Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
It has been more than three decades since Herndon and colleagues' first manuscript on inhalation injury was published. It was initially reported in 1985 that inhalation injury was a major determinant of mortality in severely burned patients. . The standard of care has evolved over time, but inhalation injury still remains a major problem. . Although sepsis is reportedly the most frequent cause of death among burned children, , roughly two-thirds of burned patients who have died at the Shriners Hospitals for Children suffer from inhalation injuries. Based on data from 506,628 admissions to burn units from 1988 to 2008, burn patients with inhalation injury are more likely to die than those without inhalation injury ( P <0.001). Furthermore when 791 burned patients from 44 hospitals were retrospectively reviewed in 2014, the mortality rate of patients with inhalation injury was 17.9% compared to 0.7% in patients without inhalation injury ( P <0.05).
Smoke inhalation causes 5000 to 10,000 deaths annually in the United States and more than 23,000 injuries, including approximately 5000 firefighter injuries. In fact, the United States has the tenth highest fire death rate per million people among industrialized countries. Approximately 15% of burned individuals with over 80% total burned surface area (TBSA) who are admitted to burn centers in the United States have a concomitant smoke inhalation injury. Greater percentages of fire victims who have sustained smoke inhalation are seen in several other countries. The lung is a critical organ, and progressive respiratory failure associated with pulmonary edema is a pivotal determinant of mortality. Although not as lethal, smoke inhalation alone is a serious problem. According to World Health Organization estimates, more than 4 million people die from household air pollution from cooking with solid fuels ( Fig. 16.1 ).

The inhalation of toxic materials has been of interest for a number of years, particularly because of the use of toxic gases in civilian mass casualty events. In the 1940s, two very large fires focused attention on smoke inhalation in fire accidents. The first was a fire at the Cocoanut Grove nightclub in Boston, Massachusetts, where a large number of people were trapped in a burning building and consequently sustained severe inhalation injury. The second disaster occurred in 1947, in Texas City, Texas. A ship loaded with ammonium nitrate fertilizer exploded in the harbor and set off a chain of explosions and fires among 50 refineries and chemical plants, resulting in more than 2000 hospital admissions. Many of the victims were burned and simultaneously inhaled smoke, while many others suffered from smoke inhalation alone. Disasters like those in Boston and Texas led to the establishment of centers for the care of burn victims and research into the pathophysiology of burn injury.
In many ways, the September 11, 2001, disaster at the Pentagon was similar to these two earlier disasters since the burn and inhalation injuries involved combustion of petroleum products. Among the 790 injured survivors of the terrorist attack on the World Trade Center in New York on September 11, 49% suffered from inhalation injury. The situation was the same as in the Pentagon attack in that inhalation injury was seen in some patients who were not burned.
Inhalation injury can be classified as follows: (1) upper airway injury, (2) lower airway injury, (3) pulmonary parenchyma injury, and (4) systemic toxicity. The extent of inhalation damage depends on the fire environment: the ignition source, temperature, and the concentration and solubility of the toxic gases generated. For instance, hot air and smoke chemical compounds usually cause upper airway injury. Water-soluble materials such as acrolein and the other aldehydes damage the proximal airways and set off reactions that inflame in the bronchi and parenchyma, whereas agents with lower water solubility such as chlorine, phosgene, nitrogen oxide, nitrogen dioxide (N 2 O 3 or even N 2 O 4 ) are more likely to cause insidious injury.
Many of the pathophysiological changes occurring after inhalation injury are related to edema formation in the oropharynx, bronchial areas, and parenchyma. This edema results from an increased transvascular fluid flux from vascular beds in these respective tissues. Before discussing the changes that occur in these structures following inhalation injury, a review of the forces responsible for the variables of the Starling-Landis equation should be reviewed :
This equation describes the physical forces and physiologic mechanisms that govern fluid transfer between vascular and extravascular compartments. J v , the transvascular fluid flux, is equal to lymph flow during equilibrium states. As transvascular fluid flux increases, interstitial volume also increases (edema formation) until a new equilibrium with lymph flow occurs. K f is the filtration coefficient, an index of the total number of pores that are filtering. The number of pores could increase if a larger area of the microcirculation were perfused or if there were more pores per given area of the microcirculation. These pores are the same size as water and electrolytes, as opposed to the larger pores associated with permeability to protein. P c and P if are the hydrostatic pressures in the microcirculation and interstitial space, respectively. The reflection coefficient, σ, is an index of microvascular permeability to protein. If σ is 1, the membrane is impermeable to protein; when σ is 0, the membrane is completely permeable to protein. COP p and COP if are the oncotic or colloid osmotic pressures in the plasma and interstitial spaces, respectively.
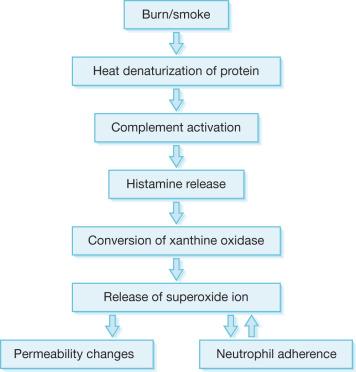
The major pathophysiology seen in the oropharynx following inhalation injury is induced by microvascular changes similar to those seen with thermal injury in other areas of the body. Heat denatures protein that, in turn, activates complement. Complement activation causes the release of histamine. Histamine then causes the formation of xanthine oxidase, an enzyme involved in the breakdown of purines to uric acid. During this conversion, reactive oxygen species (ROS) are released. ROS combine with NO, constitutively formed in the endothelium, to form reactive nitrogen species (RNS). The latter produce edema in the burned area by increasing the microvascular pressure and permeability to protein. Eicosanoids and pro-inflammatory cytokines are also released. These, along with oxygen free radicals and interleukin-8 (IL-8), attract polymorphonuclear cells to the area. These neutrophils then amplify the release of oxygen radicals, proteases, and other materials into burned areas ( Fig. 16.2 ).
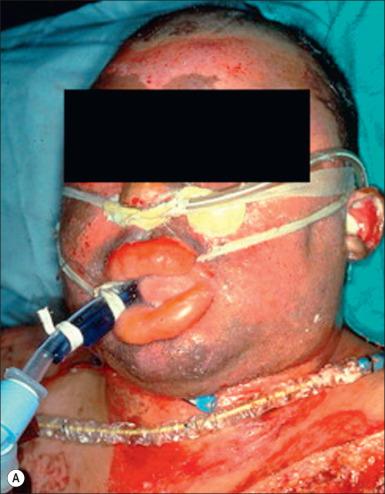
The massive edema occurring in the soft tissue of the oropharynx following burns involves most variables in the Starling equation. There is a large increase in microvascular hydrostatic pressure, a decrease in interstitial hydrostatic pressure, a fall in the reflection coefficient, and an increase in interstitial oncotic pressure. The usual treatment for burn resuscitation calls for the administration of large amounts of crystalloid solutions, which has the effect of reducing the plasma oncotic pressure. This reduction not only affects the oncotic pressure gradient in the microcirculation, but also has been reported to increase the filtration coefficient. The result of this almost complete breakdown in control of the microvascular function and the insult of fluid administration is massive edema. This is probably nowhere more apparent than in soft tissues of the face and oropharynx. The danger to the patient is extreme. The edema may obstruct the airway, making it not only laborious or impossible to breath, but also difficult for the anesthesiologist to intubate the patient ( Fig. 16.3A ). To avoid this problem, many units prophylactically perform tracheostomies on patients who have evidence of thermal injury to the upper airway on admission. However tracheostomy itself may present problems. The tube may further damage injured areas, especially the larynx. It may be time to reconsider some of these practices. Perhaps some consideration should be given to fluid resuscitation with colloids, which can prevent some of this soft tissue edema and reduce the volume of fluids required for resuscitation.
With rare exceptions such as inhalation of steam, the injury to the airway is usually from chemicals in smoke. The heat capacity of air is low, and the bronchial circulation is very efficient in warming or cooling airway gases so that most gasses are at body temperature as they pass the glottis. Flames must almost be in direct contact with the airway to induce thermal injury. The chemicals in smoke depend on the materials that are being burned; however, for the most part, the host response is similar. In most instances biological materials such as cotton fabric, wood, grass, or products of these such as cattle feces (commonly used as fuel in developing countries) are the fuel for the fire. Burning of these materials produces ROS and RNS, organic acids, and aldehydes that, upon inhalation, cause damage to the respiratory epithelium. The inhaled chemicals interact with the airway to induce an initial inflammatory response. In sheep that have inhaled cooled cotton smoke, there is damage to the tracheobronchial and alveolar epithelium. Injury and loss of these cells lead to an intense inflammatory response.
Many of the studies on bronchial circulation following smoke inhalation injury have been performed in sheep because these animals have a single bronchial artery and a single lymphatic draining the lung, thus allowing measurement of pulmonary transvascular fluid flux. In these animals, a 10-fold increase in bronchial blood flow occurs within 20 minutes of smoke inhalation. These same animals demonstrate a sixfold increase in pulmonary transvascular fluid flux and a fall in Pa o 2 /Fi o 2 to 200 or less, but these changes are delayed, occurring around 24 hours after injury. Similar findings have been reported in patients with smoke inhalation alone or a combination of large cutaneous thermal injury and smoke inhalation.
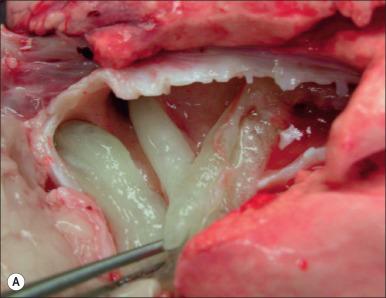
Hyperemia of the airway is such a consistent finding in smoke inhalation that it is used to diagnose the injury. Other variables that are used include injury in an enclosed space, singed nasal hair, and soot in sputum. However these latter injuries may be present but the subject may still not develop the signs of pulmonary edema characteristic of inhalation injury. Airway inflammation plays a major role in the overall response to inhalation injury ( Fig. 16.3B and C ).
As noted, there is a large sustained increase in blood flow in the airway following smoke inhalation. These changes in blood flow are associated with increased bronchial microvascular permeability to protein and small particles and pressure. Simultaneous with changes in bronchial microvasculature function, there is a loss or shedding of the bronchial columnar epithelium. These changes result in a perfuse transudate with a protein content similar to an ultrafiltrate of the plasma. There are also copious secretions from the goblet cells of the lining and mucosal epithelium. Early in the response, these secretions form a foam material in the airway that many have mistaken for severe pulmonary edema in patients. After several hours the transudate/exudate, exfoliated epithelium, secreted mucus, and inflammatory cells form obstructive materials in the airways. The degree of obstruction at this early stage statistically correlates with decreasing pulmonary function. With increasing time after injury, these obstructive materials formed in the upper airway may appear in the lower airway and alveoli. This obstructive material is problematic from several stand-points. In some rare instances of severe airway injury these materials can induce total obstruction and are life-threatening ( Fig. 16.4 ). Occlusion of some of the bronchi or bronchioles in the setting of high NO production can lead to a loss of hypoxic pulmonary vasoconstriction and thus increased shunt fraction. Loss of hypoxic pulmonary vasoconstriction has been reported with inhalation injury. If single bronchi are occluded while the patient is on a volume-limited ventilator, there may be overstretch, and barotrauma to the alveoli of the nonoccluded portion of the lung can occur. Nebulized anticoagulants have been used to combat the upper airway obstruction that can occur with severe inhalation injury. These are beneficial in reducing cast formation and improving pulmonary performance, although their use in the clinical environment has not yet been reported. Airway mucosal edema and luminal obstruction following an inhalation injury occur alongside airway smooth muscle hyperreactivity. Adrenergic and antimuscarinic bronchodilator therapies have been shown to be beneficial in reducing ventilatory peak pressure and improving pulmonary function. The initial hyperreactive response of the airways to injury and inflammation is followed by a more long-term pathogenesis of inhalation injury that includes bronchopneumonia. Pneumonia is the leading complication in the critical care of burn victims, with the incidence of pneumonia in a burn patient with inhalation injury being two- to fourfold greater than that seen in burn patients without inhalation injury. Conceptually the high incidence of pneumonia in burn patients with inhalation injury is associated with the loss of airway epithelium and the essential properties of these cells in innate defense. Studies that have focused on the repair of the epithelium after burn injury are limited. In an ovine model with a selected area of smoke injury to the tracheal epithelium, the epithelial repair process was completed at 18 days. Further study by this team of investigators revealed that nebulization of cefazolin and growth factors could improve the rate of airway healing. Because of the effects of pneumonia and inhalation injury on burn patient morbidity and mortality, further studies on the dynamics of airway damage and airway epithelial repair following toxic exposure are needed to improve critical burn care.
The airway is richly innervated with vasomotor and sensory nerve endings. These fibers are known to release neuropeptides that can engender inflammatory responses. Neuropeptides in the upper airway are also involved in mucus secretion, thus their release and interaction with the mucosal gland epithelium can increase the early obstructive pathology that occurs with inhalation injury. Neuroinflammation is responsible for pathophysiological changes associated with a number of clinical conditions including tissue injury induced by chemicals. Lange and colleagues reported that antagonists to substance P and calcitonin gene-related peptide had a marked effect on this response when administered to sheep and mice that were injured with both burn and smoke inhalation. In an ovine model, the combination of burn and smoke inhalation injury caused a 10-fold increase in pulmonary transvascular fluid flux and a reduction of Pa o 2 /Fi o 2 to 200 or less. These changes were reversed by neuropeptide receptor blocking agents. Released neuropeptides can activate nitric oxide synthase (NOS), have chemokine activity, and change microvascular permeability. The resultant activities lead to the formation of ROS and RNS. Some of the latter are very potent oxidants that can damage DNA. Damage to DNA triggers activation of a DNA repair enzyme, poly(ADP-ribose) polymerase (PARP). This enzyme depletes the cell of high-energy phosphates and causes the activation of nuclear factor-κB (NF-κB) NF-κB activation induces upregulation of inducible NOS (iNOS) and IL-8, thus accelerating production of ROS and RNS. NO and 3-nitrotyrosine (an index of RNS), and iNOS mRNA and protein have been reported to be in the airway after smoke inhalation. Compounds that catalyze the breakdown of peroxynitrite reduce the response to smoke inhalation. Poly(ADP-ribose) (PAR), the product of the constitutive enzyme PARP, is detectable in airway tissues following smoke inhalation. PARP inhibition prevents PAR formation, NF-κB upregulation, and 3-nitrotyrosine formation. Similarly, Lange and colleagues have reported that compounds that inhibit peroxynitrite by catalyzing its rapid breakdown likewise prevent the formation of these materials. It is interesting to note that mice missing the PARP genes or given a PARP inhibitor will not show the typical inflammatory changes usually observed with asthma. Thus, in many ways, inhalation of smoke may be similar to other forms of airway injury. The fact that the response to inhalation injury is driven by neuroinflammation suggests that the response to smoke from wood or cotton is similar.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here