Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Undifferentiated sarcomas – tumors for which the cell of origin and/or line of differentiation is unclear – are often subdivided based on their predominant cell shape (e.g., round cell, spindle cell, pleomorphic); while convenient, this is admittedly imprecise since many tumors contain overlapping morphologies. Small round cell sarcomas constitute a vast and heterogeneous subset of these undifferentiated neoplasms. This chapter focuses on Ewing sarcoma and entities that were previously considered so-called “Ewing-like” sarcomas ( Fig. 14.1A ). Ewing sarcoma is defined molecularly as a tumor possessing fusion of a member of the FET family of RNA binding proteins (encoded by EWSR1 or FUS ) to a member of the ETS (E26 transforming sequence [E-twenty-six-specific sequence]) family of transcription factors (encoded by FLI1 , ERG ) ( Fig. 14.1B ). Ewing-like sarcomas, on the other hand, represent a vastly heterogeneous group of morphologically overlapping neoplasms lacking FET-ETS fusions. These tumors are increasingly recognized for their unique genetic drivers that may, or may not, include FET members. Importantly, in addition to being genetically distinct, it is now known these differences are of clinical – and perhaps ultimately therapeutic – relevance. As a result, diagnostic molecular subclassification is becoming increasingly necessary.
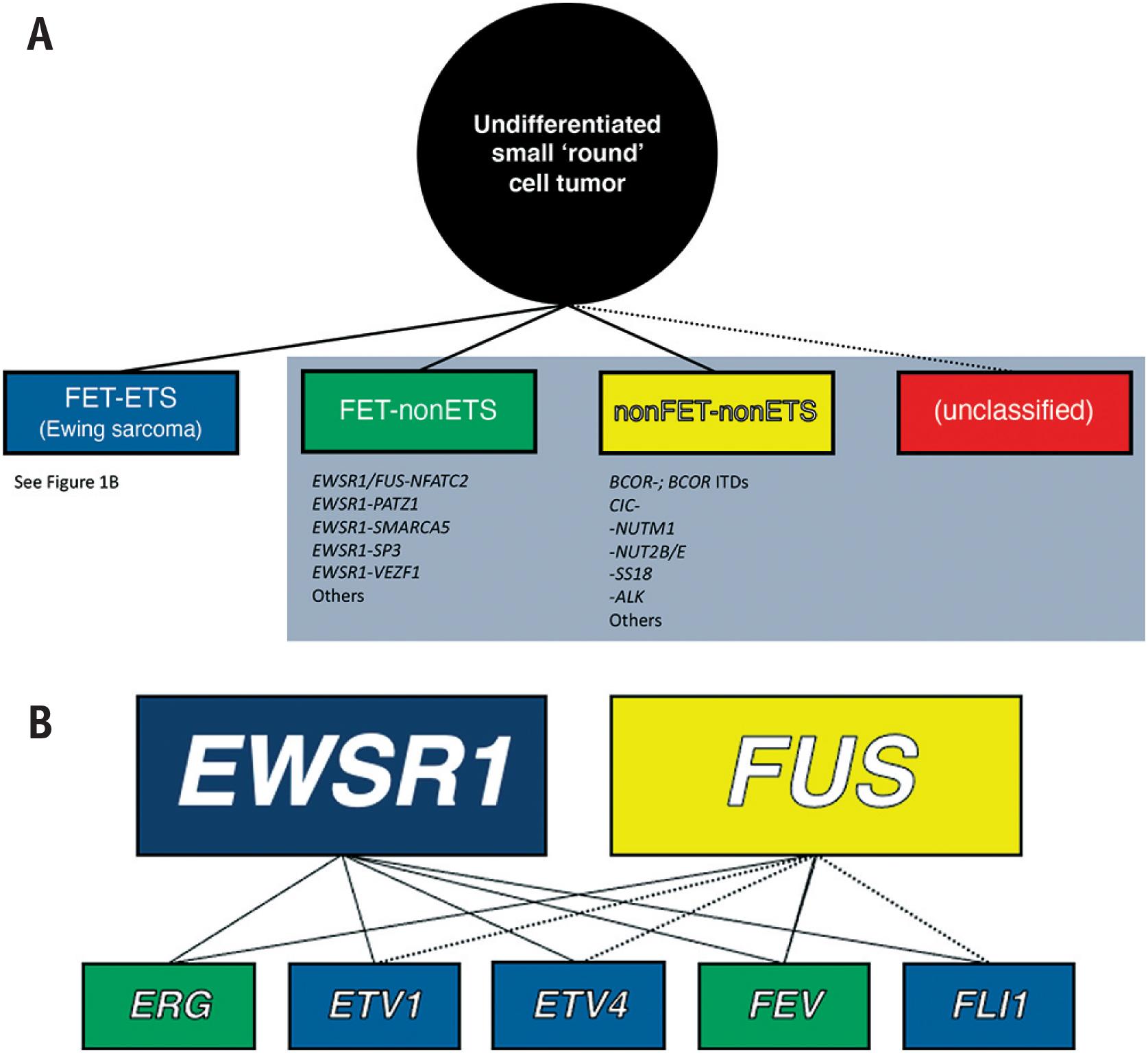
In 1921, James Ewing reported a series of round cell sarcomas occurring in young patients. He described these tumors as characterized by sheets of small polyhedral cells with monomorphic nuclei, and initially termed them “diffuse endothelioma of bone” based on his initial impression of an endothelial derivation. Related entities arising at other anatomic locations (e.g., Askin tumor, primitive neuroectodermal tumor/peripheral neuroepithelioma) were subsequently independently reported. It was not, however, until identification of a shared t(11;22)(q24;q12) event – which was subsequently shown to result in an EWSR1-FLI1 fusion gene – that coalescence of these tumors as “Ewing Family Tumors” (EFTs) was possible. Additional EWSR1 fusion partners were subsequently identified in EFTs; the subset of morphologically similar cases, which lacked ETS partners, or FET partners altogether , were tentatively classified as “Ewing-like sarcomas.”
Ewing Family Tumors are rare and aggressive sarcomas predominating in children and young adults. Tumors may occasionally arise in older patients, including the elderly. These neoplasms occur at virtually any anatomic location, and may involve bone, soft tissue, or the viscera. They are reported to have a slight male predilection, and some racial groups appear to be affected more than others (e.g., European descent). Naturally, clinical presentations vary by anatomic site of disease, while symptoms tend to be non-specific such as a painful mass or a subset of bone tumors present with pathologic fracture.
In bone, routine radiographs may demonstrate permeative growth. As tumor cells readily infiltrate Haversian and Volkmann canals there may be extensive soft tissue involvement preceding radiologic evidence of cortical destruction. Repeated expansion of the periosteum can result in a multilayered periosteal reaction – a so-called “onion-skin” appearance. Imaging modalities are often complementary; for example, computed tomography and magnetic resonance imaging are helpful in assessing the extent of bone and soft tissue involvement, and the former is additionally useful in highlighting cortical destruction. In soft tissue, imaging findings tend to be rather non-specific.
Untreated specimens are typically soft and friable, and white-grey; there is variable hemorrhage and necrosis. Most resections have undergone neoadjuvant therapy. Tumors have been reported to range from <1 cm to >20 cm.
Tumors are typically infiltrative and composed of sheets of small round cells with a lobulated pattern ( Fig. 14.2 ). The cytoplasm is scant, with indistinct cell borders. It is generally pale and eosinophilic-amphophilic clear; intracytoplasmic glycogen can usually be highlighted by periodic acid–Schiff staining. The nuclei are round-ovoid and monomorphic, with evenly dispersed chromatin; mitotic activity is variable. Interspersed between cells there is usually a delicate thin-walled vasculature. Necrosis is often present; it may be geographic, and/or limited to scattered degenerating cells. A spectrum of morphologies has been reported, for example: architecturally, tumors may contain rosettes and/or pseudorosettes (“PNET”), or hyalinized stroma; the cells may be spindled, or large (“atypical”); and, particularly in the head and neck, epithelial differentiation may be present (“adamantinoma-like”) ( Fig. 14.3 ).
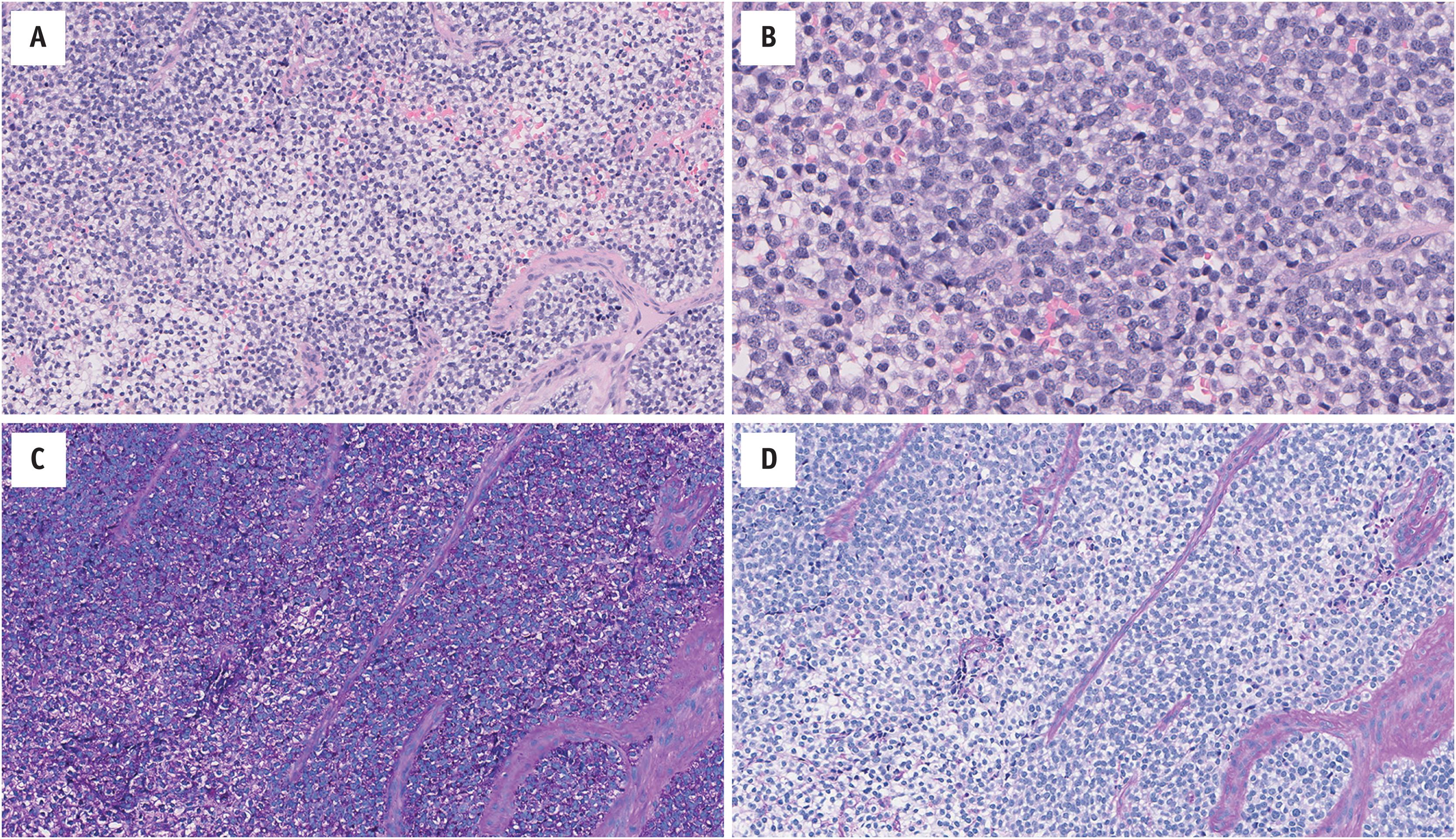
![Fig. 14.3, Ewing Family Tumors occasionally contain other morphologies. (A) Rosette and pseudorosettes (peripheral neuroectodermal tumor; EWSR1-FLI1 fusion gene); (B) large cells (atypical Ewing sarcoma; EWSR1-ERG fusion gene); and (C) epithelial differentiation (adamantinoma-like; EWSR1-FLI1 fusion gene [for full details, see Weinreb et al. 2008]). Very rarely they may have: (D) osteoid-like matrix mimicking small cell osteosarcoma ( EWSR1-FLI1 fusion gene); (E) desmoplastic stroma mimicking desmoplastic small round cell tumor ( EWSR1-FLI1 fusion gene); and (F) pseudovascular channels mimicking angiosarcoma ( EWSR1-FLI1 fusion gene), amongst other findings. Fig. 14.3, Ewing Family Tumors occasionally contain other morphologies. (A) Rosette and pseudorosettes (peripheral neuroectodermal tumor; EWSR1-FLI1 fusion gene); (B) large cells (atypical Ewing sarcoma; EWSR1-ERG fusion gene); and (C) epithelial differentiation (adamantinoma-like; EWSR1-FLI1 fusion gene [for full details, see Weinreb et al. 2008]). Very rarely they may have: (D) osteoid-like matrix mimicking small cell osteosarcoma ( EWSR1-FLI1 fusion gene); (E) desmoplastic stroma mimicking desmoplastic small round cell tumor ( EWSR1-FLI1 fusion gene); and (F) pseudovascular channels mimicking angiosarcoma ( EWSR1-FLI1 fusion gene), amongst other findings.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/UndifferentiatedSmallRoundCellSarcomasofBoneandSoftTissue/2_3s20B9780323758710000145.jpg)
There is no specific immunohistochemical marker for EFTs. The majority of cases contain diffuse membranous expression of CD99 (MIC2), and nuclear staining for NKX2.2. Most tumors also show strong nuclear staining for FLI1, while the subset of cases with ERG fusion partners have diffuse nuclear ERG expression. Approximately 20%–30% of cases show focal/patchy keratin expression. Synaptophysin, S100, and desmin staining is considerably less common ( Fig. 14.4 ).
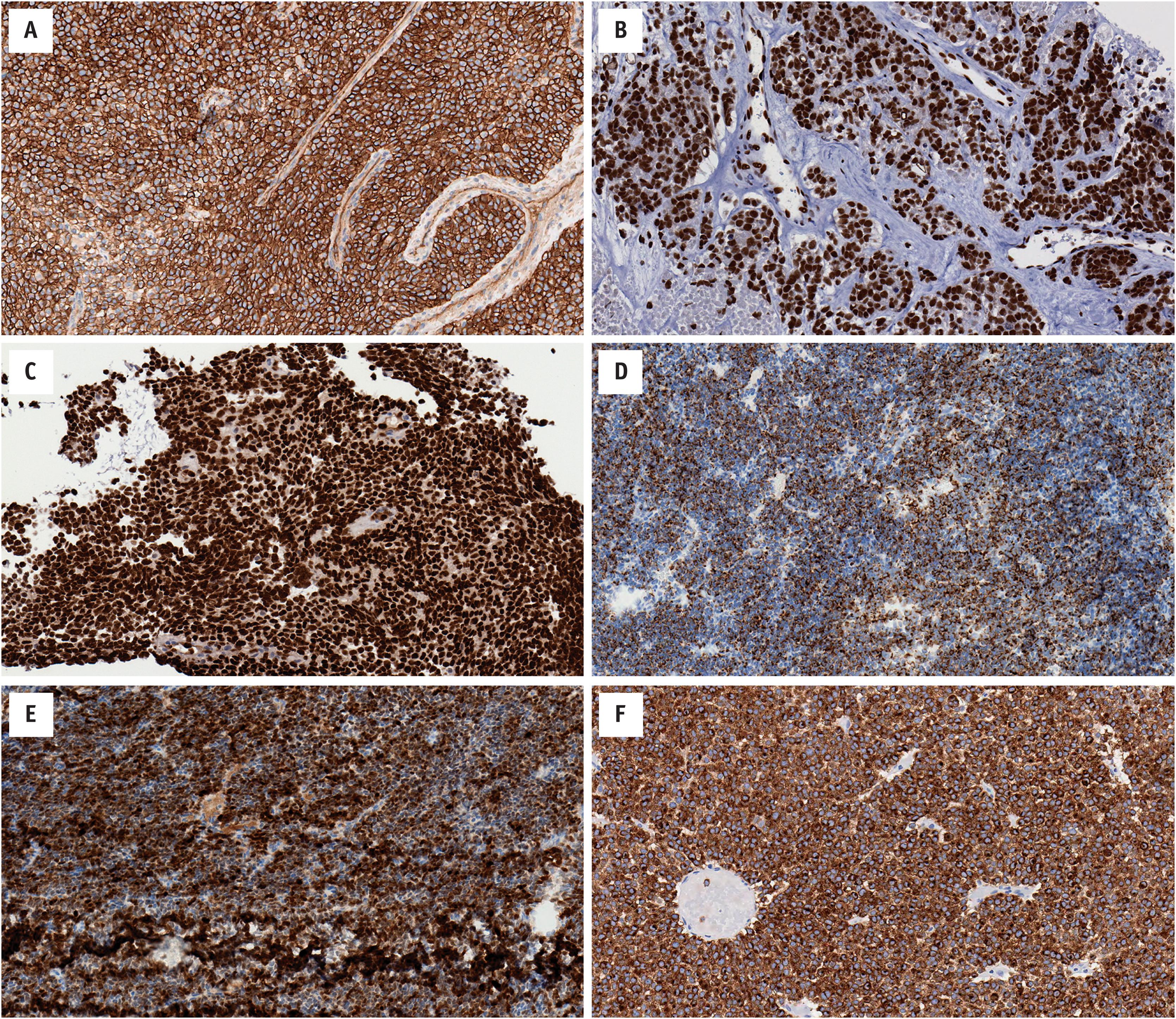
Over 90% of EFTs harbor an EWSR1-FLI1 fusion gene arising from t(11;22)(q24;q12). Approximately 5% of cases have an EWSR1-ERG fusion gene arising from t(21;22)(q22;q12). A minority contain rare EWSR1 fusion partners, including ETV1 , ETV4 , and FEV ( Fig. 14.1 ). FUS may substitute for EWSR1 ; there are reports of FUS-ERG and FUS-FEV fusion products, and other combinations are likely. These fusion genes are currently considered disease-defining for EFTs as they are not found in other tumor types. While many of these fusions can be identified using conventional cytogenetics, most laboratories currently employ either fluorescence in situ hybridization (FISH) or reverse transcriptase polymerase chain reaction (RT-PCR). Given the breadth of potential fusion genes and potential breakpoints, not to mention other fusion-associated tumors in the differential diagnosis, many diagnostic laboratories have begun moving towards diagnostic RNA-sequencing (RNA-seq).
The differential diagnosis for EFTs is broad and includes other undifferentiated round cell sarcomas (e.g., CIC -associated, BCOR -associated), poorly differentiated synovial sarcoma, mesenchymal chondrosarcoma and desmoplastic small round cell tumor, amongst many others. Identification of a disease-defining fusion product is the current gold standard for EFTs, as well as many of the entities in the differential diagnosis. This, admittedly, is not currently readily accessible in many laboratories.
CIC - and BCOR -associated sarcomas may demonstrate membranous CD99 staining; however, CIC -associated tumors generally have nuclear WT1 expression, while BCOR -associated tumors often have nuclear SATB2 expression. Poorly differentiated synovial sarcoma, while also positive for CD99, is usually positive for TLE1, a finding that is rare in EFTs; moreover, with adequate sampling, more prototypic spindle cell areas can usually be identified. Mesenchymal chondrosarcoma can express CD99 although, again with adequate sampling, this can usually be differentiated based on the presence of chondroid matrix. A number of lymphomas – notably acute lymphoblastic, acute myelogenous, and anaplastic large cell – may express CD99, but these can usually be appropriately differentiated using a broad hematolymphoid immunohistochemical panel.
Treatment depends on a multidisciplinary approach. Localized disease is usually treated with a combination of chemotherapy, and surgery and/or radiation therapy. Metastasis at diagnosis is the most important prognostic indicator. The 5-year survival for localized disease is between 65% and 75%, but considerably less with metastases.
Undifferentiated small round cell sarcoma characterized by fusion of a member of the FET family of genes (e.g., EWSR1 ) with a member of the ETS family of transcription factors (e.g., FLI1 )
Rare
May occur at ostensibly any anatomic location
Five-year survival, with localized disease on presentation, is 65%–75%
Untreated tumors are usually fatal
There appears to be a slight male predominance
Tumors are more common amongst those of European descent
May occur at any age, but predominates in children and young adults
Localized pain
Mass involving bone and/or soft tissue
Bone tumors may present with pathologic fracture
Occasionally fever and/or weight loss
Metastasis at diagnosis is the most important prognostic indicator
Treatment requires a multidisciplinary approach; localized disease is usually treated with a combination of chemotherapy, surgery and/or radiation therapy
Soft and friable
White-grey
Variable hemorrhage and necrosis
Sheets of undifferentiated small round-polygonal cells
Scant cytoplasm that is eosinophilic with occasional clear vacuoles
Monomorphic round nuclei with variable mitotic activity
Occasionally tumors may contain rosettes/pseudorosettes
Occasionally tumors may contain focal epithelial differentiation
Necrosis is typically present
Diffuse membranous CD99 staining
Diffuse nuclear FLI1 staining
Diffuse nuclear ERG staining (in cases with ERG fusion partner)
Variable keratin, synaptophysin, and S100
EWSR1-FLI1 in over 90% of cases
EWSR1- may have other partners (e.g., -ERG, - ETV1 , -ETV4, or -FEV )
FUS - can rarely substitute for EWSR1-
CIC -associated sarcoma
BCOR -associated sarcoma
Poorly differentiated synovial sarcoma
Other sarcomas, lymphoma, and carcinoma
In 1996, Richkind et al. described a primitive small cell tumor with a t(4;19)(q35;q13.1) event. Kawamura-Saito et al., reporting two morphologically similar cases with t(4;19)(q35;q13), further showed this resulted in a CIC-DUX4 fusion gene product. These tumors were tentatively classified as Ewing-like sarcoma and with the advent of next generation sequencing many other disease-defining translocations have been identified in undifferentiated round cell sarcomas, including those with the BCOR-CCNB3 fusion gene, amongst many others. The genetic signature of these tumors differs from EFT, thereby justifying their clinical distinction.
CIC -associated sarcomas are rare and highly aggressive neoplasms. They predominate in young adults but have a wide distribution ranging from childhood to the elderly. These tumors predominate in deep soft tissue; however, they may be superficial, or occur in bone or the viscera. Despite initially conflicting studies, overall there appears to be a slight male predisposition.
Imaging findings tend to be non-specific. MRI T1-weighted images yield an intermediate signal, and T2-weighted images have a high signal intensity with heterogeneous contrast enhancement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here