Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Monitoring the cerebral blood flow (CBF) is critical not only in patients with known neurological disease but also in patients at risk of neurological damage in the perioperative period. Despite the fact that virtually all interventions in neuroanesthesia and neurointensive care are directed toward maintaining adequacy of cerebral perfusion, perioperative monitoring of the same is limited because of technical and logistic issues. Introduced by Aaslid, Markwalder, and Nornes, transcranial Doppler (TCD) ultrasonography provides continuous real-time information regarding cerebral circulation. It uses a range-gated, pulsed-Doppler ultrasound beam to insonate the basal cerebral arteries through the skull and estimates the cerebral blood flow velocity (CBFV). Although TCD detects CBFV and not CBF per se, it is able to provide clinically useful information about significant changes in CBF considering the clinical setting and limitations. The practical applications include detection of intracranial vascular stenosis/spasm/hyperemia, evaluation of collateral flow, assessment of cerebral autoregulation, and cerebrovascular reactivity in addition to the detection of cerebral emboli. The major advantage of TCD ultrasonography is that it is noninvasive, nonradioactive, inexpensive, and portable.
TCD uses a range-gated, pulsed-Doppler ultrasound beam of 2 MHz frequency. As with extracranial Doppler sonography, the TCD measurement of CBFV is based on the detection of frequency shifts from insonated red blood cells moving through a small, preselected arterial region, called the sample volume . The sample volume is determined by the lateral focusing of the transducer, the duration of the transmitted sound burst at a specific pulse repetition rate (PRF), and the duration of the range-gate opening. The TCD probe (transducer) is a piezoelectric crystal, which acts as both emitter as well as receiver of ultrasound signals reflected by moving erythrocytes in cerebral vasculature. The Doppler shift in frequency (Fd) is determined by the Doppler equation:
The erythrocytes moving toward the probe cause an increase in the reflected frequency and those moving away from the probe cause a decrease in reflected frequency. The probe acting as receiver translates the reflected frequencies back into electrical signals, and the computer performs fast Fourier transformation of this signal, the information being displayed in a moving graphic manner with time represented on the “X” axis and CBFV on the “Y” axis. If flow in the vessel being insonated is directed toward the probe, it is displayed as “positive” waveform (above the baseline), and if it is directed away from the probe, it is displayed as “negative” waveform (below the baseline). The intensity of reflected signal is indicated by the brightness of the displayed velocity. Since within the insonated vessel different erythrocytes move with different velocities, the Doppler signal obtained is actually a mixture of different corresponding frequency components. The continuous moving display on the monitor represents the changes in CBFV during various phases of cardiac cycle.
For calculating the CBFV using the Doppler equation where “f” and “c” are known and “Fd” is calculated, the computer assumes the angle of insonation (θ) to be either 0 or 180 degrees since at these values of θ, the frequency shift is maximal (cos 0 = 1 and cos 180 = −1). The angle of insonation should not change during examination; hence, it is desirable to fix the probe at same position using any of the commercially available fixation devices. For calculating CBFV, a “spectral envelope” corresponding to the maximum flow velocity throughout the cardiac cycle is created, and then the various parameters are calculated from this “envelope.” The moving display is then used to calculate the CBFV in a given vessel, and further analysis of the waveform provides more detailed information about the cerebral circulation. In addition, TCD is widely used to detect cerebral microemboli. The particulate or gaseous emboli in the blood reflect ultrasound more intensely than the surrounding red blood cells. Consequently, they are visualized within the spectral display of the CBF waveform as high-intensity transient signals (HITS) that also produce a characteristic sound.
Most modern TCD machines now incorporate the power M-mode Doppler. This involves the use of several sample gates placed with 2-mm spacing configured to display Doppler signal power, colored red and blue for directionality, in an M-mode format. The ability to insonate at several depths simultaneously facilitates window location and alignment of the ultrasound beam to view CBF in multiple vessels simultaneously at the same time. This also allows a more effective detection of microemboli, which are visualized as sloping high-power tracks in the M-mode image.
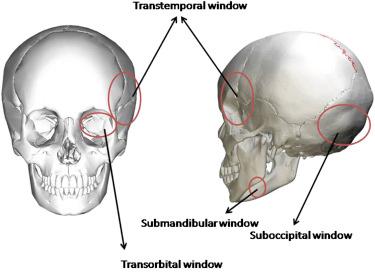
The classic TCD examination of the cerebral vasculature involves insonation through the transtemporal, suboccipital, and transorbital approaches ( Fig. 5.1 ). The Doppler signal obtained is assigned to a specific artery based on indirect parameters, typically the depth of insonation, position of the transducer, and the direction of blood flow. Transcranial color-coded duplex ultrasonography (TCCS), on the other hand, allows visualization of the basal cerebral arteries through the intact skull by color coding of blood flow velocity. Using TCCS, important cerebral structures used as landmarks are first identified: the mesencephalic brain stem and the basal cistern. Subsequently, the color mode is added to identify the arteries of the circle of Willis by their anatomical location to the brain stem structures and by the determination of their flow direction based on specific color coding of the blood flow velocity. In addition, there is also a submandibular window that provides access to the retromandibular and extradural segments of the internal carotid artery.
The TCD machine involves the visual display of the moving CBFV waveform along with the power M-mode display (when available). Typically the TCD machines are portable with small size, but often they include the regular computer displays that are mounted on a trolley. Many versions are laptop computer-based, and recently, some pocket versions have become available. The display screen also depicts important numeric values including the CBFV, the depth of insonation, the sample volume, gain, and embolic count (HITS) in addition to the indices of cerebrovascular resistance. The display might involve a one- or two-channel TCD system. It is important for the individual performing the TCD examination to be familiar with the display.
The TCD probe (transducer) is a piezoelectric crystal, which acts as both emitter as well as receiver of ultrasound signals. There are two types of probes: the diagnostic (hand-held) and the monitoring (for mounting on a probe fixation device). The typical probe is 2 MHz pulse wave (PW) and can penetrate to a depth of approximately 30–150 mm. A 1 MHz PW probe may be used to examine patients with hyperostoses and those with difficult to insonate acoustic windows. The 1 MHz probe also allows the ability to take measurements at high flow velocities at greater depths such as in the case of vasospasm or deep-seated stenoses of the basilar artery. The 1 MHz probe can also penetrate to a depth of 30–150 mm. Some manufacturers provide an option of a 2 + 2.5 MHz probe for discrimination of microemboli. This probe offers a greater bandwidth, allowing examination in dual-frequency mode. The monitoring probes are especially designed for fixations for continuous monitoring such as for carotid artery interventions or emboli monitoring. The monitoring probes allow optimal adjustment of the temporal ultrasound window around the specific fixation device.

For continuous examination, it is critical that the angle of insonation should not change because this can cause significant over/underestimation of the CBFV. Hence, it is important to use a probe fixation device to maintain a constant angle of insonation. The variety of probe fixation devices are commercially available for probe support. These include size-adjustable elastic headband, LAM support (rack), or adhesive bonding. These devices offer adjustable probe positions that enable optimal adjustment of the temporal ultrasound window. Most of these are easy to wear for the patient without any discomfort and, therefore, can be used for investigations lasting several hours. There are available options for devices with no metal components (but which may be particularly suitable for radiological applications). Most of the fixation devices are size-adjustable to the forehead, top of the skull, and back of the head and can be used for head circumferences of 55–74 cm. It is important to maintain the cleanliness using appropriate disinfectants. The head pads for some devices are disposable and can easily be changed as they are attached to the frame using Velcro.
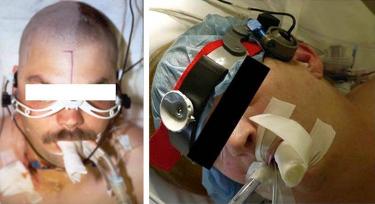
The LAM support (rack) is a light probe support with a fixation point on the nose and a pair of spectacles with two metal side pieces over the temporal ultrasound window. The support is kept firmly in position by two ear plugs. The specific advantage of the LAM support is that there are no fixation components at the back of the patient's head, allowing it to be used in anesthetized/comatose/bed-ridden patients. The patient's head does not have to be raised to put on the support; therefore, it is particularly suitable for use in operating rooms and intensive care units. Four different LAM supports are available: Standard (for patients in intensive care units and operating rooms as well as for unconscious and intubated patients, suitable for physiological tests), Neurosurgery (for use during neurosurgical procedures, the support arm is bent under and does not interfere with the surgical field), Carotid surgery (for use during carotid endarterectomies) and Oral surgery (for patients whose nose cannot be used as a fixation point, e.g., in the case of nose injuries or surgery on the nose).
Another option for probe fixation is a commercially available elastic headband made of silicone. The headband is particularly suitable for patients who are awake, e.g., during a function test or emboli monitoring. The patient's hearing is not impaired as there are no ear plugs, such as with the LAM support. The elastic headband does not contain any metal components and can therefore be worn during X-ray, CT, or MRI investigations without affecting the quality of image. Alternatively, the probes can be simply stuck on the patient's skin.
The TCD machines typically come with a remote control to allow controlling for various parameters during examination (selecting the cerebral vessel, depth of insonation, sample volume, gain, etc.). Some models offer a footswitch to control the system functions. There are also options for an integrated CO 2 module for performing the vasoreactivity testing.
The TCD examination of the cerebral vessel may be complete (involving insonation of all basal cerebral arteries) or limited (involving insonation only of a specific cerebral artery of interest). For performing the TCD ultrasonography, it is essential that the performer be well-versed with the cerebrovascular anatomy. A detailed description of the cerebrovascular anatomy is beyond the scope of this chapter. Briefly, the cerebral vasculature originates at the aortic arch with the right brachiocephalic dividing into the right common carotid and subclavian arteries. The left common carotid and left subclavian arteries arise directly from the aortic arch. The two common carotid arteries bifurcate into the internal and external carotid arteries. The anterior cerebral circulation includes branches of the internal carotid artery (ICA): the anterior cerebral artery (ACA) and the middle cerebral artery (MCA). The two ACAs are connected through the anterior communicating artery. The two vertebral arteries (VA) arise from the respective subclavian arteries and join at the pontomedullary junction to form the basilar artery (BA), which then bifurcates into the two posterior cerebral arteries (PCA), together comprising the posterior cerebral circulation. Several anastomoses supply the collateral circulation of the brain. The circle of Willis is an anastomotic ring connecting the anterior (carotid) and posterior (vertebrobasilar) systems. Importantly, only 30%–35% of normal individuals have a complete circle of Willis. A common external to internal carotid collateral channel is an anastomosis with the ophthalmic artery.
Insonation of the cerebral vessels requires the ultrasound beam to penetrate through the skull; hence, TCD relies on acoustic windows (naturally occurring foramina or areas of thinning on the skull) to measure the CBFV ( Fig. 5.5 ). The acoustic windows include the following:
Transtemporal window : area just above the zygomatic arch (line joining tragus to lateral canthus of eye), which allows insonation of terminal ICA before its bifurcation, M1 portion of MCA, A1 portion of ACA, and P1 and P2 portions of PCA;
Suboccipital window : area just below posterior aspect of skull, allowing insonation of distal portions of VA and BA through the foramen magnum;
Transorbital window : over the eyes, which allows insonation of the ophthalmic artery (OA) and the cavernous ICA (carotid siphon) through the orbit;
Submandibular window : area below the angle of mandible, allowing insonation of distal portion of extracranial ICA.
A major limitation of TCD is the absence of adequate acoustic “windows” in about 8% of individuals, more often in the elderly and in women.
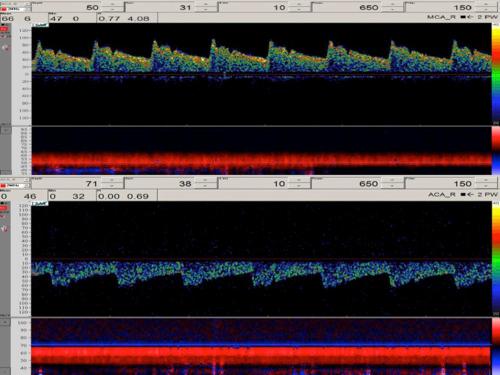
The first step in reading a TCD examination is identifying the cerebral vessel from the waveform generated. This is based on the window of insonation, depth of sample volume, direction of the probe, and the appearance of the waveform. The simplified vessel identification criteria are detailed in Table 5.1 . The TCD waveform is usually described in terms of peak systolic (FVs), end diastolic (FVd), and time averaged mean maximal velocities (FVm). The mean flow velocity is typically referenced. The normal values of CBFV are affected by a number of physiological and demographic factors, some of which include:
Age: At birth, the MCA CBFFV is about 24 cm/s, which increases to a maximum at the age of 4–6 years (100 cm/s) and decreases thereafter to be about 40 cm/s in seventh decade of life.
Gender: Women have a CBFV 10%–15% higher than males.
Pregnancy: CBFV decreases in third trimester of pregnancy.
Arousal state: CBFV during sleep is 15% lower than awake state.
PCO 2 : Increase in PCO 2 causes to dilatation of resistance vessels leading to increased CBFV. Similarly, decrease in PCO 2 causes constriction of resistance vessels leading to decreased CBFV.
Hematocrit: Decrease in hematocrit causes compensatory increase in CBFV by reducing CVR.
PO 2 : Reduction in PO 2 can lead to increased CBFV, but unless there is significant hypoxia, this effect is much less important than that of PCO 2 .
Blood viscosity: Increased blood viscosity associated with significant dehydration and hyperproteinemia causes a reduction of CBFV.
Cerebral perfusion pressure (CPP): CBFV remains fairly constant over changing CPP within autoregulatory range, but beyond this range, CBFV becomes CPP dependent.
| Artery | Acoustic window | Depth of insonation (mm) | Transducer orientation | Direction of flow | Mean flow velocity (cm/s) | Effect of ipsilateral carotid compression |
|---|---|---|---|---|---|---|
| MCA (M1) | Transtemporal | 30–60 | Perpendicular | Toward | 55 ± 12 | Ob/Dim |
| TICA | Transtemporal | 55–65 | Perpendicular | Toward | 39 ± 9 | Ob/Rev |
| ICA Bifurcation | Transtemporal | 55–65 | Perpendicular | Bidirectional | – | Same as MCA/ACA |
| ACA | Transtemporal | 60–80 | Anterior | Away | 50 ± 11 | Ob/Dim/Rev |
| PCA (P1) | Transtemporal | 60–70 | Posterior | Toward | 39 ± 10 | No change/Aug |
| PCA (P2) | Transtemporal | 60–70 | Posterior | Away | 40 ± 10 | No change |
| OA | Transorbital | 40–60 | Slightly medial | Toward | 21 ± 5 | Ob |
| Carotid Siphon | Transorbital | 60–80 | Slightly medial | Away/Bidirectional/Toward | 41 ± 11 | Ob/Rev |
| VA | Suboccipital | 60–90 | Superior, oblique | Away | 38 ± 10 | – |
| BA | Suboccipital | 80–120 | Superior | Away | 41 ± 10 | – |
| ECICA | Submandibular | 30–60 | Superior | Away | 37 ± 9 | – |
The common causes of increase or decrease in the CBFV are listed in Table 5.2 . Some important points to note while interpreting CBFV values include the following: (1) focal narrowing at the site of insonation causes an increase in CBFV at the point of narrowing (e.g., intracranial stenosis), (2) narrowing or obstruction proximal to the insonation site causes a decrease in CBFV at the insonation site, (3) a downstream decrease in cerebrovascular resistance (as in the presence of arteriovenous malformation or hypercapnia) causes an increase in CBFV at the site of insonation, and (4) a downstream increase in cerebrovascular resistance (e.g., in stenosis or obstruction) causes a decrease CBFV.
| Increased CBFV | Decreased CBFV |
|---|---|
| Hyperdynamic circulation | Hypotension (below autoregulatory limits) |
| Hypercarbia | Hypocarbia |
| Hyperemia | Hypothermia |
| Vasospasm | Raised ICP |
| Arterial stenosis | Brain death |
| Volatile anesthetics | Intravenous anesthetics |
| Arteriovenous malformation | Fulminant hepatic failure |
| Bacterial meningitis | |
| Sickle cell anemia | |
| Preeclampsia |
In addition to the CBFV, while interpreting the TCD, the following calculated indices are frequently analyzed:
Pulsatility Index (PI) of Gosling and King :
Resistance Index of Pourcelot :
Both these indices are measures of resistance to flow encountered by blood flowing in cerebral vessels, with a higher value indicating more resistance and a lower value indicating lower resistance in the vascular bed. Normal PI ranges between 0.6 and 1.0. Within the ICP ranges of 5–40 mm Hg, correlation between ICP and PI is strong, though it is less strong between CPP and PI. The advantage of PI is that being a ratio, it is not influenced by angle of insonation. However, PI and RI are influenced by physiological parameters including the systemic blood pressure and changes in PaCO 2 in addition to CVR and thus have a limited role in clinical or research settings.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here