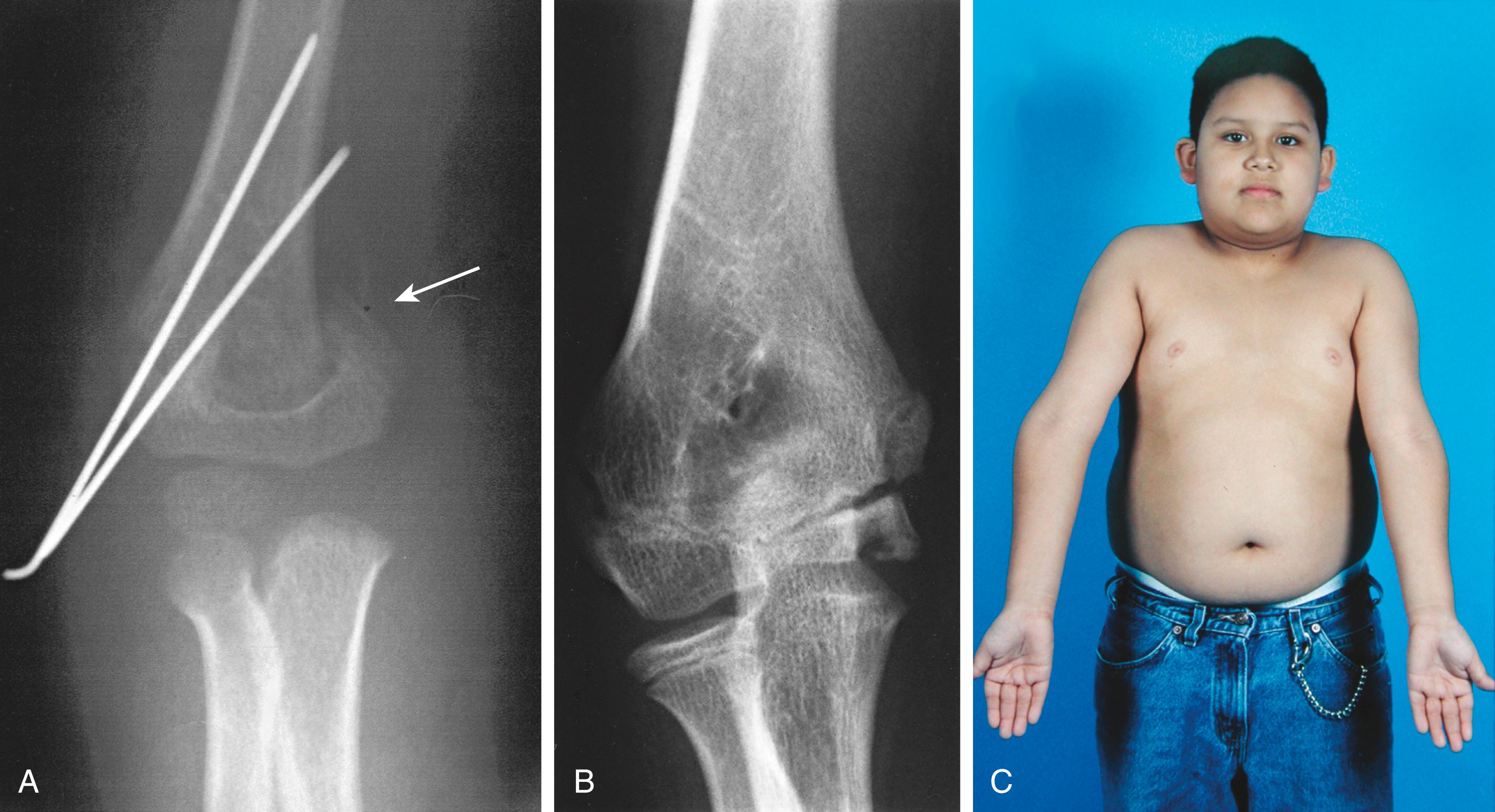
Cubitus varus or valgus is assessed by measuring the carrying angle of the arm. This is the angle created by the medial border of the fully supinated forearm and medial border of the humerus, with the elbow extended ( Fig. 29.55 ). The carrying angle exhibits considerable individual variation. Thus, comparison should be made with the contralateral side rather than with any normal standard. As the elbow extends, the carrying angle decreases (more varus); thus hyperextension tends to accentuate a cubitus varus deformity, whereas a flexion contracture can create the appearance of cubitus valgus. Smith has demonstrated that changes in the carrying angle are a result of angular displacement or tilting of the distal fragment, not translation or rotation. However, rotation of the distal fragment can contribute to the cosmetic deformity of a malunion. A residual rotational deformity is almost always present after a corrective osteotomy for cubitus varus ( Fig. 29.56 ).

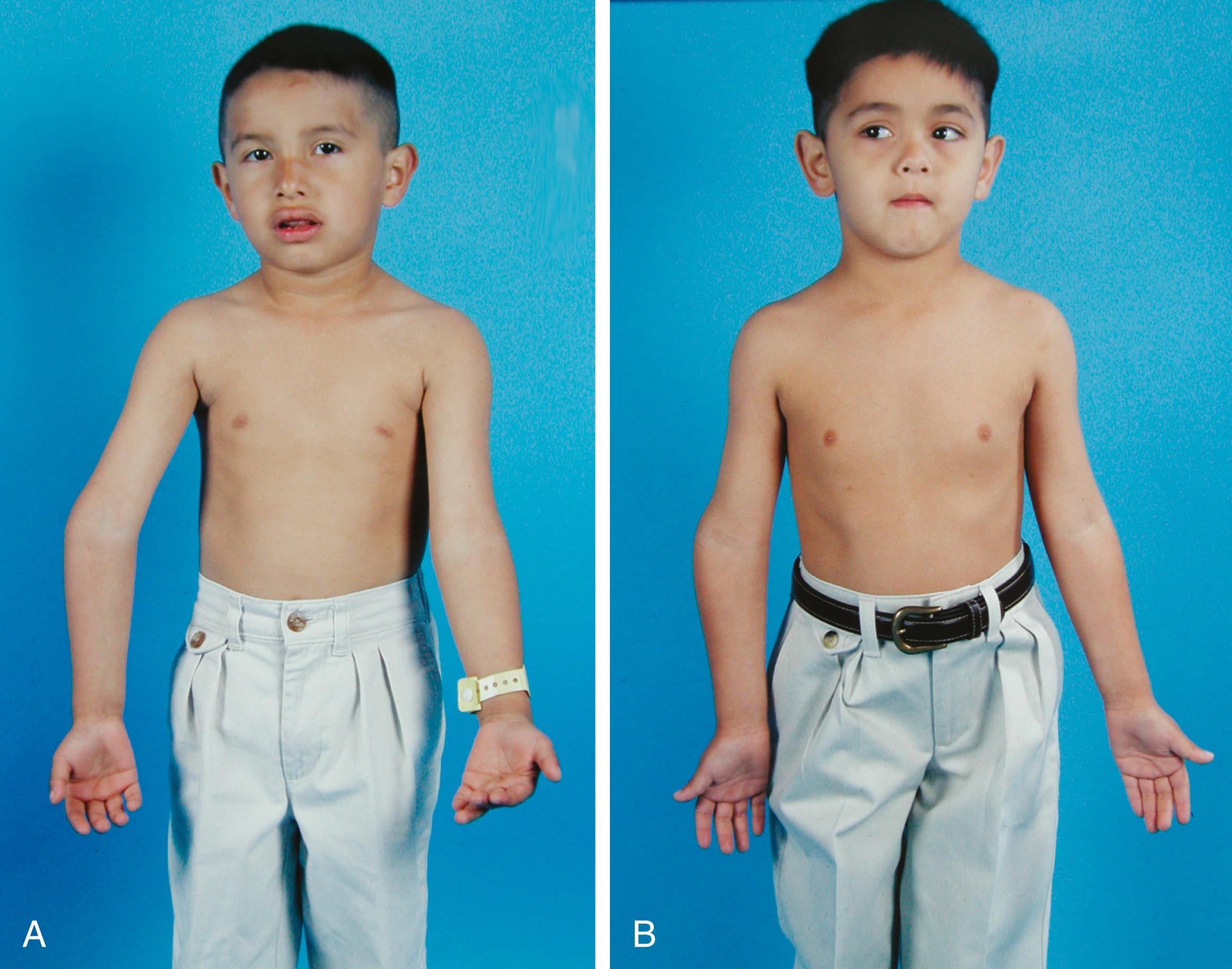
Problems arising from cubitus varus or valgus include functional limitation, recurrent elbow fracture, and cosmetic deformity. In cubitus valgus, functional problems may be related to a coexisting flexion contracture or, in extreme cases, to tardy ulnar nerve symptoms. With cubitus varus, functional problems are almost always related to limitation of flexion, although tardy ulnar nerve palsy and elbow instability have also been reported as functional complications of varus deformity. , , The limitation in flexion is a result of the hyperextension associated with varus malunion. Usually the arc of elbow motion remains constant. Thus, varus-hyperextension malunion creates a flexion deficit. If significant, this flexion deficit can interfere with activities of daily living. Lateral condyle fractures, distal humeral epiphyseal separation, and shoulder instability have also been described as potential complications of varus malunion. , , However, cosmetic deformity is the most common problem associated with malunion of supracondylar fractures.
Unfortunately, because of the limited growth and the fact that deformity is usually perpendicular to the plane of motion, little potential exists for angular malunion of the distal humerus to remodel, so the best treatment of malunion of a supracondylar humeral fracture is avoidance. Mild degrees of malunion can be treated by simple reassurance. However, if the deformity is severe, cosmetic concerns and the risk of future functional limitations and elbow instability may warrant surgical reconstruction.
The resultant cubitus varus deformity is a combined deformity of varus, extension, and internal rotation to various degrees. Most corrective osteotomies have focused on the correction of varus and extension deformity. The rotational deformity is well tolerated due to the universal motion at the shoulder and is best left untreated because rotation of the distal fragment makes the osteotomy unstable and there is little to no bony contact between the fragments. Complications from distal humeral osteotomies have been well described and include iatrogenic nerve injury, residual deformity, infection, unsightly scarring, and loss of fixation; a recent meta-analysis reporting a 14.5% complication rate should alert the surgeon and parents to the technical demands of this corrective surgery.
In an effort to limit these complications, a wide variety of osteotomy and fixation techniques have been described. Osteotomy techniques include lateral closing wedge osteotomy, step-cut osteotomy, dome osteotomy, external fixation with distraction osteogenesis, and computer-aided multiplanar osteotomy.
The author’s preferred technique is to perform two oblique lateral closing wedge osteotomies to minimize the lateral prominence that occurs with the traditional lateral closing wedge osteotomy ( Fig. 29.57 ). The radiographs of the contralateral uninjured elbow are used as a template to determine the degree of planned osteotomy and the size of the closing wedge. The apex of the osteotomy is planned to be just proximal to the medial epicondyle. Technical peals include placing lateral-entry wires up to the level of the planned distal osteotomy prior to performing the osteotomy, and leaving the medial cortex intact so as to not destabilize the osteotomy. Correction of hyperextension can also be incorporated into one of the osteotomy cuts.

These complications of supracondylar humeral fractures occur rarely. uu
References , , , , , , .
We usually assess elbow ROM 4 to 6 weeks after the cast has been removed. Mean relative arc of motion was 90% of normal by week 9 after injury in a cohort of 373 supracondylar fractures. If significant stiffness is present, we begin a supervised home program of gentle ROM exercises and continue to monitor the patient’s progress on a monthly basis. Mild stiffness generally resolves with a few months of gentle therapy, although some patients need more intensive therapy, including a splinting program. Persistent stiffness requiring surgical release is extremely uncommon. Mih and associates reported an average 53-degree increase in ROM in nine pediatric patients who underwent capsular release through a lateral and, if necessary, medial approach.
Myositis ossificans is an extremely unusual complication. This has been found to resolve spontaneously over a period of 1 to 2 years ( Fig. 29.58 ).

Transphyseal fractures are most common in children younger than 2 years. They have been reported to result from abuse in up to 50% of children younger than 2 years. In children of this age, the distal humerus is entirely cartilaginous or almost so, thus making interpretation of radiographs difficult and making diagnosis the most difficult aspect of this fracture.
The anatomic considerations for distal humeral transphyseal fractures are the same as those for supracondylar fractures of the distal humerus. The young age and consequently small anatomy of the children who typically sustain these injuries may make diagnosis and treatment difficult. Radiographically, they are often misdiagnosed as an elbow dislocation due to the unossified distal humerus. Interestingly, although transphyseal fractures share the same important anatomic considerations as supracondylar fractures, neurovascular complications are rarely reported with this type of injury.
The mechanism of injury depends on the age of the patient. In newborns and infants, there is usually a rotatory or shear force associated with birth trauma or child abuse. vv
References , , , , , , , , , .
In older children, the mechanism is usually a hyperextension force from a fall on an outstretched hand.
Although classification schemes for transphyseal separations exist, they are not clinically necessary. These fractures may be classified according to the Salter-Harris classification of physeal injuries. In infants these injuries are usually Salter-Harris type I fractures. In older children they are usually type II injuries.
The most difficult aspect of the diagnosis is distinguishing a transphyseal fracture from an elbow dislocation. Other injuries in the differential include lateral condylar and supracondylar fractures. In an elbow dislocation, the radial head does not articulate with the capitellum; however, in a transphyseal fracture, the radial head and capitellum remain congruous ( Fig. 29.59A ). In a very young patient the capitellum may not be ossified, which makes this distinction difficult. In such cases, the correct diagnosis can be made with a high degree of suspicion and the knowledge that physeal separations are more common than elbow dislocations in this age group. It may also be difficult to distinguish transphyseal separations from lateral condyle fractures that extend medial to the trochlear notch and consequently produce subluxation of the ulnohumeral joint (Milch type II fractures; see Fig. 29.33 ). In both these injuries, the radial head–capitellum relationship remains normal. Although oblique radiographs may assist in delineating these details, the distinction may require evaluation with arthrography or MRI in a small child with little ossification of the distal humeral epiphysis. ww
References , , , , , .
Supracondylar fractures usually occur at the level of the olecranon fossa, whereas transphyseal separations are more distal (see Fig. 29.33 ). A social work consult and evaluation by a qualified professional for nonaccidental trauma must be foremost in these young, nonambulatory infants who are not even yet walking to sustain an injurious fall onto their arm or elbow.
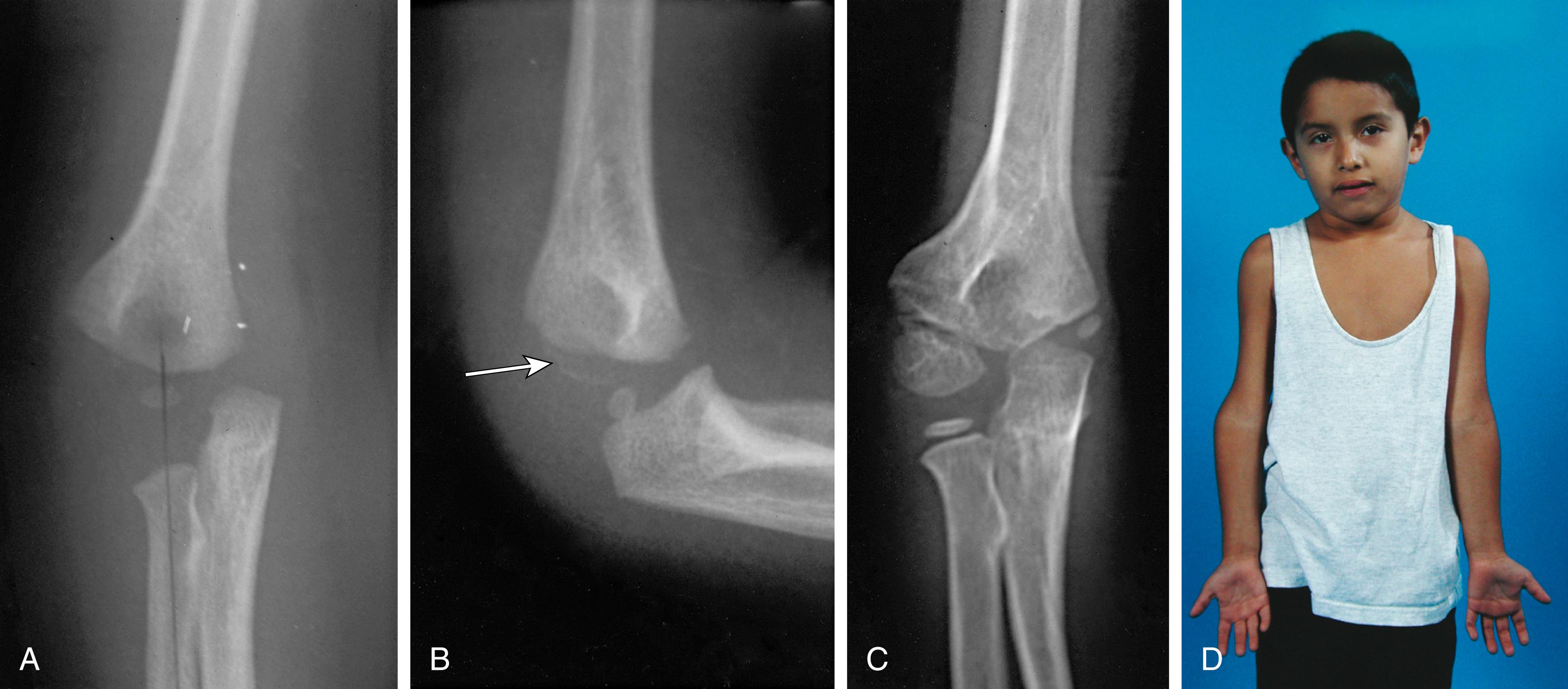
As with supracondylar fractures, obtaining good-quality radiographs of transphyseal separations is imperative but often difficult. Even under the best of circumstances, further evaluation may be required. Ultrasound, MRI, and arthrography have all been used in the evaluation of transphyseal separations. xx
References , , , , , , , .
Of these modalities, we have the most experience with arthrography because it can be performed at the time of definitive treatment.
The goal of treatment of transphyseal fractures is to achieve acceptable reduction and maintain it until the fracture unites, usually in 2 to 3 weeks. Some have advocated simple splint immobilization for transphyseal separations but a number of investigators, including some of those who advocate cast treatment, have reported cubitus varus after simple immobilization of transphyseal fractures. Our experience has paralleled that of those who reported significant cubitus varus after cast immobilization, , , particularly in patients younger than 2 years (see Fig. 29.59 ). Consequently, we favor closed reduction and pin fixation for most patients with transphyseal separations. The technique for reduction and pinning is identical to that for supracondylar fractures (see Fig. 29.42 ). We have found arthrography helpful for delineating the pathology, and we do not hesitate to perform arthrography after pin fixation or, if necessary for diagnostic purposes, before reduction and pinning ( Fig. 29.60 ). After reduction and pinning, the arm is immobilized in relative gentle extension for 2 to 3 weeks, at which time the cast and pins are discontinued.
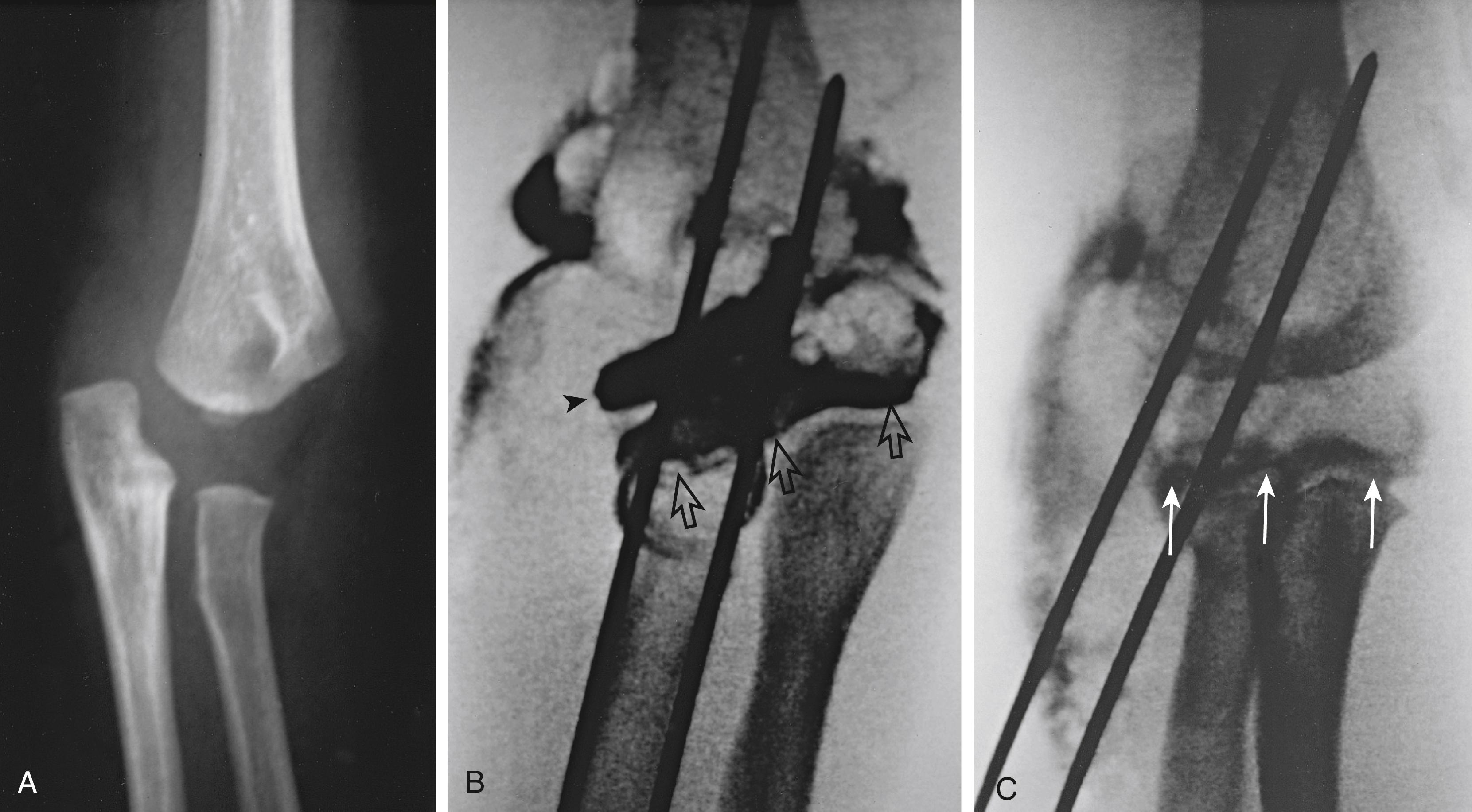
In older children the mechanism of transphyseal separation is the same as for supracondylar fractures. Not surprisingly, the potential complications are similar, although neurovascular injuries are less common. In infants, this injury is usually the result of a rotatory or shear force applied by an adult. Thus the most devastating potential complication of transphyseal separation is failure to recognize the possibility of non-accidental trauma and to return a child to a dangerous environment. The re-injury rate of abused children is between 30% and 50%, and the risk of death is 5% to 10%. , ,
The most significant and frequent orthopaedic complication of transphyseal separation is cubitus varus. The treatment of varus deformity after a transphyseal fracture is similar to that after a supracondylar fracture (see earlier, “Supracondylar Fractures of the Humerus”). Deformity secondary to avascular necrosis (AVN) has also been reported after transphyseal separation. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here