Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Beeson reported the first cases of transfusion-associated hepatitis in 1943, describing seven patients who developed illness 1 to 4 months after having received a red blood cell (RBC) or plasma transfusion. Later identified as transfusion-transmitted hepatitis B virus (HBV), the advent of the acquired immunodeficiency syndrome (AIDS) epidemic in the 1980s followed by recognition of transfusion transmission of hepatitis C virus (HCV) in the 1990s and, most recently, reports of transfusion-transmitted West Nile virus (WNV) and Zika virus (ZIKV) infections in the 21st century have continued to draw attention to the safety of the blood supply, particularly the potential risk from emerging infectious agents.
Although infections with the human immunodeficiency virus (HIV), HBV, HCV, WNV, and ZIKV have been the most publicized, a wide spectrum of other organisms, including viruses, bacteria, parasites, and prions, have been transmitted by transfusion ( Table 304.1 ). Of these, transfusion transmission of bacterial infections is most common and earliest recognized in the history of transfusion, now is most often associated with platelet transfusions, and infrequently results in sepsis and death. Recognition of the threat of Trypanosoma cruzi transmission via transfusion and the availability of suitable testing have led to screening for this pathogen, which is the cause of Chagas disease. In addition to WNV, mosquito-borne pathogens, such as dengue virus, chikungunya virus, and most recently, ZIKV, have shown potential for transfusion transmission, although the burden of disease is unknown. Tick-borne agents also are recognized to pose an increasing risk to transfusion safety, including transmission of babesiosis and, most recently, anaplasmosis and ehrlichiosis. Transmission of variant Creutzfeldt-Jakob disease (vCJD) via transfusion has occurred in the United Kingdom.
| Viruses |
|
| Bacteria |
| Parasites |
|
| Prions |
|
a Higher risk in platelets (see Table 304.3 ); in addition, Streptococcus gallolyticus (bovis) is notable for being associated with colon cancer in the donor.
b Noted in red blood cell units associated with cryophilic organisms, including Pseudomonas fluorescens, Serratia liquefaciens, and Yersinia enterocolitica.
c Not thought a current risk; last reported transfusion transmission of syphilis in the United States was in 1966.
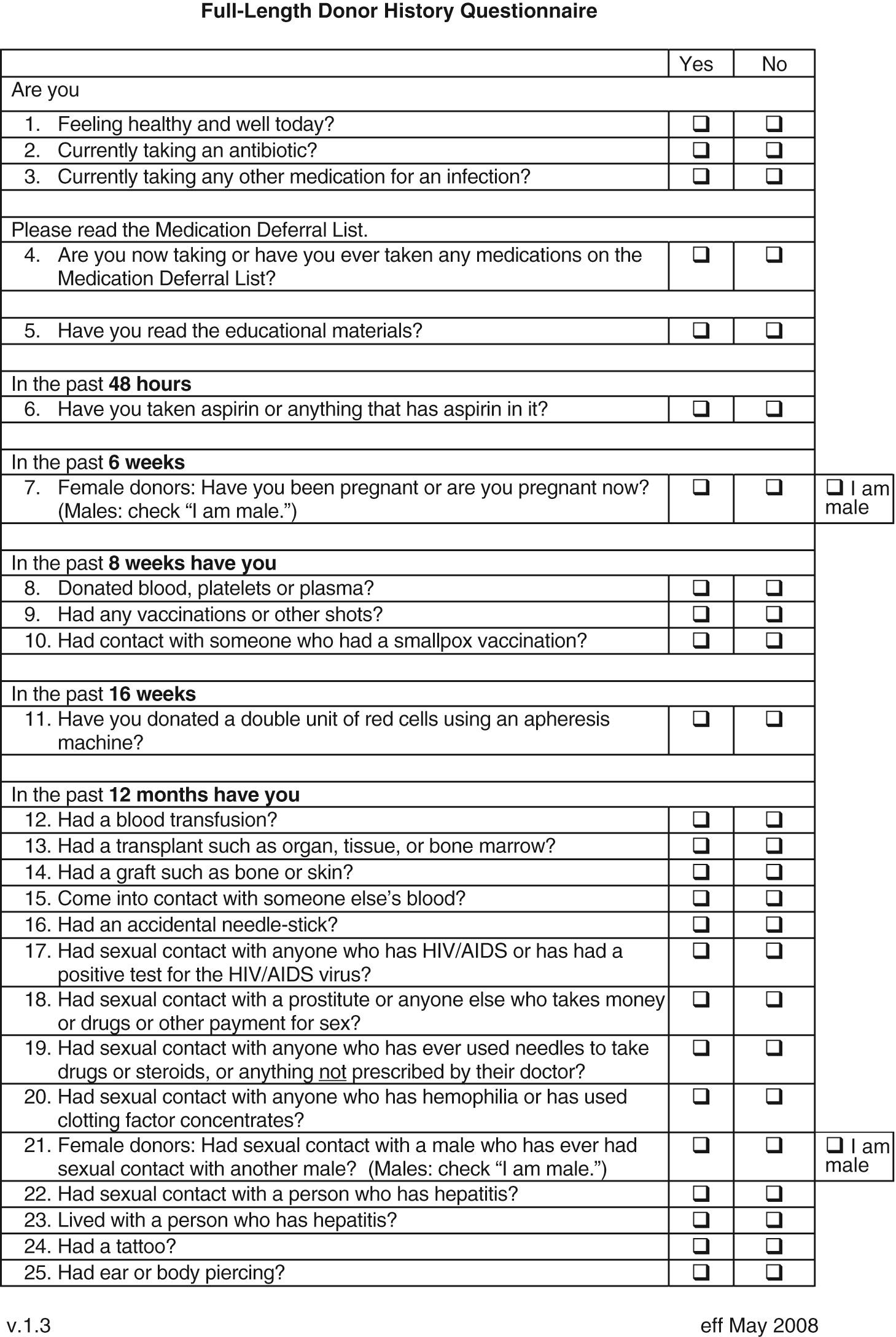
An ongoing dilemma of the blood-banking community is a mandate to ensure a maximally safe blood supply while giving consideration to the cost of such measures. The current questions for screening donors that have been adopted by most blood banks are presented in Fig. 304.1 , and the laboratory screening measures currently in place are listed in Table 304.2 . As of 2014, pathogens or diseases screened solely through donor interview include malaria and vCJD. Real or perceived threats to the blood supply occur regularly, as emerging or reemerging infectious diseases raise concern over potential transmission. A previously published review summarizes potential blood supply risks from pathogens, the majority of which are not screened for with laboratory testing.
| Required or Recommended by US Food and Drug Administration |
|
| Additionally Required by Accrediting Organizations a |
|
| Performed Voluntarily |
|
| Performed Under Investigational Protocols |
|
a Including AABB (formerly known as the American Association of Blood Banks) and the College of American Pathologists.
b In general, liquid culture media for apheresis units and pH/glucose indicators or bacterial antigen point-of-use tests for whole-blood–derived pooled units.
Pathogen reduction technology (PRT) is now available to reduce the need to develop screening measures for new infectious threats. In December 2014, the US Food and Drug Administration (FDA) approved a PRT method for plasma and apheresis platelets that uses chemicals activated by ultraviolet light to inactivate nucleic acid material comprising DNA and RNA. However, there is no approved method for treatment of RBCs. Although this technology is likely to reduce the risk of transmitted infections of viruses, bacteria, and parasites, it will not likely eliminate it, because there are organisms occurring in high titers or that have structures (e.g., non–lipid-enveloped viruses, bacterial spores, prions) that resist or are not affected by the inactivation mechanism.
According to estimates from the World Health Organization (WHO), more than 112 million units of blood were collected in 2013. Less than half of donated blood is collected in developing and transitional countries, which are home to about 80% of the world's population. Of the 156 countries providing data to WHO for 2013, 13 were not able to screen all of their donated blood for one or more of the four infections (HIV, HBV, HCV, and syphilis) that are most widely recognized to be transmitted through blood and are recommended by WHO to be screened at donation. A total of 126 countries have national guidelines on the appropriate clinical use of blood, and 70 countries have a national hemovigilance system to monitor adverse events associated with transfusion.
Surveys to determine blood product use in the United States, led by the National Heart and Lung Institute (now called the National Heart, Lung, and Blood Institute), began in 1971. In the United States, surveys have reported the frequency of blood collection and utilization since the late 1980s, most recently through the National Blood Collection and Utilization Survey (NBCUS), which has been conducted biannually since 1998. In the 2017 NBCUS report, reflecting data collected in 2015, there were nearly 11 million whole-blood and 3.7 million apheresis collections, with approximately 19 million blood components transfused. In 2015, 11.33 million whole-blood and RBC units, 2.7 million units of plasma, 1.8 million units of apheresis platelets, 0.17 million units of whole-blood–derived platelets (measured in apheresis equivalent units), and 1.2 million units of cryoprecipitate were given. The number of RBC transfusions has continued to decline since 2008, perhaps reflecting the growing adoption of more aggressive hospital blood management practices. The number of transfused platelets has remained approximately the same, but transfusion of apheresis platelets has continued to increase, while the amount of whole-blood–derived or pooled platelets has continued to decline. In addition to contributions from the voluntary donor pool, plasma units also are collected annually from paid donors and are used to prepare immune globulin, albumin, and various other plasma-derived products. By 1987, the cost of collecting, processing, and transfusing patients exceeded $3 billion; since then, costs have increased steadily with the addition of new screening tests and the implementation of leukoreduction. In 2015, the mean price paid by a hospital was $217 for a unit of leukocyte-reduced RBCs, $60 for fresh-frozen plasma, and $537 for apheresis platelets, so current costs are likely to exceed $4 billion annually.
It has been estimated that the annual likelihood of an individual's receiving a transfusion increases dramatically with age; in 2015, the transfusion rate in the US population was 35.3 units per 1000 people. Because of concern about contracting an infectious disease, there has been historic interest in autologous and donor-directed blood donation. However, donor-directed units, usually given by family members for a specific patient, have been shown to have higher rates of various infectious agents. There has been movement away from donor-directed donation and toward building a dedicated, voluntary repeat donor population. Viral infections are much less common among repeat donors compared with first-time whole-blood donors, and they may be even less common among donors associated with apheresis collection.
A majority of, but not all, blood components transfused in the United States are leukoreduced, a modification that is performed to filter out the majority of white blood cells. Leukoreduction is performed to reduce nonhemolytic transfusion reactions, but it may also reduce the risk for infectious disease transmission through removal of infected white blood cells, particularly for cell-associated agents.
In 2015, only about 0.42% of the donated allogeneic blood supply was discarded on testing, usually because of detection of a potentially transmissible infection. This reflects a continued steady decline in units discarded owing to testing, perhaps indicating improved accuracy of screening tests and retention of repeat donors. The incidence of viral markers in donated units was increased among volunteers who donated after September 11, 2001, primarily owing to a large cohort of first-time and infrequent repeat donors.
Transfusion-associated transmission risk has persisted for HIV, HBV, and HCV for two distinct reasons: (1) the incomplete sensitivity of the available screening tests, and (2) the “window” period, which is defined as the period between acute infection (and potential infectivity) and the point at which serologic tests can reliably detect infection.
The development and implementation of routine nucleic acid amplification testing (NAAT) has transformed blood bank screening for viral pathogens. NAAT can detect viral RNA after the first 10 to 14 days of infection, narrowing the window period by 7 to 10 days for HIV and by 50 to 60 days for HCV compared with serology. NAAT for blood donor screening, in addition to HIV, HBV, and HCV, is now additionally required for WNV and ZIKV, and is voluntarily performed by plasma manufacturers in pools for parvovirus B19.
With introduction of NAAT, the risk of acquiring HIV or HCV per unit of blood transfused has plummeted from approximately 1 per 500,000 for HIV and 1 per 100,000 for HCV before NAAT to a current rate of 1 per 2 million for both viruses. Detected cases are so unusual that the incidence can only be estimated statistically. As remarkable as this advance is, a small window period remains, meaning that the risk for receiving one of these viruses in a unit of blood is not zero. Four cases of HIV transmission and many more cases of HCV transmission through transfusion have been recognized and reported to public health authorities since NAAT was implemented; the modeling data estimate more cases than the number reported, so some cases likely go unrecognized.
The advent of NAAT also has had a substantial impact on the choice of serologic tests. In addition to sensitivity, blood bankers seek optimal specificity: False-positive or indeterminate test results exclude otherwise appropriate donors from subsequent donations and create anxiety due to incorrect diagnosis of infection. The introduction of an extremely sensitive test such as NAAT allows blood banks to focus on the specificity of other screening tests, thereby limiting the number of persons potentially excluded from the donor pool because of misleading test results. Although sensitivity varies little between the second- and third-generation antibody tests, the third-generation assay has superior specificity, decreasing the proportion of persons with indeterminate antibody tests for HCV. HBV NAAT has been adopted for blood donor screening.
More than 8000 persons in the United States have developed AIDS from receipt of blood or tissue. In addition, at least 50% of all hemophiliacs in the United States and Europe became infected with HIV from 1978 to 1985, most from receipt of infected plasma factors, with the highest incidence in 1982, when there were 22 infections per 100 person-years. The first HIV screening test was introduced in 1985, and since 1987 few new infections among hemophiliacs have been reported. As noted, the introduction of NAAT screening has substantially lowered the risk for HIV transmission, with the last reported case occurring in 2008.
Redundant methods of testing also have been useful in confirming the status of infection for blood donor follow-up. NAAT has been useful in clarifying the HIV serostatus of persons with indeterminate results of Western blot tests. These reactions occur in about 1 of every 5000 donations and usually represent false-positive test results.
In June 1992, the FDA mandated screening for HIV-2. Since then, few HIV-2–positive donors have been identified, and no cases of transmission have occurred in the United States, although transfusion-related HIV-2 cases have occurred elsewhere.
HTLV-1 and HTLV-2, unlike HIV-1 and HIV-2, are cell associated and therefore predominantly transmissible only with blood component transfusions. Screening for both types is done with a single test. The rate of transmission of HTLV-1 decreased almost 10-fold (from 1 per 8,500 to 1 per 69,000) after introduction of the screening test. Transplantation of solid organs from an HTLV-1–infected donor resulted in rapid progression to subacute myelopathy in three recipients; this phenomenon has not been described in transfusion recipients.
A short-lived perceived threat to the blood supply was raised when xenotropic murine leukemia virus–related virus (XMRV) was linked to prostate cancer and then chronic fatigue syndrome. Furthermore, evidence of viral infection, either by serology or NAAT, was found in controls, including in over 5% of healthy blood donors. XMRV later was proved to be a laboratory contaminant and is not thought to be associated with pathogenic human infection.
HBV remains the virus with the highest residual risk of transfusion transmission despite screening; HBV is transmitted in approximately 1 in every 200,000 to 300,000 transfusions. Risk persists both because the screening test is incompletely sensitive and because of the prolonged window period of about 2 months. In countries that screen for hepatitis B core antibody (HBcAb), most transmissions derive from the window period, whereas in those that do not check HBcAb status, half derive from the window period and the others are due to the insensitivity of the hepatitis B surface antigen (HBsAg) test as a screening test.
NAAT for HBV recently has been implemented for blood screening using minipool testing. With current sensitivity of minipool testing, NAAT shortens the window period by about 7 days, leaving a residual risk for HBV of about 1 per 250,000 to 350,000 transfusions. Sensitive screening tests are particularly needed in HBV-endemic areas, such as Taiwan, where HBcAb testing is not routinely performed and transfusion-acquired HBV is a relatively frequent event. Testing for HBcAb, in addition to HBsAg, remains important; in some cases, it is more sensitive than NAAT, so implementation of new testing does not obviate the use of these other tests for donor HBV infection. Increasing use of hepatitis B vaccination may further reduce the risk for transmission.
Screening of donors for HBsAg excludes most, but not all, carriers of hepatitis D, also known as delta virus. Identification of donors with antibody to the delta agent is not done routinely, so there is a residual risk for transmission of delta virus to HBsAg-positive recipients.
Hepatitis C, previously known as non-A, non-B hepatitis, was associated with a lifetime risk of 7% to 10% in transfusion recipients in the late 1970s and early 1980s. The transmission rate of HCV has decreased with improved screening tests to identify the virus, providing an excellent example of how implementation of each new generation of screening technology has improved blood safety. More than 90% of recipients of HCV-contaminated blood products develop HCV infection. An older survey using the second-generation test found that 3.6 per 1000 US donors were positive for HCV, a prevalence lower than that in the US population. HCV infection in blood donors usually is the result of intravenous drug use, although other health care–associated risks have been identified, including questionable injection practices.
Before any testing, 0.45% of all transfusions transmitted HCV. With the introduction in 1986 of surrogate marker testing (alanine aminotransferase and HBcAb), the rate decreased to 0.19%, and with the introduction of the first-generation antibody test for HCV in 1990, the rate fell to 0.03%. The second-generation test lowered the rate even further, mostly by identifying chronic infections more accurately (i.e., increasing sensitivity) rather than by shortening the window period. Despite the continuing relatively high prevalence of HCV infection in the US population, the introduction of NAAT has reduced the transmission risk to about 1 in 1.5 million transfusions.
Transmission of hepatitis A virus (HAV) via transfusion has been described with RBCs, particularly in infants, and with factor VIII concentrate. Donors with early, asymptomatic infection may transmit HAV infection. Factor VIII–associated transmission has occurred despite appropriate use of organic solvent and detergent to inactivate the virus. The lack of a lipid envelope around HAV may have contributed to incomplete virus killing during preparation of the concentrate. Because of this small but persistent risk, vaccination for HAV has been recommended for chronic recipients of products made from pooled plasma. Review of all cases of hepatitis from 1998 to 2002 in the United States among persons with bleeding disorders revealed no acquisition of HAV via factor concentrates, although as the incidence of HAV infection increases in the US population, some plasma manufacturers voluntarily screen donation pools using NAAT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here