Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Implantable cardioverter-defibrillators (ICDs) provide important therapy for patients at high risk for ventricular fibrillation (VF) or life-threatening ventricular tachycardia (VT). This chapter complements Chapter 121 on technical aspects and Chapter 123 on the subcutaneous (S) ICD.
ICDs are the preferred treatment for secondary prevention of VT/VF when no reversible cause can be identified. Presently, however, more than 80% of ICDs are implanted for primary prevention. The Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) and Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) demonstrated 5% to 7% absolute mortality reductions over 2 to 4 years in high-risk, primary prevention patients and established ICDs as a standard of care. Guidelines based on these trials identify candidates for ICDs with ischemic or nonischemic cardiomyopathy primarily by heart failure class (nonambulatory patients excluded) and left ventricular (LV) ejection fraction (LVEF).
Guidelines and expert consensus based on limited evidence identify subgroups of high-risk patients with less common diseases, including hypertrophic cardiomyopathy, ion channelopathies, and other specific arrhythmogenic cardiomyopathies. Some patient groups at a high risk for VT/VF have not been studied in clinical trials. Expert consensus documents provide recommendations for ICD implantation in them ( Table 122.1 ). ,
| Disease | Class of Recommendation | Details |
|---|---|---|
| Based on Guideline | ||
| Ischemic CM a | I | EF ≤35%, NYHA 2, 3 |
| I | EF ≤30%, NYHA 1 | |
| Nonischemic CM | I | EF ≤35%, NYHA 2, 3 |
| IIB | EF ≤35%, NYHA 1 | |
| Specific conditions and risk factors | IIA/B | HCM LQTS Brugada syndrome Awaiting transplant Myocarditis Sarcoid Chagas disease CM due to lamin A/C, phospholamban, or filamin-c mutation ARVC |
| Based on Expert Consensus (Ischemic Cardiomyopathy) | ||
| <40 days post MI | Needs nonelective pacing, meets primary prevention for ICD ICD at RRT, generator change indicated |
|
| <90 days post PCI/CABG | Needs nonelective pacing, meets primary prevention for ICD ICD at RRT, generator change indicated Previous primary prevention indication; EF unlikely to improve >35% Previous secondary prevention indication, either abnormal EF postrevascularization or previous VT/VF arrest unlikely related to ischemia |
|
In the United States, the Center for Medicare Studies (CMS) has complex reimbursement rules that identify specific exclusions for primary prevention ICD implantation: coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) within 3 months, myocardial infarction (MI) within 40 days, treatment of heart failure for less than 3 months in nonischemic patients, or findings that favor coronary revascularization. Both CMS and practice guidelines require shared decision making before primary prevention ICD implantation. Smartphone apps facilitate following these multiple guidelines, consensus statements, and rules.
ICDs implanted based on primary prevention guidelines increase survival for many patients but suffer from a lack of both specificity and sensitivity: The number of patients who need to receive ICDs to save one life is high (15 to 20), and most patients who experience sudden cardiac death (SCD) do not qualify to receive ICDs. Thus the two great unmet needs are identifying the patients who qualify for ICDs under present guidelines but do not benefit and the patients who do not qualify but would benefit.
Identifying the patients who qualify for ICDs but do not benefit is important beyond reducing unnecessary cost and morbidity. Patients and physicians vary in their acceptance of an invasive therapy that may compromise daily quality of life only to reduce a low future risk. Despite a decade of essentially unchanged guidelines, only a minority of qualifying primary prevention patients receive ICDs. Research in identifying guideline-indicated patients who do not need ICDs has taken two approaches. The first seeks to identify patients in whom advanced competing comorbidities cause early death that negates the long-term benefit of ICDs. Retrospective analyses showed that ICDs did not prolong life in MADIT II and SCD-HeFT patients with advanced comorbidities including heart failure and renal failure. , A subsequent matched cohort study reported no benefit in dialysis patients who qualified for primary prevention ICDs. Present guidelines exclude New York Heart Association (NYHA) class IV heart failure patients unless they are a candidate for cardiac resynchronization therapy (CRT), an LV assist device (LVAD), or heart transplantation.
The second approach reassesses risk for SCD for guideline-indicated patients. For example, the Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality (DANISH) randomized controlled ICD trial of patients with nonischemic cardiomyopathy reported that mortality was low in the control group and ICDs did not reduce overall mortality, although subgroup analysis showed benefit in patients younger than 68 years. Future analysis may elucidate the relative roles of patient selection, compliance with contemporary medical therapy, and CRT pacemakers to reduce mortality in the control group. Nevertheless, DANISH sends a signal about the heterogeneous population of patients with nonischemic cardiomyopathy: Not only are more restrictive guidelines needed, but (at least with contemporary therapy) the overall benefit of ICDs is sufficiently low that it is ethical to test more restrictive criteria in future studies. More specific risk markers are needed. Late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) imaging has been proposed as a candidate.
The paradox of primary prevention guidelines is that they exclude most patients who could benefit. Epidemiologic studies show that up to 20% of people who die suddenly qualified for primary prevention ICDs. About 70% had LVEF greater than 35%, but the absolute risk for arrhythmic SCD is low in these patients. Thus there is an unmet need for accurate risk stratification of low-risk patients. Given the vast number of such patients, research has focused on low-cost methods, including modifiable risk factors for heart disease, genetic markers, other biomarkers, and electrocardiographic markers related to depolarization or repolarization. Statistically significant correlations have between identified. Nevertheless, even combinations of markers identify only patients with two to five times the baseline risk, far short of the approximately 100 times multiple needed to justify primary prevention ICDs.
Transvenous ICDs permit antibradycardia pacing and antitachycardia pacing (ATP) and cardiac resynchronization pacing (see Chapter 125 ). Fig. 122.1 provides an overview of system selection. See Chapter 123 for selection between S-ICD and transvenous ICDs.
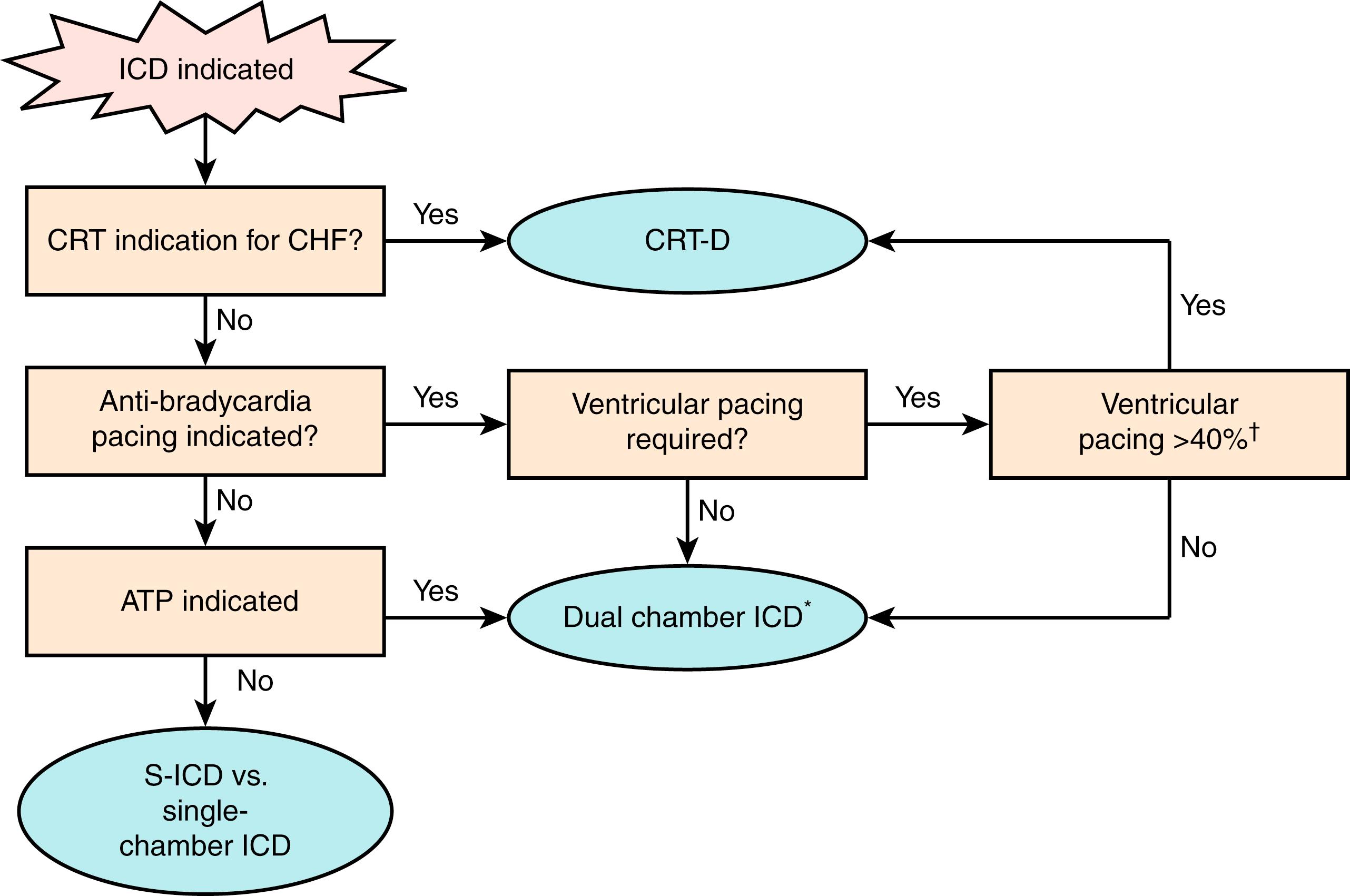
Dual-chamber ICDs provide pacing modes that minimize ventricular pacing to reduce the risk for right ventricular (RV) pacing-induced/exacerbated cardiomyopathy. They permit both diagnostics for atrial fibrillation (AF) and dual-chamber algorithms to discriminate supraventricular tachycardia (SVT) from VT. An atrial electrogram (EGM) improves SVT-VT discrimination by clinicians, so it seems logical that the same should be true for ICDs. Nevertheless, clinical trials have reported inconsistent results. There are three primary reasons for lack of consistent superiority of dual-chamber algorithms. First, for many patients, single-chamber strategic programming results in a sufficiently low rate of inappropriate detection of SVT as VT and an even lower rate of inappropriate shocks. Second, many failures of modern dual-chamber algorithms relate to atrial sensing problems. Although clinical studies were based on assigned treatment, in clinical practice, dual-chamber discrimination is not programmed if atrial sensing is unreliable. Third, early trials tested technically deficient dual-chamber algorithms.
Disadvantages of dual-chamber ICDs include higher cost, atrial lead complications, and decreased longevity. Present consensus favors reserving dual-chamber ICDs for patients who need atrial pacing or secondary prevention patients who have SVT and monomorphic VT with similar ventricular rates.
Excluding leads with known high failure rates, the overall incidence of clinical failure of defibrillation leads is about 1.3 per 100 lead-years. New engineering standards for preclinical testing of leads are expected to improve reliability.
Dual-coil leads (see Chapter 121 ) defibrillate with slightly lower shock strengths than single-coil leads, but there is no difference in first-shock efficacy or all-cause mortality. Atrial cardioversion is more effective with dual-coil than with single-coil leads. EGMs recorded between the proximal coil and ICD housing record atrial signals that are useful for physician interpretation of single-chamber EGMs. Nevertheless, single-coil leads are usually preferred because dual-coil leads increase the difficulty and risk for extraction.
Integrated bipolar leads require one less conductor than dedicated bipolar leads (see Chapter 121 ), so conceptually they may be more reliable. In practice, reliable leads have been developed with both designs. Oversensing of P waves and extracardiac signals is more common with integrated bipoles; T wave oversensing is more common with dedicated bipoles.
Implant testing was mandatory for early ICDs to ensure adequate sensing of VF EGMs and reliable defibrillation. Improved ICD performance and inherent risks for inducing VF have led to evidence-based omission of defibrillation testing for many primary prevention patients.
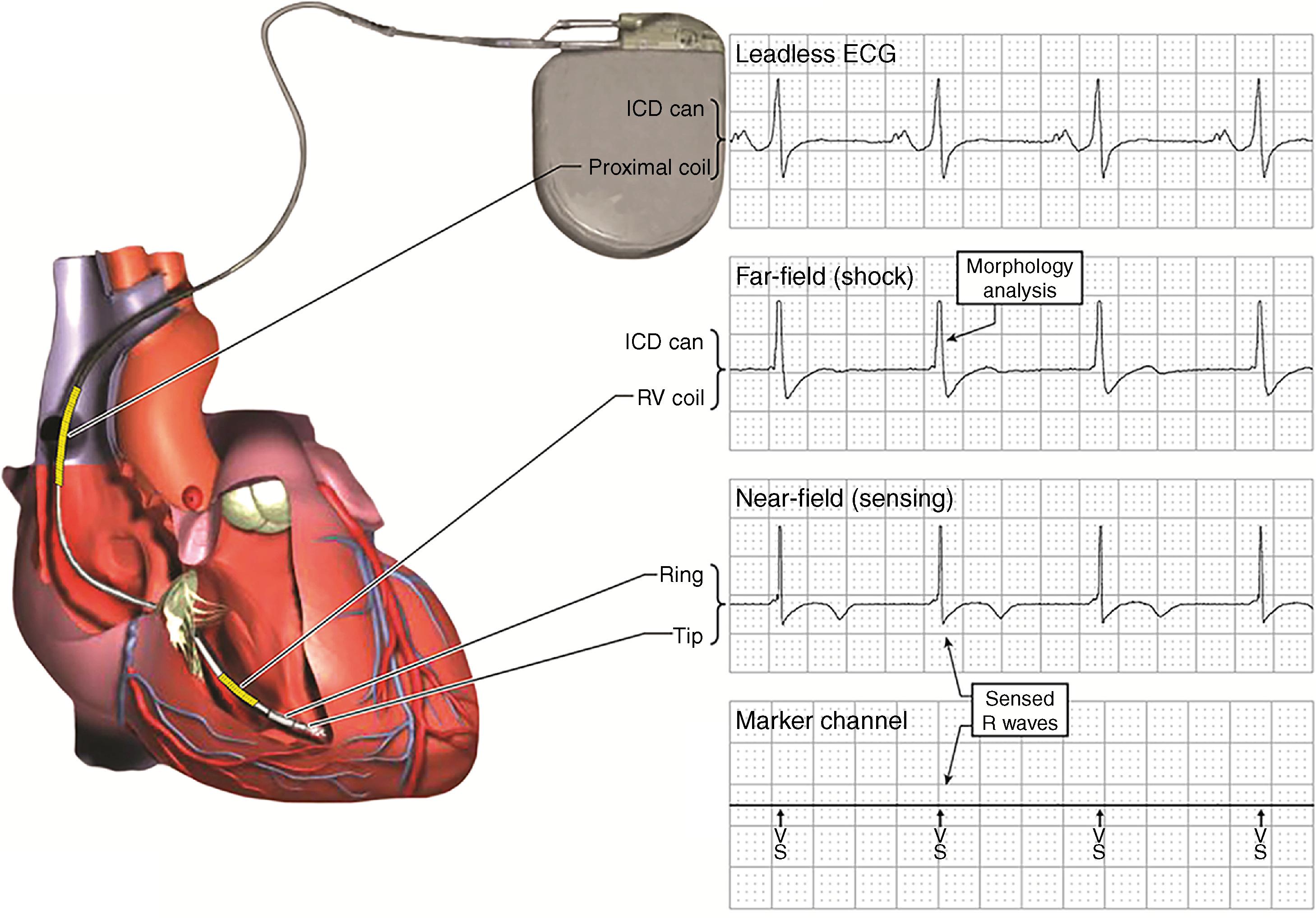
Sensing is the process of identifying EGMs that correspond to individual cardiac depolarization ( Fig. 122.2 ). The combination of dynamic adjustment of sensitivity and short blanking periods produces reliable sensing during VF if the R wave during native rhythm is 5 mV or greater and perhaps as low as 3 mV with dedicated bipolar sensing. Presently, undersensing of VF is caused primarily by variations in VF EGM amplitude that occur faster than dynamically adjusting sensitivity can track them ( Fig. 122.3A ). Thus induction of VF is not required to test sensing for most implants, but it may be important in circumstances that promote undersensing, such as low R wave amplitudes (<3–5 mV), minimum sensitivity reprogrammed above the nominal value or other programming to reduce oversensing, or risk of interdevice or intradevice sensing interaction.
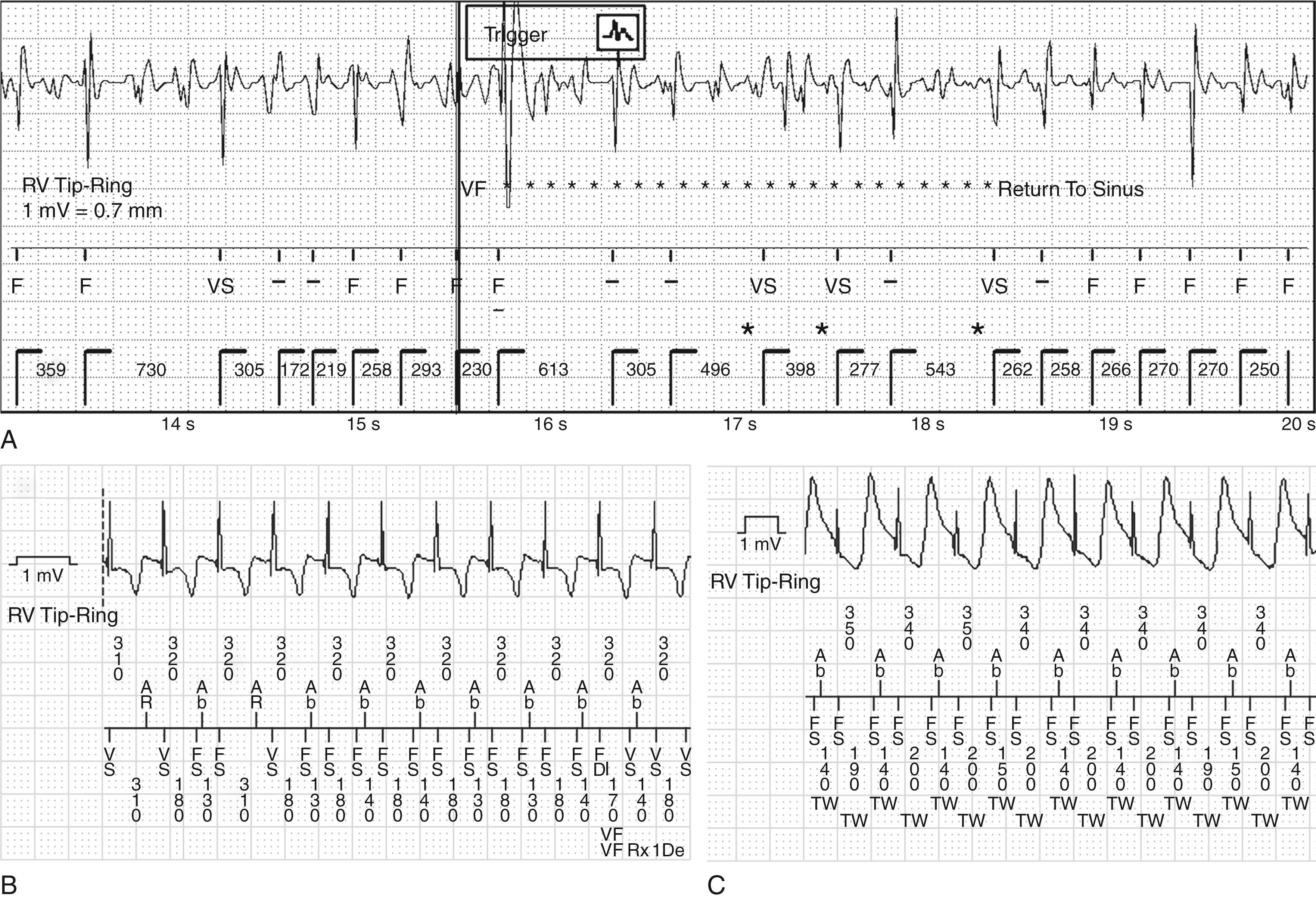
Based on the SIMPLE (Shockless Implant Evaluation) and NORDIC-ICD (No Regular Defibrillation Testing in Cardioverter Defibrillator Implantation) randomized controlled trials, the 2016 consensus statement determined that it is reasonable to omit routine testing of defibrillation efficacy for new implants of left-pectoral ICDs with satisfactory lead parameters and fluoroscopic positioning. Testing is still recommended, however, under other conditions. Present consensus does not provide guidance on how to test, but it is important to understand testing methods because they differ in strengths and limitations.
Defibrillation efficacy is usually assessed directly by fibrillation-defibrillation (defibrillation) testing; it can also be assessed indirectly by vulnerability testing (see Chapter 121 ). Either method can estimate the minimum shock strength that defibrillates (patient-specific strategy) or estimate if the maximum output of the ICD will defibrillate (safety margin strategy).
Clinical testing usually determines the safety margin between the ICD’s maximum shock strength and an estimated high point on the defibrillation probability of success curve. A commonly used implant criterion is two successful, consecutive defibrillations at 10 J below maximum shock strength. Patient-specific defibrillation threshold (DFT) testing is usually reserved for research studies that compare different methods of defibrillation. Statistical modeling of clinical data shows that safety margin testing is more reproducible than DFT testing for clinical implants, in part because the calculated DFT depends on the testing method (shock protocol, initial shock strength, and shock step size). ,
The upper limit of vulnerability (ULV), the weakest shock strength that does not induce VF when delivered during the vulnerable period of the T wave, is linked mechanistically to reinitiation of VF after a shock. It corresponds closely to the 85% to 90% point on the defibrillation probability of success curve. An advantage of vulnerability testing is that it can confirm a defibrillation safety margin without inducing VF in 80% to 95% of patients by testing at 10 to 15 J below maximum output. A disadvantage is that it requires timing measurements to ensure that the T wave shock is delivered at the most vulnerable interval.
Identifying ICD systems with insufficient defibrillation safety margins is challenging because of their low prevalence (5%–10%). Further, because defibrillation is probabilistic, measurements are subject to regression to the mean: If repeated measurements are performed under the same conditions, outlier values (extremely low or high DFTs) are likely to be followed by values nearer the patient’s true mean. A corollary is that a single defibrillation failure may be a low-probability outcome of a shock that has a high probability of successful defibrillation. Additionally, clinical conditions may alter DFTs for spontaneous VF relative to induced VF (e.g., autonomic differences between awake and anesthetized states, hemodynamic and ischemic effects of preceding VT).
Box 122.1 summarizes a stepwise approach to this problem. Attention to the patient’s preoperative condition and technical aspects of the implant reduce the need for troubleshooting. This includes positioning the distal defibrillation coil deep in the RV and confirming that DF-1 high-voltage connections are correct using low-voltage pulses. Before troubleshooting, the diagnosis should be confirmed to ensure that a defibrillation failure was not a chance event caused by the probabilistic nature of defibrillation. Failure of a test shock at 10 J below maximum and the subsequent maximum-output rescue shock is sufficient confirmation if detection of VF is rapid. Nevertheless, the test shock should be repeated if the rescue shock succeeds. Once inadequate defibrillation is confirmed, the first step is to identify reversible causes. If there are none, the clinician may alter the shock vector by inserting an additional defibrillation lead, either subcutaneously or transvenously (e.g., azygous vein, coronary venous system, left subclavian vein for right-sided implants). Alternatively, testing may be repeated on another day after treatment with a drug that lowers DFT, such as sotalol or dofetilide.
Consider risks and benefits of continuing or withdrawing drugs that might increase DFT
Optimize therapy for heart failure and ischemia
Drain left pleural effusion
Position RV electrode tip distally (before testing)
For dual-coil leads, ensure position of proximal coil in high SVC or innominate vein; exclude proximal coil if low in atrium
Set right ventricular polarity to anode for phase 1 if not nominal
Optimize waveform duration for shock pathway impedance if programmable
For DF-1 connectors, confirm correct connections of RV and SVC electrodes
Confirm integrity of current paths using shock impedance assessed by low-voltage pulses
Metabolic: hyperkalemia, hypoxemia, hypercapnia, and acidemia because of deep sedation
Alternate current path (low-shock impedance; e.g., left pleural effusion/hemothorax; pericardial effusion/hemopericardium)
Retained guidewire
Fragments of old intracardiac or intravascular electrodes
High shock impedance (e.g., pneumothorax)
Myocardial depression
Ischemia
Pharmacologic (e.g., amiodarone, verapamil, lidocaine)
Prolonged procedure with multiple fibrillation-defibrillation episodes
Proximal coil in SVC, innominate vein, or high right atrium (exclude proximal coil if low)
For a right-sided implant, exclude can or use inactive can
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here