Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Approximately 15% of all blood transfusions in the United States are used in the care of patients who have sustained traumatic injury. Blood transfusion in trauma is lifesaving for those patients in hemorrhagic shock, as the median time to hemorrhagic death in trauma is 2.0 to 2.6 hours, and approximately 85% of hemorrhagic deaths occur within 6 hours after hospital admission. Importantly, concomitant attempts at prompt cessation of hemorrhage and definitive hemorrhage control are also necessary. Earlier time to hemostasis is associated with decreased 30-day mortality, acute kidney injury, ARDS, multiple organ failure and sepsis in bleeding trauma patients, and should be considered an important quality indicator.
Trauma patients in hemorrhagic shock may develop trauma-induced coagulopathy (TIC) which consists of both acute trauma coagulopathy (ATC) related to tissue injury and shock and occurs immediately, and resuscitation coagulopathy (RC) related to fluid/blood product administration, hypothermia, acidosis and hypocalcemia. Early administration of blood and blood products (plasma, platelets, cryoprecipitate, fibrinogen concentrate [FC]) in ratios close to whole blood composition (hemostatic resuscitation, damage control resuscitation), with attempts to correct the ATC, is associated with improved outcomes in prospective and retrospective cohort studies.
Blood transfusion in trauma has also been identified as an independent predictor of multiple-organ failure (MOF), systemic inflammatory response syndrome (SIRS), increased postinjury infection, and increased mortality rate in multiple studies. The cumulative risks of blood transfusion have been related to the number of units of packed red blood cells (PRBCs) transfused, increased storage time of transfused blood, and possibly donor leukocytes. Lack of efficacy of RBC transfusion for anemia in critically ill patients who are hemodynamically stable has also been documented. Therefore, once hemorrhage control has been established in acute trauma we should attempt to minimize the use of blood transfusion for the treatment of asymptomatic anemia in trauma patients.
A number of potential mechanisms that may mediate adverse effects associated with blood transfusion in trauma have been proposed, including increased systemic inflammatory response, immunomodulation, microcirculatory dysfunction due to altered RBC deformability, increased nitric oxide binding by free hemoglobin and vasoconstriction, and others. These data have led some to conclude that blood transfusion in the injured patient should be minimized whenever possible.
The majority of potentially preventable early trauma deaths still result from uncontrolled hemorrhage including noncompressible torso hemorrhage. Trauma patients in hemorrhagic shock have an absolute indication for PRBC transfusion if they are unresponsive to isotonic crystalloid if they have ongoing significant hemorrhage, and manifest physiologic signs of persistent shock (hypotension, tachycardia, oliguria, lactic acidosis, abnormal base deficit [BD])—indicating that oxygen consumption is dependent on hemoglobin concentration (critical oxygen delivery). In these patients, the prompt transfusion of PRBCs and blood products in conjunction with prompt hemorrhage control is lifesaving.
Patients with hemorrhagic shock, identified by a metabolic acidosis and increasing BD, have been documented to require increased blood and plasma transfusion ( Table 1 ). A single-institution study documented that 8% (479 of 5645) of acute trauma patients received PRBCs, using 5219 units. The majority (62%) of transfusions were administered in the first 24 hours of care. Only 3% of patients ( n = 147) received more than 10 units of PRBCs, and these patients also received plasma and platelet transfusions to treat actual or anticipated dilutional coagulopathy. Mortality rates in trauma patients who require blood transfusion is high, ranging from 27% to 39%.
| Admission Base Excess | Interpretation | Units PRBCs in First 24 Hours | Total Units PRBCs | Total Units FFP |
|---|---|---|---|---|
| ≥ −2 | Normal | 0–1 | 1–2 | 0–1 |
| −3 to −5 | Mild base deficit | 1–2 | 2–3 | 0–1 |
| −6 to −9 | Moderate base deficit | 3–4 | 5–6 | 1–2 |
| ≤ –10 | Severe base deficit | 8–9 | 9–10 | 3–4 |
Massive blood transfusion is most commonly defined as complete replacement of a patient’s blood volume within a 24-hour period or more than 10 units of PRBCs in 24 hours. Newer definitions include an ongoing blood loss of more than 150 mL/minute, or the replacement of 50% of the circulating blood volume in 3 hours or less. These newer definitions have the benefit of allowing early recognition of major blood loss and of the need for effective intervention to prevent hemorrhagic shock and other complications of massive hemorrhage and transfusion.
Massive transfusion (MT) therapy for the treatment of hemorrhagic shock requires a coordinated and detailed approach with the fundamental components listed in Table 2 . In the absence of a predefined MT protocol, access to the appropriate blood products (and adequate volume of these products) may be significantly delayed. Without prompt replacement of these blood products, the resultant coagulopathy may worsen and bleeding will continue. In fact, the implementation of an organized “Massive Transfusion policy” to address exsanguinating hemorrhage in the trauma population has proved to be of benefit in patient outcomes and in reducing blood product use. Studies have demonstrated an increase in survival rate (16% to 45%) in patients with exsanguinating hemorrhage following the implementation of such an MT protocol. Guidelines for the treatment of acute massive blood loss are listed in Table 3 .
| Provide adequate ventilation and oxygenation |
| Expedite control of the source of hemorrhage |
| Restore the circulating volume |
| Minimize or discontinue crystalloid fluid resuscitation |
| Hypotensive resuscitation, systolic blood pressure 80–100 mm Hg |
| Start blood component therapy: RBCs, FFP, Plts, Cryo |
| Initiate massive transfusion protocol |
| Anticipate and treat coagulopathy, thrombocytopenia |
| Maintain or restore normothermia |
| Evaluate the therapeutic response |
| Test for coagulopathy, thrombocytopenia, and DIC |
| Consider TEG or ROTEM |
| Know and implement specific local procedures for dealing with the logistic demands of massive transfusion |
| Goal | Procedure | Comments |
|---|---|---|
| Arrest bleeding |
|
|
|
|
|
| Request laboratory testing |
|
|
| Request red blood cells |
|
|
|
|
|
|
|
|
| Request cryoprecipitate |
|
|
| Suspect DIC |
|
|
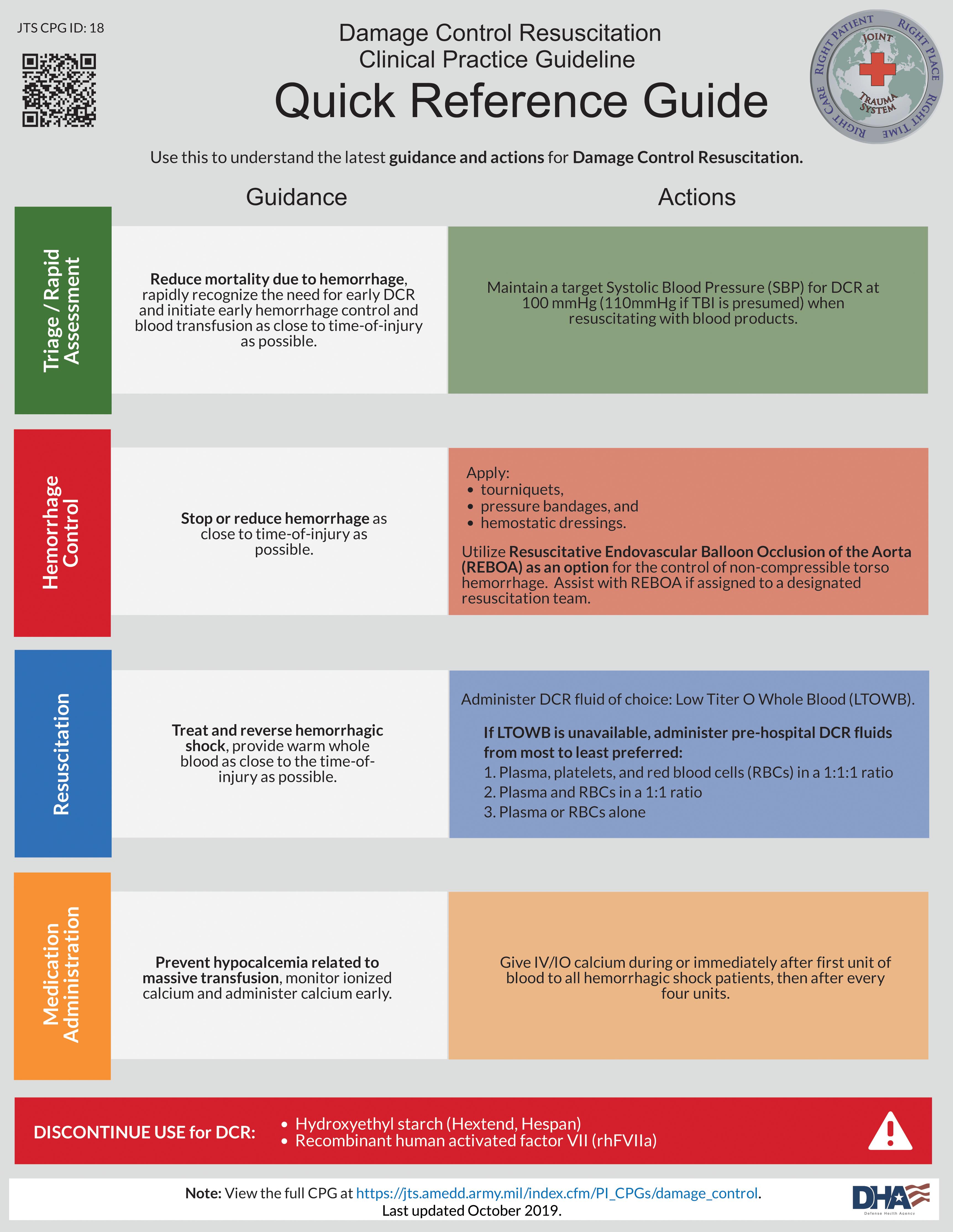
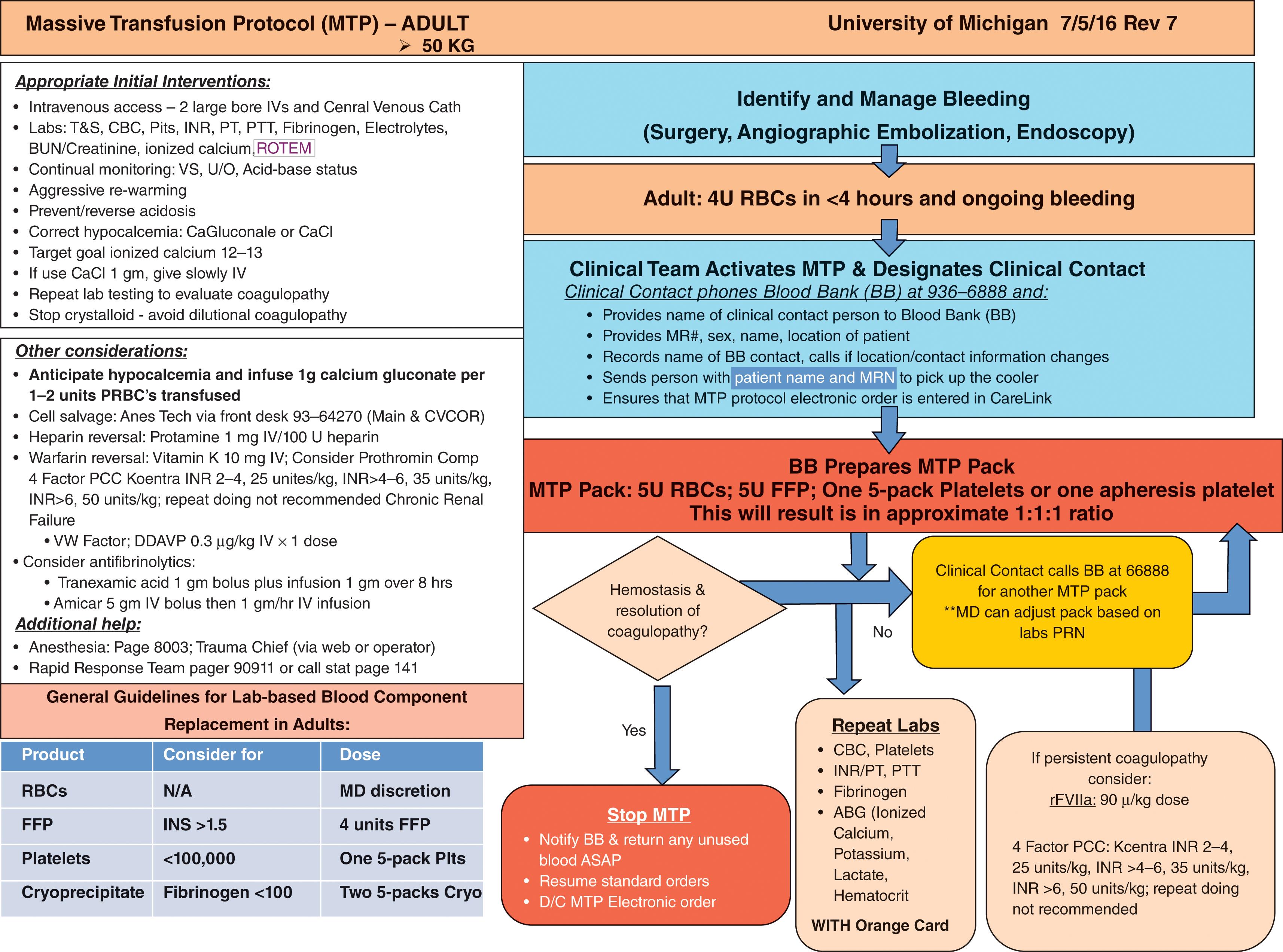
The Joint Trauma System Clinical Practice Guidelines for damage control resuscitation in military combat casualty care are an excellent resource as an evidence-based guideline and provide a template for MT protocol ( Fig 1 ). Our University of Michigan Trauma Massive Transfusion protocol is provided as a template for a civilian MT protocol ( Fig. 2 ).
Data from a large prospective multicenter cohort study evaluating outcomes in blunt injured adults with hemorrhagic shock was used to examine resuscitation strategies over time (2004–2009) and determined that the percentage of patients who required MT overall significantly decreased over time. Over the study period for the MT group ( n = 526), initial BD and admission international normalized ratio (INR) were unchanged, but Injury Severity Score (ISS) was significantly higher. No significant differences were found over time for 6-hour, 12-hour, and 24-hour FFP:PRBC and Plt:PRBC (platelet:PRBC) transfusion ratios in MT patients. Sub-MT patients ( n = 344) had significantly higher 6-hour FFP:PRBC ratios and significantly higher 6-hour, 12-hour, and 24-hour Plt:PRBC ratios in the recent time period. The 6-hour/24-hour percent total for FFP and platelet transfusion was significantly greater in the recent time period (FFP: 54% vs.70%, P = .004; and Plt 46% vs. 61%, P = .048). This study documented that in a severely injured cohort requiring MT, FFP:PRBC and Plt:PRBC ratios have not changed over time, whereas the rate of MT overall has significantly decreased. During the recent time period (after December 2007), significantly higher transfusion ratios and a greater percent of 6-hour/24-hour FFP and platelet transfusion were found in the sub-MT group, those patients just below the PRBC transfusion threshold definition of MT. These data suggest early, more aggressive attainment of high blood product transfusions ratios may reduce the requirement for MT and may shift overall blood requirements below those that currently define MT. Further prospective evidence is required to verify these findings.
Time to initiation of hemostatic/damage control resuscitation is thought to be associated with improved outcomes in MT patients. The identification of early predictors of MT in trauma would prevent undertriage of trauma patients likely to require MT. The Assessment of Blood Consumption (ABC) score is recommended for use by ACS TQIP to trigger MTP since it is easy to remember and use, and includes four simple variables: SBP less than 90 mm Hg, heart rate (HR) 120 beats per minute (bpm) or greater, positive result for focused assessment with sonography in trauma (FAST), and penetrating mechanism of injury. An ABC score ≥ 2 is indicative of possible need for MT, with a score of 3 associated with 45% change of MT, and a score of 4 associated with 100% chance of MT in the initial report. Comparisons of the many predictive scores for MT in trauma still confirm the utility of the ABC score ( Table 4 ).
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUROC | Studies cited | |
|---|---|---|---|---|---|---|
| ABC ≥ 2 | 47.0–90.0 | 67.0–89.8 | 13.2 | 98.0 | 0.74–0.90 | Brockcamp et al; Schroll et al; Nunez et al; Cotton et al |
| TASH | 84.4 | 78.4 | 18.9 | 98.8 | 0.842–0.889 | Brockcamp et al; Nunez et al |
| PWH/Rainer | 80.6 | 77.7 | 17.7 | 98.5 | 0.860 | Brockcamp et al |
| Vandromme | 78.9 | 76.2 | 16.5 | 98.4 | 0.840 | Brockcamp et al |
| Schreiber | 85.8 | 61.7 | 11.8 | 98.7 | 0.800 | Brockcamp et al |
| Larson | 70.9 | 80.4 | 17.4 | 97.9 | 0.823 | Brockcamp et al |
| Shock Index | 67.7 | 81.3 | — | — | 0.83 | Schroll et al |
* The American College of Surgeons Trauma Quality Improvement Project (TQIP) recommends use of the ABC score.
For severe hemorrhagic shock, uncrossmatched type O blood should be transfused due to immediate availability. Advanced Trauma Life Support (ATLS) encourages a transition to RBC products immediately after failure to achieve hemodynamic stability with 2 L of crystalloid solution. Because emergent transfusions are typically needed before identifying a patient’s specific blood type, uncrossmatched RBC (URBC) products are commonly used. Rh-positive blood is commonly used in male trauma patients, with minimal transfusion-related complications, and a low rate of seroconversion of Rh-negative patients.
Rh-negative blood should be used in women of childbearing age if possible. A prompt transition to the use of type-specific (ABO, Rh-matched) blood should be accomplished as quickly as possible (approximately 10 minutes for type-specific RBCs), and use is continued until fully crossmatched units of blood are available (approximately 30 to 40 minutes for full crossmatching). Once a trauma patient has been administered more than one blood volume and the initial antibody screen is negative, there is no point attempting compatibility testing except for ABO matching.
Fresh frozen plasma (FFP) is administered to trauma patients with hemorrhage in an attempt to correct the ACOT. A systematic review of 37 observational studies documented that in patients undergoing MT, plasma infusion at high FFP:RBC ratios was associated with a significant reduction in the risk of death (OR, 0.38; 95% CI, 0.24 to 0.60) and multiorgan failure (OR, 0.40; 95% CI, 0.26 to 0.60). However, the quality of this evidence was very low due to significant unexplained heterogeneity and several other biases. In patients undergoing surgery without MT, plasma infusion was associated with a trend toward increased mortality rate (OR, 1.22; 95% CI, 0.73 to 2.03). Plasma transfusion was associated with increased risk of developing acute lung injury (OR, 2.92; 95% CI, 1.99 to 4.29). The study conclusion was that very low quality evidence confirms that FFP infusion in the setting of MT for trauma patients is associated with a reduction in the risk of death and multiorgan failure but a threefold increased risk of acute lung injury. We therefore keep thawed plasma available in the emergency department for use in hemostatic/damage control resuscitation in trauma.
Although FFP is widely available as a potential source of fibrinogen, it has multiple shortcomings such as extended administration time, transfusion-related complications, and limited efficacy. Blood group matching is required, and because FFP is stored at − 20° C it needs to be thawed before administration. The average concentration of fibrinogen in FFP is around 2.5 g/L, although there is considerable variation. The low concentration of fibrinogen in FFP limits the extent to which the fibrinogen level can be raised. FFP is not typically subjected to viral inactivation procedures, so there are risks of viral transmission. Treatment with methylene blue or solvent detergent unfortunately reduces the level of fibrinogen in the end product (particularly in the case of methylene blue treatment, in which the reduction is around 30%). Finally, numerous publications have documented serious adverse events following FFP transfusion including volume overload and transfusion-related acute lung injury (TRALI).
Platelet transfusion is administered early, along with RBCs and FFP, as a part of hemostatic/damage control resuscitation, particularly in patients requiring MT. Platelets should be transfused in bleeding trauma patients with a goal to keep the platelet count higher than 100,000 to establish a stable clot.
The impact of platelet transfusion in trauma patients undergoing MT was evaluated in a retrospective single-institution study. For injured patients requiring MT, as the apheresis Plt:RBC ratio increased, a stepwise improvement in survival was seen. Similar to recently published military data, transfusion of Plt:RBC ratios of 1:1 was associated with improved early and late survival, decreased hemorrhagic death, and a concomitant increase in MOF-related mortality rate in a large multicenter retrospective study. Based on these data, increased and early use of platelets may be justified, pending the results of prospective randomized transfusion trials in trauma.
Similar to data with RBC transfusion, increased storage age of platelets has been associated with adverse outcome. In a study of 380 critically ill trauma patients who received platelet transfusions, there was a stepwise increase in complications, in particular sepsis, with exposure to progressively older platelets. Further evaluation of the underlying mechanism and methods for minimizing exposure to older platelets is warranted, as is further prospective evaluation of the role of platelet transfusion in massively transfused patients.
Cryoprecipitate contains a higher concentration of fibrinogen than FFP, typically around 15 g/L. However, it shares many of the disadvantages of FFP. The risk of viral transmission is similar to that of FFP, but the risk is enhanced by the fact that each pool of cryoprecipitate contains human plasma from multiple donors. Despite internal quality control protocols in blood banks, the fibrinogen concentration is variable and blood group matching is needed. Finally, time is also required for thawing cryoprecipitate. Cryoprecipitate was withdrawn from most European countries some years ago on the basis of safety concerns, though it remains available in Scandinavia, the United Kingdom, and the United States. Cryoprecipitate is unsuitable for pathogen-reduction steps, but it can be produced from plasma that has undergone treatment.
Current therapeutic options for supplementing plasma fibrinogen are FFP, cryoprecipitate, and FC. FC is currently indicated for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia. FC is increasingly being used in severe trauma patients with hemorrhagic shock, but there is significant controversy regarding its efficacy.
A systematic review of studies to date compared efficacy of FFP and FC in trauma or perioperative patients. The weight of evidence does not appear to support the clinical effectiveness of FFP for surgical and massive trauma patients and suggests that it can be detrimental. Perioperatively, FC was generally associated with improved outcome measures, although more high-quality, prospective studies are required before any definitive conclusions can be drawn.
The single-center R Eversal of T rauma- I nduced C oagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC) study focused on treatment of coagulation in major trauma and reported that coagulation factor concentrates with FC at a dose of 50 mg/kg was more effective than FFP with early hemorrhage control, correction of TIC, reduced blood component transfusion, and decreased MT rate. FFP patients had a 3-fold higher rate of MT, but no difference in hospital mortality. The Fibrinogen Early in Severe Trauma Study (FEISTY) is an exploratory, multicenter, randomized controlled trial comparing FC to cryoprecipitate for fibrinogen supplementation in traumatic hemorrhage when viscoelastic testing confirms fibrinogen deficiency ( http://www.feisty.org.au/ ).
Thromboelastography (TEG) functional fibrinogen assay studies indicate that fibrinogen is critical in correcting abnormal clot strength following trauma. In vitro studies demonstrated that the addition of FC resulted in an increase in functional fibrinogen, clot strength, and percent of fibrinogen contribution to clot strength. These data suggest that fibrinogen should be addressed early in trauma patients manifesting ACOT, but additional studies are warranted to determine dose and timing.
Dilution is inevitable when giving blood component therapy ( Fig. 3 ). The administration of 1 unit each of PRBC, FFP, and platelets does not reconstitute into the amount of coagulation factors in a unit of whole blood. Therefore, dilutional coagulopathy and dilutional thrombocytopenia are common even with early aggressive transfusion of blood components, even with hemostatic/damage control 1:1:1 RBC:FFP:Plt blood component resuscitation, particularly in patients requiring MT.
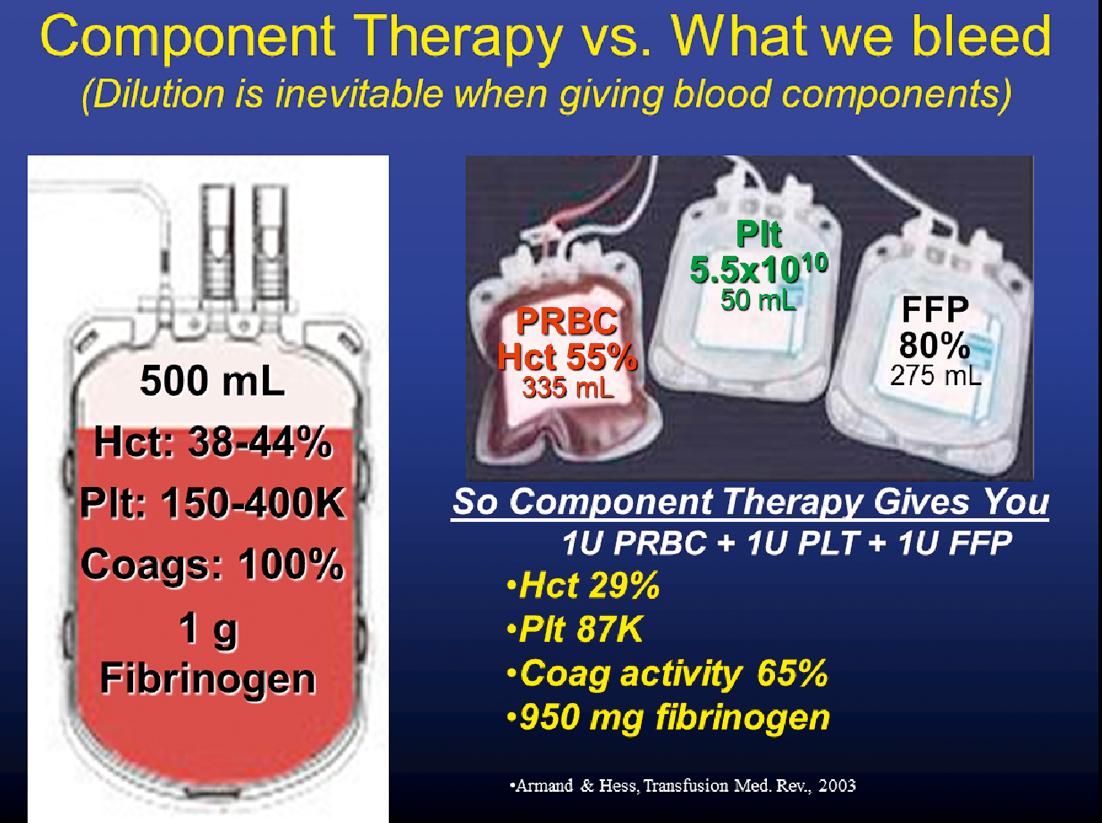
The administration of early balanced resuscitation of RBCs, plasma, and platelets (hemostatic resuscitation, damage control resuscitation) has been shown in numerous retrospective investigations to have a mortality benefit for patients ultimately requiring MT. Increasing evidence suggests that high FFP:PRBC and Plt:PRBC transfusion ratios may prevent or reduce the morbidity associated with early coagulopathy, which complicates MT. Despite studies demonstrating an advantage for hemostatic/damage control resuscitation in severely injured patients, it remains difficult to readily identify those most likely to benefit from this approach early after injury.
Coagulopathy and thrombocytopenia are a common occurrence during major trauma resuscitation, and hemorrhage remains a major cause of traumatic deaths. Current coagulation factor replacement practices vary, and some may be inadequate. A recent pharmacokinetic model was used to simulate the dilutional component of coagulopathy during hemorrhage and compared various FFP transfusion strategies for the prevention or correction, or both, of dilutional coagulopathy. This study documented that once excessive deficiency of factors has developed and bleeding is unabated 1 to 1.5 units of FFP must be given for every unit of PRBCs transfused. If FFP transfusion should start before plasma factor concentration drops below 50% of normal, an FFP:PRBC transfusion ratio of 1:1 would prevent further dilution. They concluded that during resuscitation of a patient who has undergone major trauma the equivalent of whole-blood transfusion is required to correct or prevent dilutional coagulopathy.
Ultimately, additional blood product administration for the treatment of dilutional and consumptive coagulopathy and thrombocytopenia in trauma must be guided by blood coagulation testing, with regular monitoring of hemoglobin, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen levels (see Table 3 ) and viscoelastic testing with the use of TEG and thromboelastometry (ROTEM) for more detailed analysis of the acute coagulopathy of trauma (ACOT).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here