Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A heartbeat results from the added output of millions of cells that contract in synchrony. To achieve this function, complex molecular networks work in concert with exquisite temporal precision. The accurate timing of the molecular events demands a comparable precision on the location of each molecule within the cell. Indeed, molecular networks organize within well-confined microdomains, where physical proximity allows for prompt and efficient interaction. In turn, the loss of molecular organization at a nanoscale can be a core component in the pathophysiology of a disease.
This chapter focuses on the intercalated disc (ID), a region of specialization formed at the end-end site of contact between cardiomyocytes (CMs). When first observed using light microscopy (in 1866), the ID was considered “a cementing material” at cardiac cell boundaries. The 1893 article by Przewoski “Du mode de reunion des cellules myocardiques de l’homme adulte” supported the idea that the ID was necessary for cell-cell adhesion. The scientific community at the time, however, was divided on whether cardiac cells were separate from each other or fused into a single syncytium. The latter hypothesis was, in fact, favored by most during the early twentieth century. The advent of electron microscopy tilted and eventually settled this debate. The studies of Sjostrand and Andersson and others showed that the ID consists of a double membrane flanked by the termination of myofibrils in the dense material. Their observations led Muir to conclude that “the discs represent the junctions between neighboring cardiac muscle cells.” He also wrote “… there is no valid evidence to contest the statement that the IDs are specialized regions of cellular adhesion.” Since then, and as a result of the pioneering electrophysiologic experiments of Weidmann and other ultrastructural observations, the ID has been recognized as an area of specialization that provides a physical continuum between cardiac cells through mechanical junctions (desmosomes, adherens junctions, and area composita) and intercellular channels (gap junctions).
The availability of immunofluorescence microscopy demonstrated that other molecular complexes, not detectable by electron microscopy, are also present in the ID. Of particular relevance to this chapter is the fact that channel protein complexes involved in both depolarization and repolarization localize preferentially to the ID. This physical proximity allows for a key functional consequence: molecules traditionally defined as junctional, such as connexin43 (Cx43) and plakophilin-2 (PKP2), actually regulate the function of ion channels responsible for the action potential (AP). In turn, molecules accessory to ion channels are also relevant for cell adhesion and for gap junction function. When taken together, the data support the notion that the ID is not just the site of residence of independent “junctional” and “nonjunctional” complexes that are oblivious to the presence and function of others. It is, rather, the home of a protein-interacting network (a connexome ) where molecules multitask to achieve jointly, intimately related functions—that is, the entry and exit of charges into cells, transfer of charges between cells, and anchoring of cells to each other; this provides a mechanically stable environment critical for ion channel functions. In recent years, this has been complemented with the observation of dyadic structures at the ID, suggesting that the components of the ID may be affected by events occurring during excitation-contraction coupling.
This chapter outlines current knowledge on the composition of selected molecular complexes of the ID, their interactions, and the possible mechanisms by which the dysfunction of ID molecules may lead to arrhythmia, focusing mostly on arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited condition linked to dysfunction of desmosomes and other ID proteins. In the end, we converge with other investigators to challenge the notions that (1) connexins are only involved in the formation of gap junctions, (2) sodium channels are only important for single cell excitability, (3) desmosomal molecules are only relevant to cell adhesion, (4) the dyad and the ID are separate and independent structures, and (5) it is only through modifications of those functions that these proteins participate in the genesis of lethal cardiac arrhythmias or are potentially valuable as targets for antiarrhythmic therapy.
In its classical definition, the ID is composed of three electron-dense structures: adherens junctions, desmosomes, and gap junctions ( Fig. 22.1 ). Additional reviews on the characteristics of these structures can be found elsewhere. , These structures are described briefly later, and the structural/molecular definition of the ID is expanded to include the area composita, intercellular space, and so-called nonjunctional ion channel complexes.
Adherens junctions are specialized structures essential for mechanical coupling between neighboring cells. The three morphologically different forms of adherens junctions are puncta adherentia, zonula adherens, and fascia adherens, with the last name corresponding to the morphology found in the cardiac ID. Cell-cell mechanical anchoring occurs at two crucial points: the extracellular space, within which cadherins tightly bind to each other, and the intracellular space, within which the cytoplasmic end of the cadherin is indirectly attached to the actin cytoskeleton. The association between cadherins and the cytoskeleton involves at least two molecular “hinges.” Cadherin binds to β-catenin and plakoglobin, and both molecules in turn bind to α-catenin (among others), the latter being in direct contact with actin.
A desmosome (macula adherens) appears as two parallel tripartite plaques, containing an intercellular gap of approximately 30 nm bisected by a distinct line, parallel to the apposed cell membranes (see Fig. 22.1 for an example). Desmosomes contribute to the mechanical continuity between cardiac cells. Whereas adherens junctions link the actin cytoskeleton of adjacent cells, desmosomes provide continuity to the intermediate filament network (desmin in the case of the heart). , In the extracellular space, desmosomal cadherins (desmocollins and desmogleins) bind tightly to each other. In the intracellular space, the intermediate filaments bind to desmoplakin (DSP). The interaction between DSP and desmosomal cadherin can be, in some cases, direct but mostly occurs through the association with PKP2 and plakoglobin. , The topologic organization of desmosomal molecules was studied by North and associates using quantitative immunogold electron microscopy. More recently, Al-Amoudi and coworkers solved the three-dimensional molecular structure of the desmosomal plaque. Overall, the structural and biochemical evidence combined show that DSP binds to PKP2 through its N-terminal domain, , whereas DSP binds to the intermediate filament by its C-terminal domain, , yielding a highly organized structure.
Hatsell and Cowin once described the desmosome as “a system as staid and solid as the queen’s corsets.” With that analogy, it is easy to imagine the loss of containment that would follow in its absence. Mice deficient in plakoglobin, DSP, or PKP2 die during embryonic development as a result of severe myocardial rupture. More relevant from the point of view of clinical cardiology, ARVC (also referred to as arrhythmogenic cardiomyopathy [ACM]) in humans has been linked to mutations in desmosomal proteins. The relationships between the various complexes of the ID and ARVC are discussed later in this chapter and in other review articles. ,
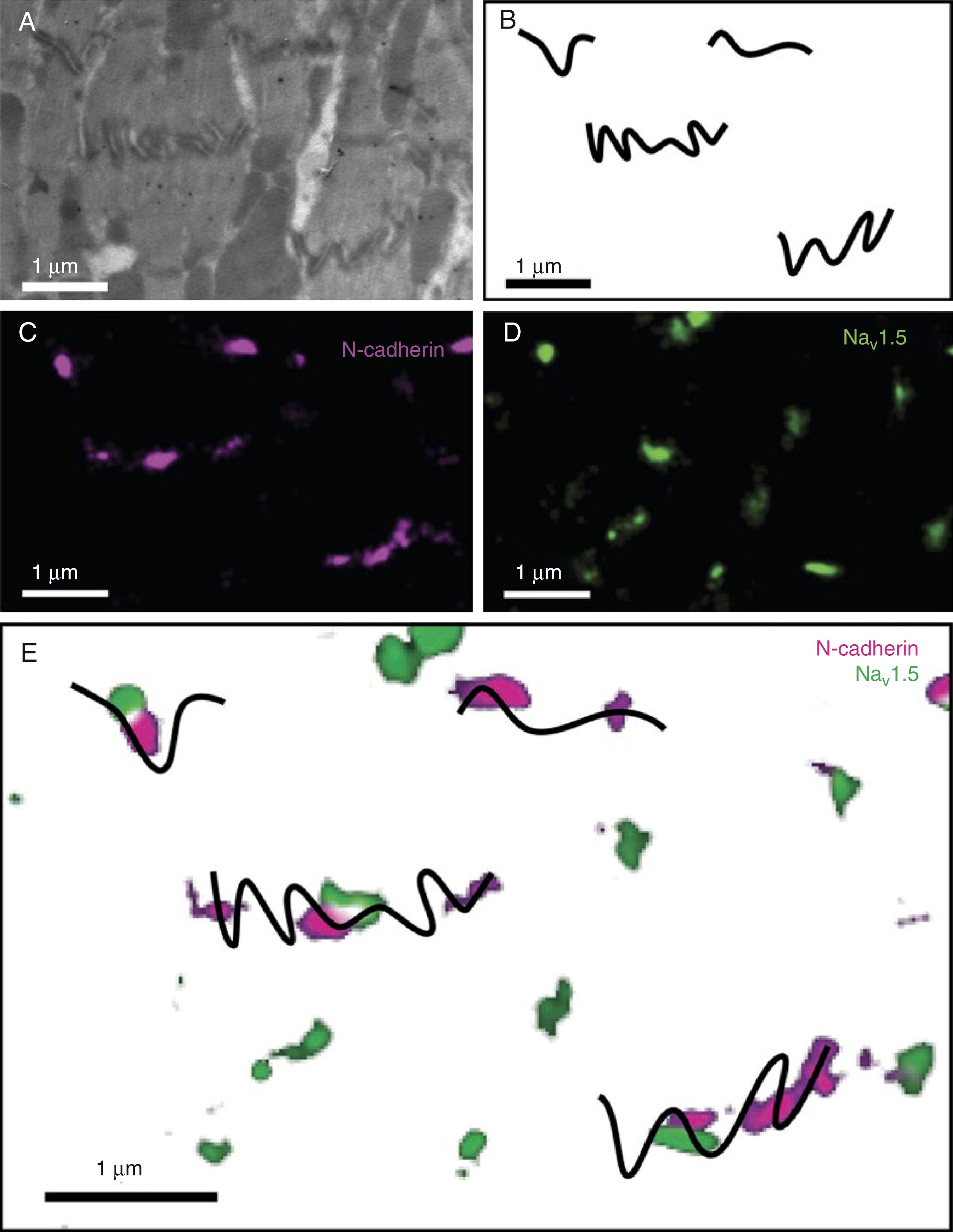
Immunoelectron microscopy studies have revealed the presence of a structure with mixed features of desmosomes and adherens junctions, dubbed the area composita. This structure is only found in the heart of higher vertebrate species, including mice and humans. , The combination of components of desmosomes and adherens junctions allows the anchorage of actin and desmin filaments to the same point, perhaps providing additional strength and flexibility to muscle cells. Knockdown of PKP2 in neonatal CMs leads to the remodeling of the area composita. Additional studies suggest a role for α-T-catenin in the maintenance of these hybrid junctions. Interestingly, knockdown of α-Tcatenin leads to a PKP2 decrease only at the area composita and not at desmosomes, suggesting that the molecular composition of the area composita and its regulation can be independent from that of other junctions. The area composita may represent a physical space where mechanical junction proteins interact with ion channel complexes. The work of Agullo-Pascual and associates has demonstrated that Cx43 can also be found in the area composita and that PKP2 and Cx43 can be found in close physical proximity. The separate work of Leo-Macias et al. showed Na V 1.5 closely localized with N-cadherin, forming an “adhesion-excitability node” or “mininode of Ranvier” (see Fig. 22.1 ). The collective evidence suggests that these structures represent the home of the cardiac connexome, where interactions between structural and ion channel complexes occur. ,
Catenins are a part of both adherens junctions and desmosomes, thus participating in cell adhesion. Yet these proteins also act as transcriptional activators. A prominent example is the participation of β-catenins in the canonical Wnt signaling. Binding of Wnt to its frizzled receptor leads to an increase in the levels of cytosolic β-catenin and a consequent translocation of the protein to the nucleus, where it binds to the Tcf/Lef complex, promoting the expression of various genes, such as c-Myc or c-Fos . In the heart, the Wnt signaling and the activation of genes by β-catenin/Tcf/Lef have been associated with the regulation of the physiologic and pathologic growth of CMs. Plakoglobin, another protein of the armadillo family, shows high homology with β-catenin and has been also associated with Wnt signaling. Different studies have shown that plakoglobin interacts and competes with β-catenin at multiple levels, acting as an antagonist of the Wnt/β-catenin signaling. The fact that desmosomal proteins are involved in the regulation of the Tcf/Lef complex has been invoked as a possible mechanism for the fibrofatty infiltration common in hearts affected with ARVC. The participation of the Hippo pathway in this process has been proposed.
In 1958 Sjostrand and colleagues described an area of specialization in the cardiac ID composed of “three dark lines with two intervening less dense lines.” This structure, which was similar to the one previously identified in the giant axon of the crayfish, was named the longitudinal connexion by these investigators. Years later, Revel coined the term gap junctions , thus emphasizing two key features: a gap between the cells and yet a junction between them.
Gap junctions form intercellular channels that provide a low-resistance pathway for direct cell-to-cell passage of electrical charges between CMs. Each gap junction channel is composed of two hexameric structures called connexons that dock across the extracellular space and form a permeable pore isolated from the extracellular space. Each connexon results from the oligomerization of an integral membrane protein, connexin. The most abundant connexin isotype in the heart, brain, and other tissues is the 43-kDa protein Cx43.
The importance of Cx43 in the propagation of the cardiac action potential is well established. If Cx43 channels are not present, normal propagation is disrupted and lethal arrhythmias can ensue. , Gap junction remodeling has been studied for various inherited and acquired diseases. The implications of connexin remodeling to electrophysiology are discussed later in this chapter.
Gap junctions are formed by two connexin hexamers (also referred to as connexons or hemichannels ). Each cell provides a hemichannel; when two hemichannels dock in the intercellular space, they form a full gap junction channel. For a number of years, Cx43 hemichannels (Cx43-Hs) were considered electrically silent, maintaining a tight closed configuration until docking. This view has been progressively changing. Earlier studies showed that Cx43-Hs open upon metabolic inhibition. , More recent data demonstrated active Cx43-Hs in adult ventricular myocytes, but their activation seemed to require extreme depolarization (V m +40mV), questioning their potential physiologic role. In another study, Lissoni et al used an ingenious experimental approach to demonstrate that Cx43-Hs in adult ventricular myocytes can be opened within a physiologic range of intracellular calcium ([Ca 2+ ] i ) and V m , and contribute to set action potential duration. Cx43-Hs can be activated by various triggers such as changes in Cx phosphorylation status and by oxidative stress. , When open, a Cx43-H provides a nonselective, large-conductance conduit that can allow the passage of molecules and ions up to 1000 Da across the membrane.
It is important to mention that the structure, regulation, and pharmacologic susceptibility of Cx43-Hs are not the same as those of gap junctions. As such, when it comes to studies on Cx43-Hs, gap junction biology is not an adequate point of reference. Cx43-Hs are not simply “half gap junctions.” A few fundamental differences are outlined. (1) The relation between gap junctions and [Ca 2+ ] i is sigmoidal: junctional conductance decreases as [Ca 2+ ] i increases. In contrast, the relation between Cx43-Hs and [Ca 2+ ] i is bell-shaped: there is a range of [Ca 2+ ] i (250 to 500 nM) at which Cx43-Hs are active, but higher and lower [Ca 2+ ] i lead to a closed state. (2) Inhibitors of Cx43-Hs such as αCT1, RRNYRRNY, and GAP19 do not close gap junctions. In other words, if a molecule brings Cx43-Hs from the open to the closed state, one should not assume that gap junctions will close as well. (3) Effects of posttranslational modifications on Cx43-Hs versus gap junctions may differ. The effects of Cx43 phosphorylation on gap junction conductance do not predict Cx43-Hs function. Overall, the notion that Cx43-Hs can be active during the cardiac cycle and modulated in pathologic conditions opens a new window to understanding the role of Cx43 in electrophysiology, not only as a building block of gap junctions but also as a functional membrane channel that if activated in excess, could be a substrate for arrhythmias.
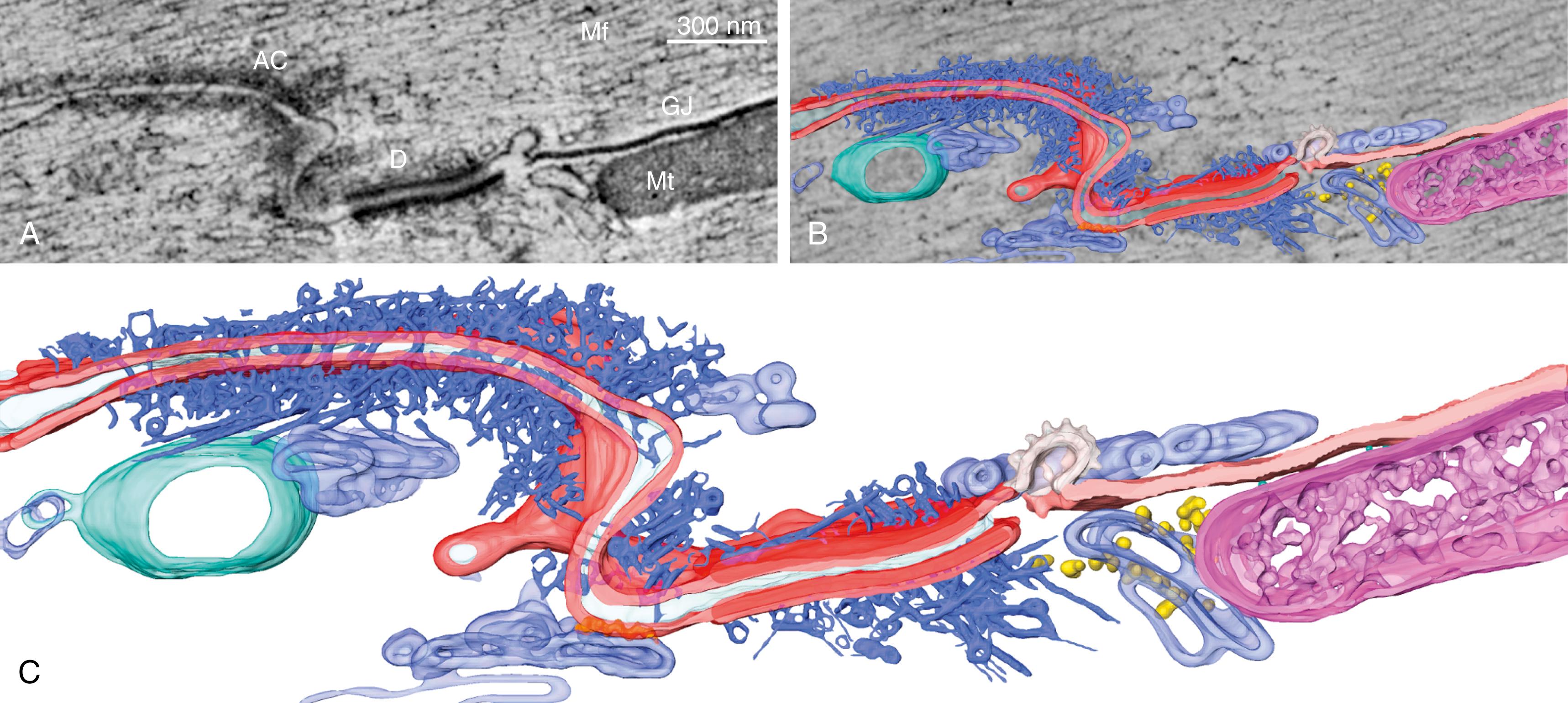
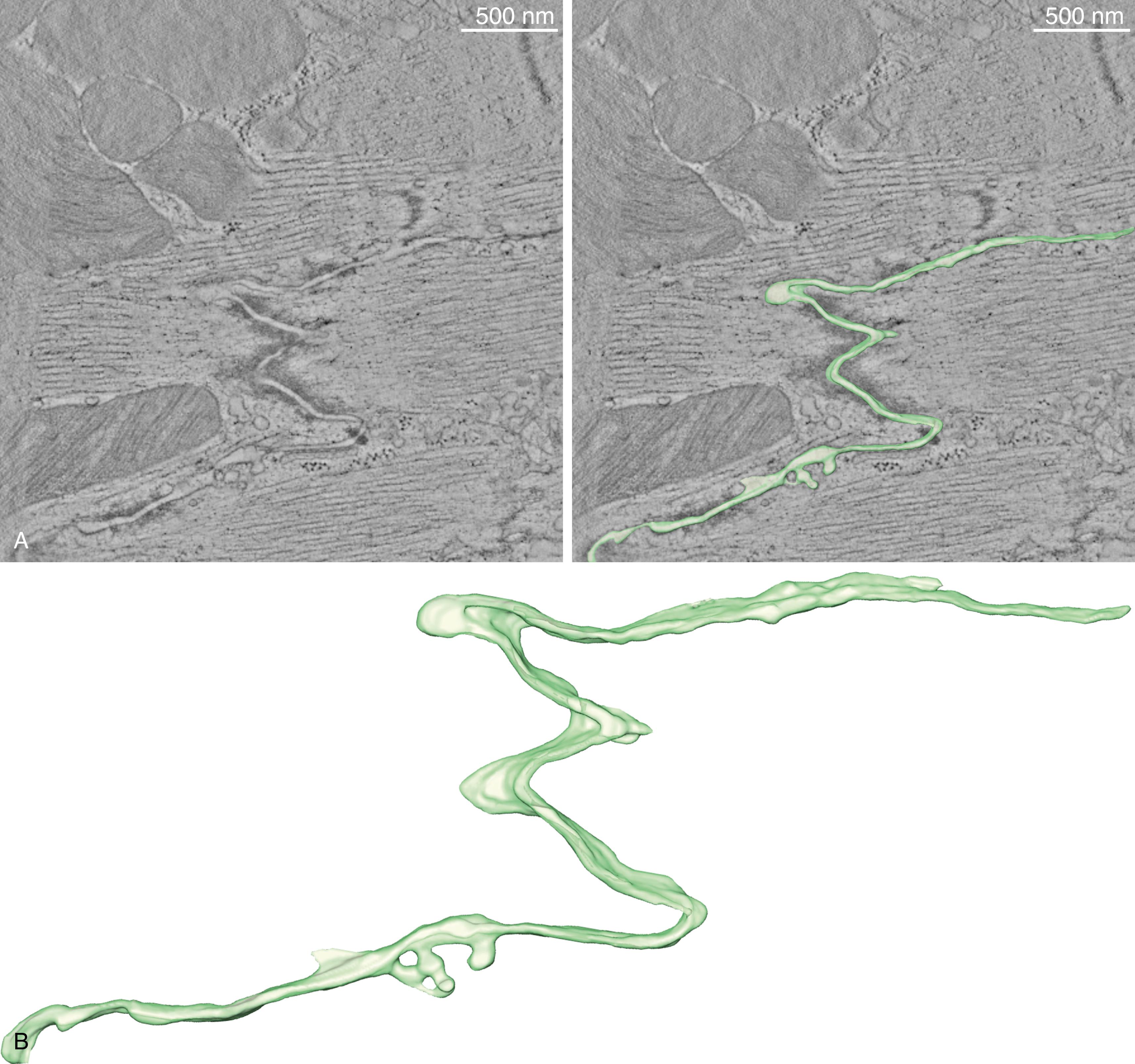
The size of the space separating two cardiac cells at the ID changes depending on its proximity to the various structures and the vesicular activity between the two cells ( Fig. 22.2 ). It is a common view that the intercellular space is not relevant for electrophysiology. This view, however, is changing. Mathematical modeling studies , and experimental evidence support the idea that the intercellular space may be critical to propagation via an electric field mechanism. Of note, increased size of the intercellular space has been reported in animal models of ARVC. Recently, Leo-Macias and associates used tomographic electron microscopy to obtain a structural reconstruction of the intercellular space ( Fig. 22.3 ). Their findings suggest that at the sites occupied by the area composita, the intercellular membranes may separated by a gap of approximately 90 nm.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here