Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver transplantation has become an important component of treatment algorithms in children diagnosed with primary hepatic malignancy. Interestingly, liver transplantation for malignancy in the pediatric population has historic significance. A 3-year old child with biliary atresia and an incidentally discovered hepatocellular carcinoma (HCC) underwent transplantation on January 2, 1970, and that individual is the oldest surviving liver transplant recipient.
Hepatoblastoma and HCC are the two most common primary hepatic malignancies found in children. Liver transplantation of a child afflicted with a hepatoblastoma unresectable by conventional approach results in an excellent outcome with multiple institutional and cooperative studies reporting survival rates greater than 80%. The role of liver transplantation for HCC in the pediatric population remains more controversial.
In this chapter we will discuss the management of children with primary hepatic malignancy, focusing on recent developments in the use of liver transplantation. In addition, we will discuss the role of transplantation for vascular neoplasms of the liver and the evolving role of transplantation in patients with preexisting metabolic disease who are at risk for the development of HCC.
Hepatoblastoma is the most common primary malignancy found in the liver in childhood with an annual incidence of one per million children. The median age at diagnosis is 1 year, and it is found more frequently in males. A child who has a hepatoblastoma can present in a variety of fashions ranging from an asymptomatic abdominal mass found by a primary caregiver to an acute abdomen secondary to tumor rupture. On occasion the size of the tumor will be so large as to cause respiratory distress or failure to thrive because of loss of abdominal domain. Rarely, a hepatoblastoma may produce β-human chorionic gonadotropin (β-HCG) hormone, resulting in the paraneoplastic process of precocious puberty.
Hepatoblastomas arise from immature hepatic epithelium and are classified histologically into epithelial, anaplastic, or macrotrabecular cell types. The epithelial cell type can be further divided into fetal, embryonal, and small cell undifferentiated variants. Children with a hepatoblastoma consisting of the fetal cell type have the best prognosis. Children with tumors that contain components of small cell undifferentiated histological characteristics have the worst prognosis.
The cause of a hepatoblastoma remains unknown. As with many malignancies, abnormalities in gene expression are thought to play a role; however, the specific mechanism by which the tumor develops remains unclear. Patients who are afflicted with the genetic conditions of Beckwith-Wiedemann syndrome, its variant hemihypertrophy, and familial adenomatous polyposis are found to have an increased incidence of hepatoblastoma and need close surveillance in childhood. Recent studies have suggested the β-catenin pathway may be aberrantly activated, whereas other studies using genomic sequencing have identified profiles that may portend a worse prognosis. These studies require validation.
Blood tests obtained from a child with a hepatoblastoma will often show anemia, thrombocytosis, and leukocytosis. Hepatocellular transaminase levels are usually within normal limits. Serum levels of α-fetoprotein (AFP) are elevated in over 90% of patients with a hepatoblastoma. On occasion an elevated β-HCG level will be found.
Radiographic analysis is essential in the diagnosis and treatment of a child in whom a hepatoblastoma is suspected. Cross-sectional imaging of the abdomen using either computed tomography (CT) or magnetic resonance imaging (MRI) will identify the site from which a mass arises and will assist in the determination of the resectability of the lesion. The proximity of the tumor and the presence of thrombus within the portal vein and major hepatic veins or inferior vena cava can be determined by the same imaging modalities or by ultrasonography. A CT scan of the chest should be used to rule out metastatic lung disease.
Tissue diagnosis by definitive resection or biopsy obtained via a percutaneous, open, or laparoscopic approach is necessary to establish the diagnosis.
Over the past 15 years the staging systems for patients afflicted with hepatoblastoma have evolved significantly. The Pret reatment ext ent of disease (PRETEXT) staging system has been accepted by multiple oncology collaborative study groups as the optimal method to classify patients. In this system, tumors at the time of diagnosis are staged by radiographic analysis according to the number of sectors of the liver in which tumor is present. The liver is divided into a right anterior, right posterior, left medial, and left lateral sector. Patients are classified in four stages: PRETEXT I, tumor in only one sector; PRETEXT II, tumor involving two adjoining sectors; PRETEXT III, tumor involving three contiguous sectors or in two nonadjoining sectors; PRETEXT IV, tumor in all four sectors ( Fig. 28-1 ). In addition to the intrahepatic extent of disease, the involvement of a hepatic or portal vein, the presence of extrahepatic spread, and the presence of metastatic disease are documented. The PRETEXT staging system has been shown to be a predictor of outcome in multiple clinical trials.
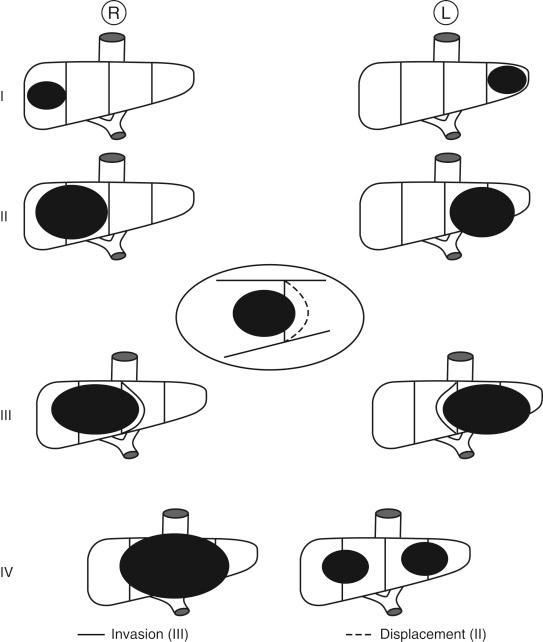
In the recent Children’s Oncology Group (COG)-sponsored trial AHEP 0731, a combination of both PRETEXT and the Evans classification, the traditional North American staging system ( Table 28-1 ), was used to stratify patients. It is anticipated that this combination will be used in future trial design.
| I | Complete resection |
| II | Microscopic residual tumor |
| III | Gross residual tumor Biopsy without resection |
| IV | Metastatic disease at diagnosis |
Complete surgical resection of the primary liver lesion remains the most crucial intervention required to achieve long-term survival. In children who present with tumor confined to a single lobe of the liver without vascular involvement, standard lobectomy followed by adjuvant chemotherapy is indicated. Historically over 60% of children presented with lesions unresectable by conventional surgery, and the outcome was poor due to residual disease after attempted resection. In 1982 Evans et al reported a significant improvement in the outcome of children treated with a combination of adjuvant chemotherapy followed by surgical resection. This finding dramatically altered the strategy for treatment of children with hepatoblastoma. Over 75% of lesions initially felt to be unresectable will decrease sufficiently in size with neoadjuvant chemotherapy to allow conventional resection. The combination of adjuvant chemotherapy followed by conventional resection has improved the prognosis of children with hepatoblastoma such that 70% to 80% will achieve long-term survival. Recent trials have further defined the role of chemotherapy in management, identifying other active agents, including doxorubicin and irinotecan. These agents have improved treatment algorithms with the goal of allowing more conventional resection.
In spite of these improvements, some patients after adjuvant chemotherapy will have tumor that remains unresectable by conventional resection. It is these patients who benefit from total hepatectomy and orthotopic liver transplantation. Although initial studies on the outcome of orthotopic liver transplantation for hepatoblastoma reported mixed results, multiple studies have documented the efficacy of this form of treatment ( Table 28-2 ). Transplantation can also be used for salvage after attempted conventional resection in which residual disease remains, although some studies suggest that these patients have worse outcome.
| Author | No. of Patients | Preoperative Chemotherapy | Prior Resection (%) | Recurrence (%) | Mortality (%) | Overall Survival (%) |
|---|---|---|---|---|---|---|
| Penn | 18 | No | NA | 50 | NA | 50 |
| Koneru et al | 12 | No | 33 | 25 | 25 | 50 |
| Al-Qabandi et al | 8 | Yes | 25 | 25 | 12 | 63 |
| Reyes et al | 12 | Yes | 8 | 17 | 0 | 83 |
| Srinivasan et al | 13 | Yes | 8 | 8 | 7 | 93 |
| Molmenti et al | 9 | Yes | 33 | 13 | 33 | 63 |
| Pimpalwar et al | 12 | Yes | 0 | 17 | 8 | 83 |
| Tiao et al | 8 | Yes | 38 | 0 | 12 | 88 |
On occasion the tumor size at the time of presentation is so large that it may compromise the respiratory status of the patient. The time required for adjuvant chemotherapy to induce tumor shrinkage may leave the patient ventilator dependent for a prolonged period of time. In these patients, early transplantation may be indicated.
The current COG treatment algorithm in the management of a child who presents with a hepatoblastoma uses a combination of conventional resection, chemotherapy, and transplantation (AHEP 0731; Fig. 28-2 ). The treatment algorithm is tailored to the individual child. In those children who present with unresectable lesions at the time of diagnosis, adjuvant cisplatin-based chemotherapy is administered, and after every two cycles of chemotherapy, the patient is restaged radiographically. If the lesion has decreased in size to allow for conventional resection, surgery is performed. At least two cycles of chemotherapy are administered after resection.
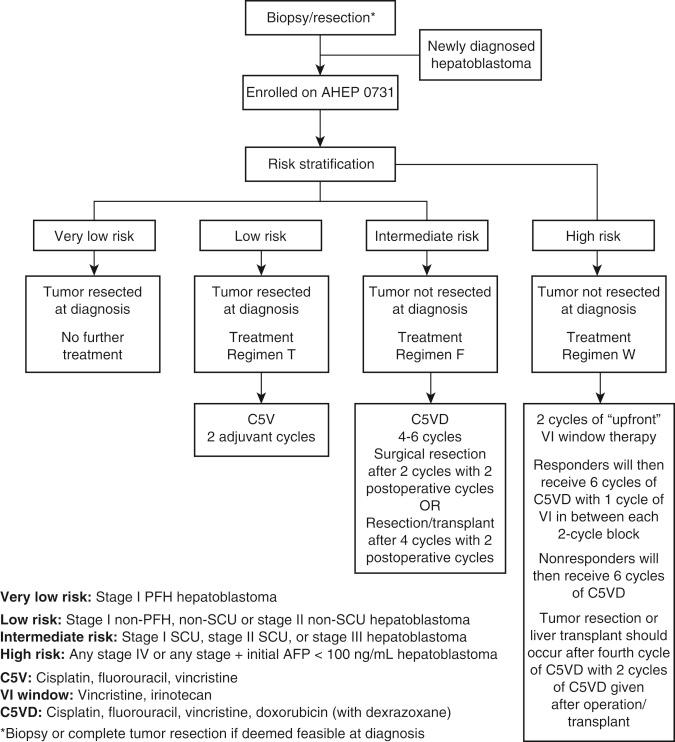
In children diagnosed with a PRETEXT III or IV tumor, early referral to an experienced center with liver transplantation capacity is recommended so that should transplantation be warranted, the number of chemotherapy cycles administered while awaiting transplantation is limited. If the lesion remains too large to resect after four cycles of chemotherapy, the patient is listed for transplantation. Chemotherapy is continued while the patient is waiting for transplantation. In some of our patients we have used living related transplantation so as not to prolong the use of chemotherapy while waiting for an appropriate organ. Ideally, two rounds of chemotherapy are administered after transplantation.
One area of controversy in the management of hepatoblastoma is the role of aggressive conventional resection (i.e., trisegmentectomy or central liver resection) in those children who have bulky tumors despite adjuvant chemotherapy. Surgical radicality as defined by trisegmentectomy was shown to be a negative predictor of outcome in the German Cooperative Pediatric Liver Tumor Study HB 94. In contrast, a recent study suggests aggressive conventional resection did not negatively affect outcome. Even in the patients who had positive microscopic margins after resection, none developed local tumor recurrence.
Patients with extensive hepatic disease not amenable to conventional resection after chemotherapy who also had metastatic lung lesions at diagnosis may still be considered for transplantation. Although the overall prognosis is worse for this group of patients, there is a subset that responds well to adjuvant chemotherapy and if complete clearance of their lung disease can be achieved, may do well following transplant. Thoracoscopic or open resection may be necessary to remove all metastatic lesions. In the SIOPEL (International Childhood Liver Tumours Strategy Group) 1 study performed in Europe, there were three patients who presented with metastatic lung disease and large lesions that responded well to chemotherapy and subsequently underwent transplantation who were disease free 3 years after transplant. In our own experience, we performed transplantation in several children who had lung lesions at the time of diagnosis, all of whom have done well since transplant. The length of time a patient needs to be free of metastatic disease before transplantation remains uncertain.
Initial reports of liver transplantation for hepatoblastoma found a 50% survival rate with half of the poor outcomes due to tumor recurrence. Most patients did not receive adjuvant chemotherapy. More recent studies, in which patients received chemotherapy both before and following transplantation have reported improved outcome after liver transplantation such that the 5-year survival after transplant ranges from 63% to 93%. Variables previously thought to be predictors of poor outcome, such as vascular invasion or metastatic disease at the time of presentation, have not been shown to affect outcome after transplantation. Transplant-related complications were the cause of mortality in less than 10% of the patients since 1999. These studies demonstrate the efficacy of transplantation in patients who had what was considered unresectable hepatoblastoma.
Tumor recurrence remains a significant problem following transplantation, with recurrence rates of up to 25% in recent series. The outcome is usually poor if recurrence occurs. Recurrence rates following transplantation, however, are similar to those following conventional resection. A recent study by Pimpalwar et al addressed this observation. They found that tumor susceptibility to chemotherapy, as manifest by decreasing AFP levels or reduction in tumor size indicated by cross-sectional imaging, better predicted outcome than the manner by which the tumor was completely removed. In patients who had a poor response to adjuvant chemotherapy, the outcome was worse, regardless of whether the patient underwent conventional resection or transplantation, when compared with those who had a good response to chemotherapy before surgery. The number of patients within this study was small, and a larger study will be necessary to confirm these findings.
Close follow-up is essential for all children who have undergone treatment for a hepatoblastoma. Serial measuring of AFP levels and radiographic evaluation during the first 3 years after treatment are important so that early detection of recurrent disease is possible.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here