Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The principal functional unit of the central nervous system (CNS) is the neuron . Neurons of different types and in different locations have distinct properties, including diverse functional roles, patterns of synaptic connections, neurotransmitters used, and metabolic requirements, which vary with electrical activity. A set of neurons, not necessarily clustered together in a region of the brain, may thus show selective vulnerability to particular insults because they share one or more of these properties. Because brain functions are anatomically compartmentalized, the pattern of clinical signs and symptoms that follow injury depend as much on the region of brain that is affected as on the pathologic process. Mature neurons are incapable of cell division, so destruction of even a small number of neurons essential for a specific function may produce a neurologic deficit.
In addition to neurons, the CNS contains other cells, such as astrocytes and oligodendrocytes , which make up the glia . Different cellular components of the CNS are affected by distinct unique neurologic disorders and also respond to common insults (e.g., ischemia, infection) in a manner that is distinct from other tissues. We start our discussion of diseases of the CNS with an overview of the patterns of injury of different cells and the reactions of these cells to various insults.
Neurons and glia of the CNS display a range of functional and morphologic changes following injury. Understanding these patterns can provide information about the mechanism of cellular injury and type of disease.
Neuronal injury may be an acute process, often as a consequence of depletion of oxygen or glucose or trauma, or may develop over a period of years, as occurs in degenerative disorders of the brain. Neurons are highly metabolically active and require a continuous supply of oxygen and glucose to meet their metabolic needs. Moreover, since neurons are postmitotic cells that are incapable of proliferation, they must be maintained throughout life. Perhaps because of their lengthy lifespan and high metabolic activity, neurons are unusually susceptible to the accumulation of misfolded proteins, which trigger the unfolded protein response ( Chapter 2 ) and appear to be central to the pathogenesis of many degenerative disorders of the CNS.
Acute neuronal injury (“red neurons”) refers to changes seen following acute CNS hypoxia/ischemia, severe hypoglycemia, and other acute insults, and are the earliest morphologic markers of neuronal cell death (see Fig. 28.15A ). “Red neurons” are evident by 6 to 12 hours after an irreversible hypoxic/ischemic insult. Typical features include shrinkage of the cell body, pyknosis of the nucleus, disappearance of the nucleolus, loss of Nissl substance, and intense cytoplasmic eosinophilia.
Subacute and chronic neuronal injury (“degeneration”) refers to neuronal death that occurs as a result of a progressive disease of months to years duration, as is seen in slowly evolving neurodegenerative diseases such as amyotrophic lateral sclerosis and Alzheimer disease. Prior to death, neurons often suffer a loss of synapses (sites of interneuronal communication), which may stem from aberrations in synaptic pruning (a process responsible for normal brain development and plasticity). Loss of synapses is followed by cell death (often selectively involving functionally related groups of neurons) and reactive gliosis. At an early stage, the cell loss is difficult to appreciate; the associated reactive glial changes are often the best indicator of neuronal injury. In many of these diseases, the predominant mechanism of cell death appears to be apoptosis.
Axonal reaction is a change observed in the cell body during regeneration of the axon; it is best seen in anterior horn cells of the spinal cord when motor axons are cut or seriously damaged. The increase in protein synthesis that occurs in response to the injury is reflected in enlargement and rounding up of the cell body, peripheral displacement of the nucleus, enlargement of the nucleolus, and dispersion of Nissl substance from the center to the periphery of the cell (central chromatolysis).
Neuronal damage may be associated with a wide range of subcellular alterations in the neuronal organelles and cytoskeleton. Neuronal inclusions may occur as a manifestation of aging, and consist of intracytoplasmic accumulations of complex lipids (lipofuscin), proteins, or carbohydrates. Abnormal cytoplasmic accumulations of lipids and other substances also occur with certain inborn errors of metabolism, genetic disorders caused by mutations that lead to the loss of specific enzyme activities ( Chapter 5 ). Viral infection can lead to the appearance of viral inclusions, e.g., in herpetic infection (Cowdry A or B bodies), rabies (Negri body), and cytomegalovirus infection.
Some degenerative diseases of the CNS are associated with neuronal intracytoplasmic inclusions, such as neurofibrillary tangles of Alzheimer disease and Lewy bodies of Parkinson disease; others cause abnormal vacuolization of the perikaryon and neuronal cell processes in the neuropil (Creutzfeldt-Jakob disease).
Wallerian degeneration refers to degeneration of axons after disruption of nerve fibers (see Chapter 27 ).
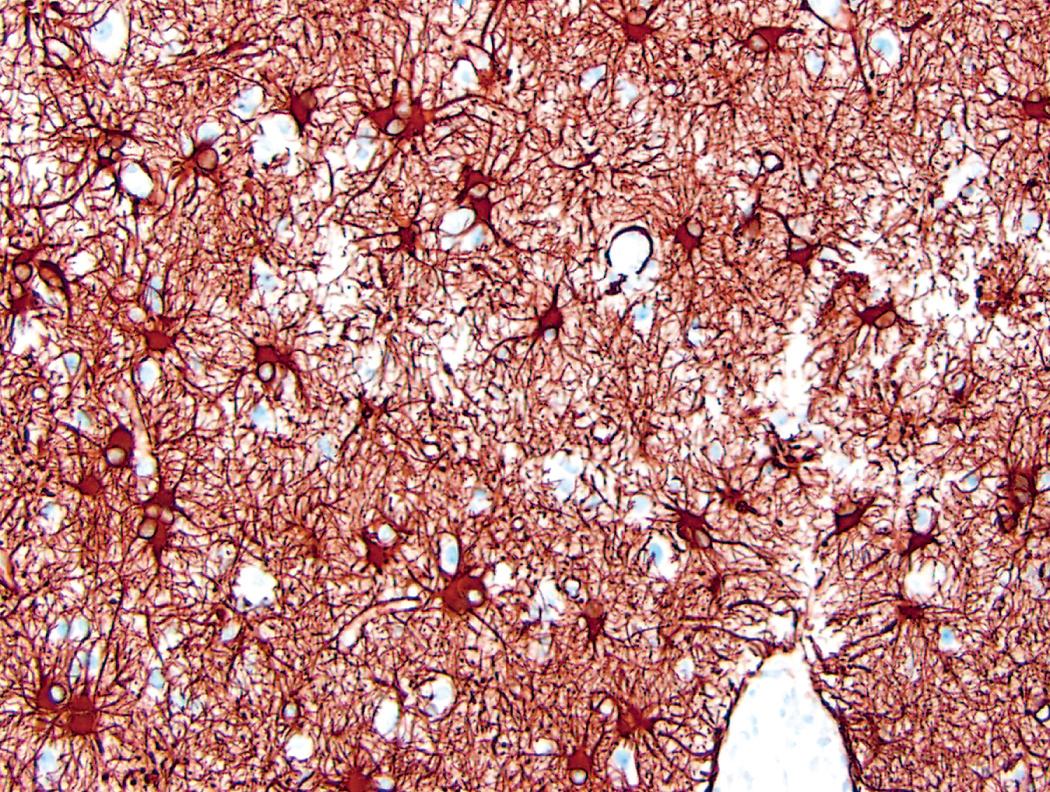
Gliosis is the most important histopathologic marker of CNS injury, regardless of etiology, and is characterized by hypertrophy and hyperplasia of astrocytes. The astrocyte derives its name from its star-shaped appearance. These cells have multipolar, extensively branched cytoplasmic processes that emanate from the cell body and express glial fibrillary acidic protein (GFAP), a cell type–specific intermediate filament. Astrocytes act as metabolic buffers and detoxifiers within the brain. Additionally, through the foot processes (which surround capillaries or extend to the subpial and subependymal zones), they contribute to barrier functions by controlling the flow of macromolecules between the blood, the cerebrospinal fluid (CSF), and the brain.
In gliosis, the nuclei of astrocytes (which are typically oval with evenly dispersed, pale chromatin) enlarge, become vesicular, and occasionally develop prominent nucleoli. The previously indistinct cytoplasm becomes bright pink due to increased expression of GFAP, and the cells develop numerous stout, ramifying processes; these cells are called reactive or gemistocytic astrocytes ( Fig. 28.1 ). There are at least two subtypes of reactive astrocytes; although morphologically indistinguishable, these cells form in response to different injurious stimuli, have distinct gene expression patterns, and have different effects on neuronal health and survival, with one subtype promoting CNS injury and the other contributing to CNS repair.
Acute astrocytic injury, as occurs in hypoxia, hypoglycemia, and toxic injuries, is manifested by cellular swelling, as in other cells ( Chapter 2 ). The Alzheimer type II astrocyte (unrelated to the disease but first described by the same neuroscientist) is a gray matter cell with a large (two to three times normal) nucleus, pale-staining central chromatin, an intranuclear glycogen droplet, and a prominent nuclear membrane and nucleolus. Alzheimer type II astrocytes are mainly seen in individuals with hyperammonemia due to chronic liver disease, Wilson disease, or hereditary metabolic disorders of the urea cycle.
Other types of astrocyte injury lead to the formation of cytoplasmic inclusion bodies. Rosenthal fibers are thick, elongated, brightly eosinophilic, irregular structures that occur within astrocytic processes and contain two heat-shock proteins (αB-crystallin and hsp27) as well as ubiquitin. Rosenthal fibers are typically found in regions of long-standing gliosis; they are also characteristic of one type of glial tumor, pilocytic astrocytoma (see Fig. 28.49C ). In Alexander disease, a leukodystrophy associated with mutations in the gene encoding GFAP, abundant Rosenthal fibers are found in periventricular, perivascular, and subpial locations. More commonly seen are corpora amylacea, or polyglucosan bodies. These are round, faintly basophilic, periodic acid-Schiff (PAS)–positive, concentrically lamellated structures of 5 to 50 µm in diameter that are located wherever there are astrocytic end processes, especially in the subpial and perivascular zones. They consist primarily of glycosaminoglycan polymers, as well as heat-shock proteins and ubiquitin. Corpora amylacea occur in increasing numbers with advancing age and are thought to represent a degenerative change in the astrocyte. Lafora bodies , which are seen in the cytoplasm of neurons (as well as hepatocytes, myocytes, and other cells) in a variant of myoclonic epilepsy, have a similar structure and biochemical composition.
Microglia are phagocytic cells derived early in embryonic development from the yolk sac or fetal liver that serve as the resident macrophages of the CNS and share many surface markers with bone marrow–derived peripheral monocytes/macrophages. At rest, microglia are tiled (i.e., cover nonoverlapping territories) and have highly branched, amoeboid processes. During development, microglia prune unused synaptic connections, most likely through a phagocytic process that may involve the complement system; aberrant reactivation of this developmental process has recently been implicated in a number of different brain diseases, including schizophrenia, encephalitis, Alzheimer disease, and frontotemporal dementia. Microglia respond to injury by (1) proliferating; (2) developing elongated nuclei; (3) forming aggregates around small foci of tissue necrosis (microglial nodules); or (4) congregating around cell bodies of dying neurons (neuronophagia) . In addition to resident microglia, blood-derived macrophages may also be present in inflammatory foci.
Oligodendrocytes are cells that wrap their cytoplasmic processes around axons and form myelin. Each oligodendrocyte myelinates numerous internodes on multiple axons, in contrast to the myelinating Schwann cell in peripheral nerves, which only myelinates the internode of a single axon. Injury or apoptosis of oligodendrocytes is a feature of acquired demyelinating disorders and leukodystrophies. Oligodendroglial nuclei may harbor viral inclusions in progressive multifocal leukoencephalopathy (PML). Glial cytoplasmic inclusions, primarily composed of α-synuclein, are found in oligodendrocytes in multiple system atrophy (MSA).
Ependymal cells , the ciliated columnar epithelial cells lining the ventricles, do not show specific reaction patterns. When there is inflammation or marked dilation of the ventricular system, disruption of the ependymal lining is paired with proliferation of subependymal astrocytes to produce small irregularities on the ventricular surfaces (ependymal granulations) . Certain infectious agents, particularly CMV, may produce extensive ependymal injury, with viral inclusions in ependymal cells. However, neither oligodendrocytes nor ependymal cells mediate significant responses to most forms of injury in the CNS.
Each cellular component of the nervous system responds to injury in a distinctive way.
Neuronal injury commonly results in cell death, either by apoptosis or necrosis. Loss of neurons or synapses is often difficult to detect morphologically, but is a major contributor to neurologic dysfunction.
Astrocytes respond to injury through apparent hypertrophy (increased cytoplasm due to accumulation of the intermediate filament protein GFAP) and hyperplasia; this response can be beneficial or harmful, depending on the type of injurious stimulus and the exact nature of astrocytic response.
Microglia, the resident monocyte-lineage population of the CNS, proliferate and accumulate in response to injury. Aberrant activation of microglia- and complement-mediated synaptic pruning plays an important role in the pathogenesis of many different neurologic disorders.
The brain and the spinal cord are encased and protected by the rigid skull, dural reflections, and the bony spinal canal. The pressure within the cranial cavity may rise in any one of three commonly observed clinical settings: generalized brain edema, increased CSF volume, and focally expanding mass lesions. Depending on the degree and rapidity of the pressure increase and the nature of the underlying lesion, the consequences range from subtle neurologic deficits to death.
Cerebral edema (more precisely, brain parenchymal edema) is the result of increased fluid leakage from blood vessels and injury to various cells of the CNS. There are two main pathways of edema formation in the brain.
Vasogenic edema is an increase in extracellular fluid caused by blood-brain barrier disruption and increased vascular permeability, allowing fluid to shift from the intravascular compartment to the intercellular spaces of the brain. The paucity of lymphatics greatly impairs the resorption of excess extracellular fluid. Vasogenic edema may be either localized, e.g., adjacent to inflammation or neoplasms, or generalized, as may occur following a global ischemic injury.
Cytotoxic edema is an increase in intracellular fluid secondary to neuronal, glial, or endothelial cell membrane injury, as might be encountered with a generalized hypoxic/ischemic insult or with a metabolic derangement that prevents maintenance of the normal membrane ionic gradients.
In practice, conditions associated with generalized edema often have elements of both vasogenic and cytotoxic edema. In generalized edema, the gyri are flattened, the intervening sulci are narrowed, and the ventricular cavities are compressed. As the brain expands, herniation may occur.
Hydrocephalus is the accumulation of excessive CSF within the ventricular system ( Fig. 28.2 ). The choroid plexus within the ventricular system produces CSF, which normally circulates through the ventricular system and enters the cisterna magna at the base of the brainstem through the foramina of Luschka and Magendie. Subarachnoid CSF bathes the superior cerebral convexities and is absorbed by the arachnoid granulations. Most cases of hydrocephalus are a consequence of impaired flow and resorption of CSF; overproduction is a rare cause that can accompany tumors of the choroid plexus. In all forms, the increased volume of CSF expands the ventricles and can elevate the intracranial pressure.

When hydrocephalus develops in infancy before closure of the cranial sutures, the head enlarges. By contrast, after the sutures close, hydrocephalus is associated with expansion of the ventricles and increased intracranial pressure, without a change in head circumference. If the ventricular system is only focally obstructed, due to a mass in the third ventricle or to aqueductal stenosis, it is called noncommunicating (obstructive) hydrocephalus . In communicating hydrocephalus, the ventricular system remains in continuity with the subarachnoid space and there is enlargement of the entire ventricular system; causes include overproduction of CSF from a choroid plexus tumor and arachnoid fibrosis following meningitis. The term hydrocephalus ex vacuo refers to a compensatory increase in ventricular volume secondary to a loss of brain parenchyma.
Herniation is the displacement of brain tissue past rigid dural folds (the falx and tentorium) or through openings in the skull because of increased intracranial pressure. As the volume of the brain increases, CSF is displaced, leading to increasing pressure within the cranial cavity; herniation occurs when this pressure exceeds the brain's limited capacity to accommodate the increased intracranial pressure. Brain herniation is mostly caused by mass effects, either diffuse (generalized brain edema) or focal (tumors, abscesses, or hemorrhages). Elevated intracranial pressure may also compress the vasculature and reduce perfusion of the brain, causing ischemic injury and further exacerbating cerebral edema.
The brain may herniate through different openings, and if the expansion is sufficiently severe, herniation may occur simultaneously in several locations ( Fig. 28.3 ):
Subfalcine (cingulate) herniation occurs when unilateral or asymmetric expansion of a cerebral hemisphere displaces the cingulate gyrus under the falx. This may lead to compression of the anterior cerebral artery and its branches, resulting in secondary infarcts.
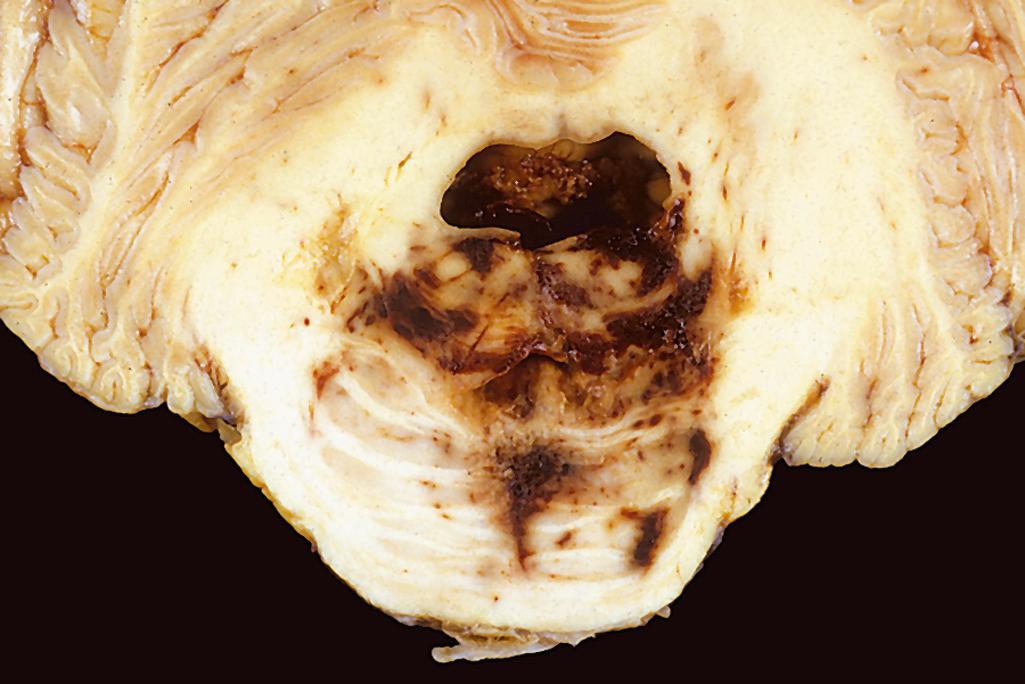
Transtentorial (uncal, mesial temporal) herniation occurs when the medial aspect of the temporal lobe is compressed against the free margin of the tentorium. With increasing displacement of the temporal lobe, the third cranial nerve is compromised, resulting in pupillary dilation and impaired ocular movements on the side of the lesion. The posterior cerebral artery may also be compressed, resulting in an infarct of its territory (which includes the primary visual cortex). When the extent of herniation is large enough, the contralateral cerebral peduncle may be compressed, resulting in hemiparesis ipsilateral to the side of the herniation. Progression of transtentorial herniation is often accompanied by secondary hemorrhagic lesions in the midbrain and pons, termed Duret hemorrhages ( Fig. 28.4 ). These linear or flame-shaped lesions usually occur in the midline and paramedian regions and are believed to be due to distortion or tearing of penetrating veins and arteries supplying the upper brainstem.
Tonsillar herniation refers to displacement of the cerebellar tonsils through the foramen magnum. This pattern of herniation is life-threatening because it causes brainstem compression and compromises vital respiratory and cardiac centers in the medulla.
Cerebral edema is the accumulation of excess fluid within the brain parenchyma. Hydrocephalus is an increase in CSF volume within all or part of the ventricular system.
Increase in the intracranial volume (due to increased CSF volume, parenchymal edema, hemorrhage, or tumor) raises intracranial pressure.
Increased intracranial pressure may result in cerebral herniation, decreased perfusion, and secondary infarction of the affected areas.
Although the pathogenesis and etiology of many CNS malformations remain unknown, both genetic and environmental influences appear to be involved. Genomic sequencing has begun to uncover a range of genetic variants that are associated with certain malformations. The causal relationship between these genetic alterations and the pathogenesis of the malformations is the subject of active research. Besides genetic factors, many toxic compounds and infectious agents also have teratogenic effects and may cause brain malformations.
Neural tube defects are midline malformations that involve some combination of neural tissue, meninges, and overlying bone or soft tissue; collectively, they are the most common CNS malformations. Two distinct pathogenic mechanisms are contributory: (1) failure of neural tube closure, in which secondary mesenchymal tissue defects stem from aberrant skeletal modeling around the malformed tube (e.g., anencephaly and myelomeningocele); and (2) primary bony defects that are caused by abnormal axial mesoderm development and lead to secondary CNS abnormalities (e.g., encephalocele, meningocele, and spina bifida).
Anencephaly is a malformation of the anterior end of the neural tube that leads to absence of most of the brain and calvarium. Forebrain development is disrupted at approximately 28 days of gestation, and all that remains in its place is the area cerebrovasculosa, a flattened remnant of disorganized brain tissue with admixed ependyma, choroid plexus, and meningothelial cells. The posterior fossa structures may be spared, depending on the extent of the skull deficit; descending tracts associated with disrupted structures are, as expected, absent.
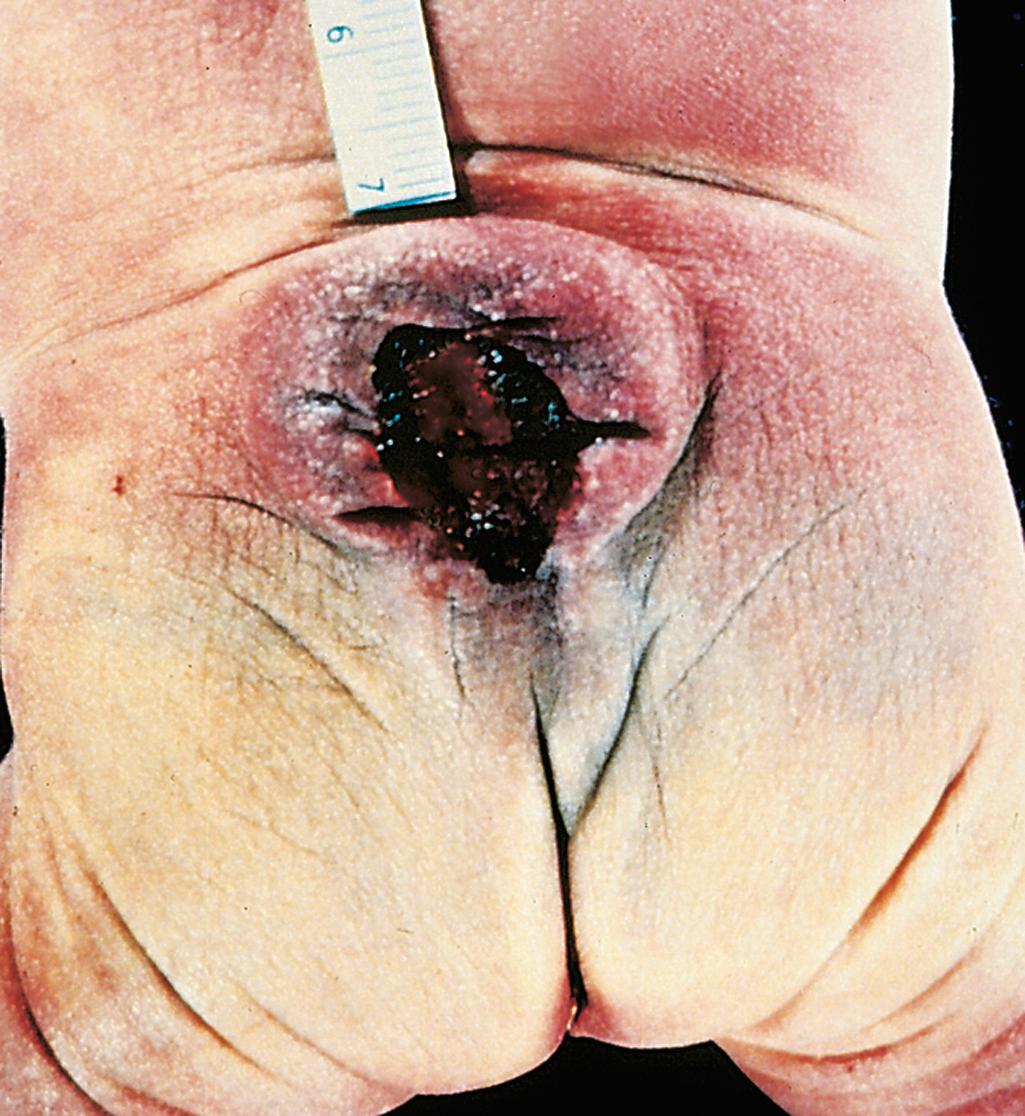
Myelomeningocele (or meningomyelocele) refers to extension of CNS tissue through a defect in the vertebral column; the term meningocele applies when there is only a meningeal extrusion. Myelomeningoceles occur most commonly in the lumbosacral region ( Fig. 28.5 ). Affected individuals have motor and sensory deficits in the lower extremities as well as disturbances of bowel and bladder control. They are often complicated by superimposed infection of the cord due to defective barrier function of the thin, overlying skin.
Encephalocele refers to an extrusion of malformed brain tissue through a midline defect in the cranium. It most often occurs in the occiput, although nasofrontal variants involving the orbit, ethmoid, or cribriform plate (sometimes misleadingly referred to as a “nasal glioma”) also are seen.
Spinal dysraphism or spina bifida (the most common neural tube defects) may be an asymptomatic bony defect (spina bifida occulta) or a severe malformation with a flattened, disorganized segment of spinal cord, associated with an overlying meningeal outpouching.
The frequency of neural tube defects varies widely among different ethnic groups, with the overall recurrence rate for a neural tube defect in subsequent pregnancies estimated at 4% to 5%. Folate deficiency during the first several weeks of gestation is a well-established risk factor; differences in rates of neural tube defects among populations can be attributed in part to polymorphisms in enzymes involved in folic acid metabolism. Folate supplementation can lower the risk of neural tube defects, but because neural tube closure is normally complete by day 28 of embryonic development (before most pregnancies are recognized), it must be given to women throughout their reproductive years to be fully effective. Precisely how folate deficiency increases the risk is uncertain; effects on DNA methylation (an important epigenetic mode of gene regulation) are suspected.
Abnormalities in the generation and migration of neurons result in malformations of the forebrain that may be focal or involve entire structures . The pool of proliferating precursor cells in the developing brain lies in the germinal matrix adjacent to the ventricular system; the total number of neurons is determined by the fraction of proliferating cells that undergo transition into migrating cells with each cell cycle. The migration of neurons from the germinal matrix to the cerebral cortex follows two paths: radial migration, for progenitor cells destined to become excitatory neurons; and tangential migration, for those that will become inhibitory interneurons.
A range of different anatomic malformation patterns has been defined. Recently, it has become clear that many of these patterns are caused by mutations in genes that are required for proper cerebral development. Changes may be seen in the complexity of the brain surface (either too few or too many gyri present), the organization of the brain into normal lobes, the structure of the cerebral cortex, or the distribution of neurons within the brain.
The volume of brain may be abnormally large (megalencephaly) or abnormally small (microencephaly) . Microencephaly, by far the more common of the two, is typically accompanied by a small head circumference. It is associated with a number of conditions, including chromosome abnormalities, fetal alcohol syndrome, and viral infection acquired in utero (e.g., with human immunodeficiency virus 1 [HIV-1] or Zika virus). It is postulated that the underlying anomaly is a reduction in the number of neurons that reach the neocortex, which leads to a simplification of gyral folding, a mechanism supported by experimental results in mouse models.
Lissencephaly is a malformation characterized by reduction in the number of gyri, which in the extreme case may show no gyral pattern (agyria) . Two general patterns are observed, a smooth-surface form (type 1), and a rough- or cobblestone-surface form (type 2). In general, type 1 forms are associated with mutations that disrupt mechanisms involved in cell migration, such as mutations in the cytoskeletal “motor” proteins that drive migration of neuroblasts. In contrast, type 2 lissencephaly is most commonly associated with genetic alterations that disrupt the “stop signal” for migration. This signal depends on a set of specifically glycosylated proteins, and mutations in the enzymes that place sugars onto these proteins are the most common causes of this form of lissencephaly.
Polymicrogyria is characterized by numerous small, irregularly formed cerebral convolutions with shallow sulci. The cerebral cortex is composed of four or fewer layers (instead of the normal six layers), with fusion of the molecular layers between gyri. Polymicrogyria can be induced by localized tissue injury toward the end of neuronal migration, although genetically determined forms, which are typically bilateral and symmetric, are also recognized.
Neuronal heterotopias are a group of migrational disorders defined by collections of subcortical neurons in inappropriate locations along the pathway of migration. As might be expected, one location is along the ventricular surface—as though the cells never left their place of birth. Periventricular nodular heterotopias can be caused by mutations in the gene encoding filamin A, an actin-binding protein responsible for assembly of complex meshworks of filaments. This gene is on the X chromosome, and the mutant allele causes male lethality; in females, the process of X inactivation separates neurons into those with a normal allele (in the correct location) and those with the mutant allele (in the heterotopia). Another microtubule-associated protein, doublecortin (DCX), is also encoded by a gene on the X chromosome; mutations in this gene result in lissencephaly in males and in subcortical bandlike heterotopias in females (this parallel layer of gray matter imparts the impression of a “double cortex”). Nodular subcortical heterotopias may also be encountered.
Holoprosencephaly is a spectrum of malformations characterized by incomplete separation of the cerebral hemispheres across the midline. Severe forms manifest midline facial abnormalities, including cyclopia; less severe variants (arrhinencephaly) show absence of the olfactory cranial nerves and related structures. Intrauterine diagnosis of severe forms by ultrasonography is now possible. Holoprosencephaly is associated with trisomy 13 as well as other genetic syndromes. Mutations in genes that encode components of the sonic hedgehog signaling pathway may also produce holoprosencephaly.
Agenesis of the corpus callosum, a relatively common malformation, is the absence of the white matter bundles that carry cortical projections from one hemisphere to the other ( Fig. 28.6 ). Radiologic imaging studies show misshapen lateral ventricles (“bat-wing” deformity); on coronal whole-mount sections of the brain, “Probst bundles” of anteroposteriorly oriented white matter can be demonstrated. Agenesis of the corpus callosum is sometimes associated with intellectual disability but may also be found in normal individuals. It may be sporadic or familial and can be present in isolation or in association with a range of other malformations.
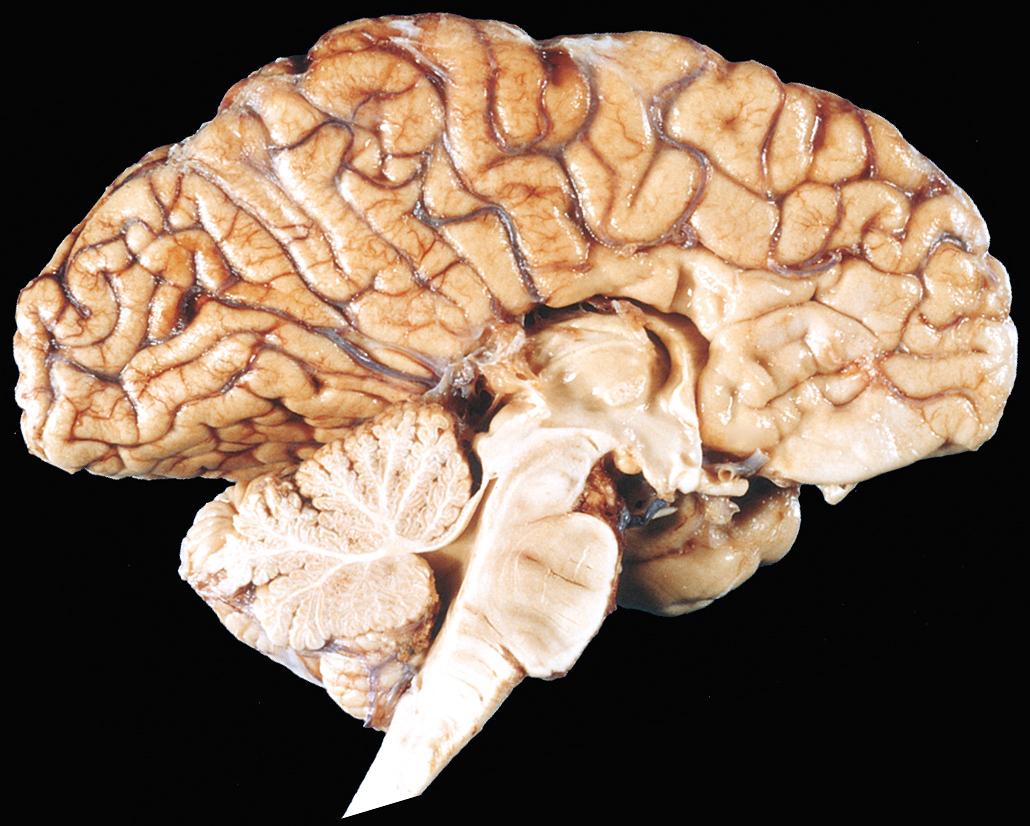
A distinct set of malformations primarily affect the brainstem and the cerebellum, which often show dramatic changes in size and shape. These may be accompanied by morphologic changes in other regions of the brain.
Arnold-Chiari malformation (Chiari type II malformation) consists of a small posterior fossa, a misshapen midline cerebellum with downward extension of vermis through the foramen magnum ( Fig. 28.7 ), and, almost invariably, hydrocephalus and a lumbar myelomeningocele. Other associated changes may include caudal displacement of the medulla, malformation of the tectum, aqueductal stenosis, cerebral heterotopias, and hydromyelia (see later).
Chiari type I malformation is a less severe disorder in which low-lying cerebellar tonsils extend down into the vertebral canal. It may be a silent abnormality or may become symptomatic because of impaired CSF flow and medullary compression; if present, these symptoms can usually be corrected by neurosurgical intervention.
Dandy-Walker malformation is characterized by an enlarged posterior fossa. The cerebellar vermis is absent or present only in rudimentary form in its anterior portion; in its place is a large midline cyst that is lined by ependyma and is contiguous with leptomeninges on its outer surface. This cyst represents the expanded, roofless fourth ventricle in the absence of a normally formed vermis. Dysplasias of brainstem nuclei are commonly found in association with Dandy-Walker malformation.
Joubert syndrome and related disorders share hypoplasia of the cerebellar vermis with apparent elongation of the superior cerebellar peduncles and an altered shape of the brainstem; together these changes give rise to the “molar tooth sign” on imaging. This group of malformations has been found to be caused by diverse mutations affecting genes that encode components of the primary (nonmotile) cilium.
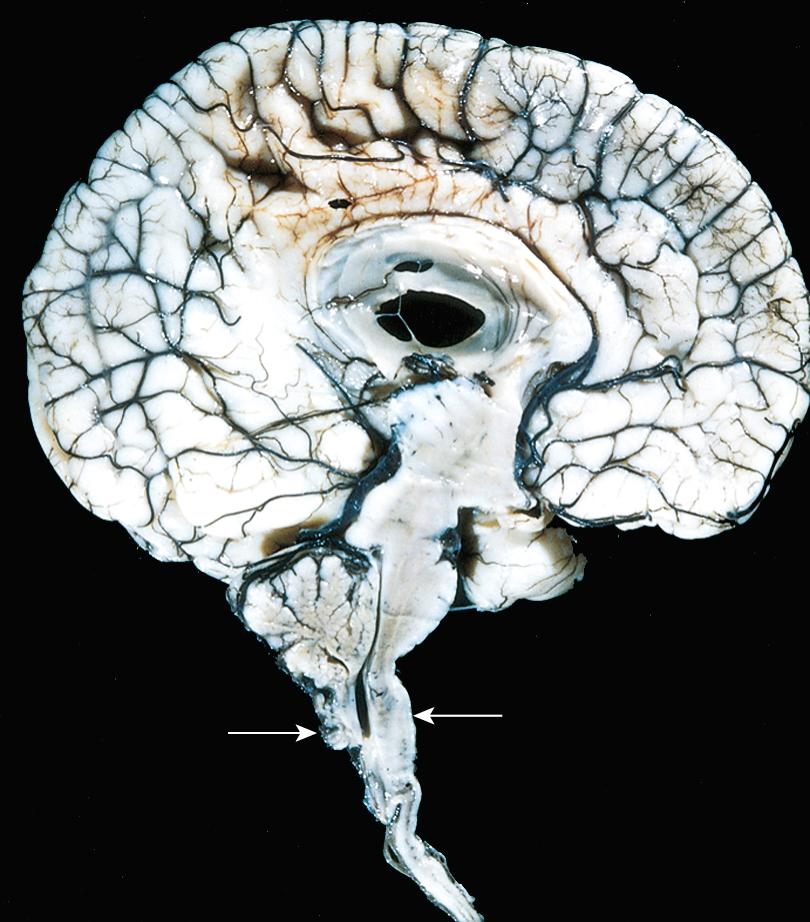
These disorders are characterized by expansion of the ependyma-lined central canal of the cord (hydromyelia) or by the formation of a fluid-filled cleftlike cavity in the inner portion of the cord (syringomyelia, syrinx) that may extend into the brainstem (syringobulbia) .
Syringomyelia may be associated with a Chiari malformation; it may also occur in association with intraspinal tumors or following traumatic injury. In general, the histologic appearance is similar in all of these conditions, with destruction of the adjacent gray and white matter, surrounded by dense reactive gliosis. The disease generally becomes manifest in the second or third decade of life. The distinctive symptoms and signs of a syrinx are the isolated loss of pain and temperature sensation in the upper extremities because of disruption of the crossing anterior spinal commissural fibers of the spinal cord.
Malformations may be associated with single-gene mutations, larger-scale genetic alterations, or exogenous factors.
Overall, the earlier in development a malformation occurs, the more severe the morphologic and functional phenotype.
Neural tube defects are caused by the failure of the tube to close or by skeletal bony defects that lead to secondary tube abnormalities; they range from incidental findings to severe malformations.
Cortical development depends on proper orchestration of progenitor cell proliferation in the germinal matrix and migration of progenitors upward into the developing cortex. Disruption of these processes can alter the size, shape, and organization of the brain.
Malformations involving the posterior fossa are typically distinct from those that affect the cerebral hemispheres.
Brain injury occurring in the perinatal period is an important cause of childhood-onset neurologic disability. Injuries that occur early in gestation may destroy brain tissue without eliciting the reactive changes observed in adult brain and, therefore, may be difficult to distinguish from malformations. Different patterns of injury may occur.
The term cerebral palsy refers to a nonprogressive neurologic motor deficit characterized by combinations of spasticity, dystonia, ataxia/athetosis, and paresis, attributable to brain injury occurring during the prenatal and perinatal periods. Signs and symptoms may not be apparent at birth and only declare themselves later, as development proceeds. Postmortem examinations of children with cerebral palsy have shown a wide range of neuropathologic findings, including destructive lesions traced to remote events that may have caused hemorrhage and infarction.
In premature infants, there is an increased risk of germinal matrix hemorrhage, often near the junction between the developing thalamus and caudate nucleus. Hemorrhages may remain small and localized or extend into the ventricular system and subarachnoid space, sometimes leading to hydrocephalus and death in severe cases.
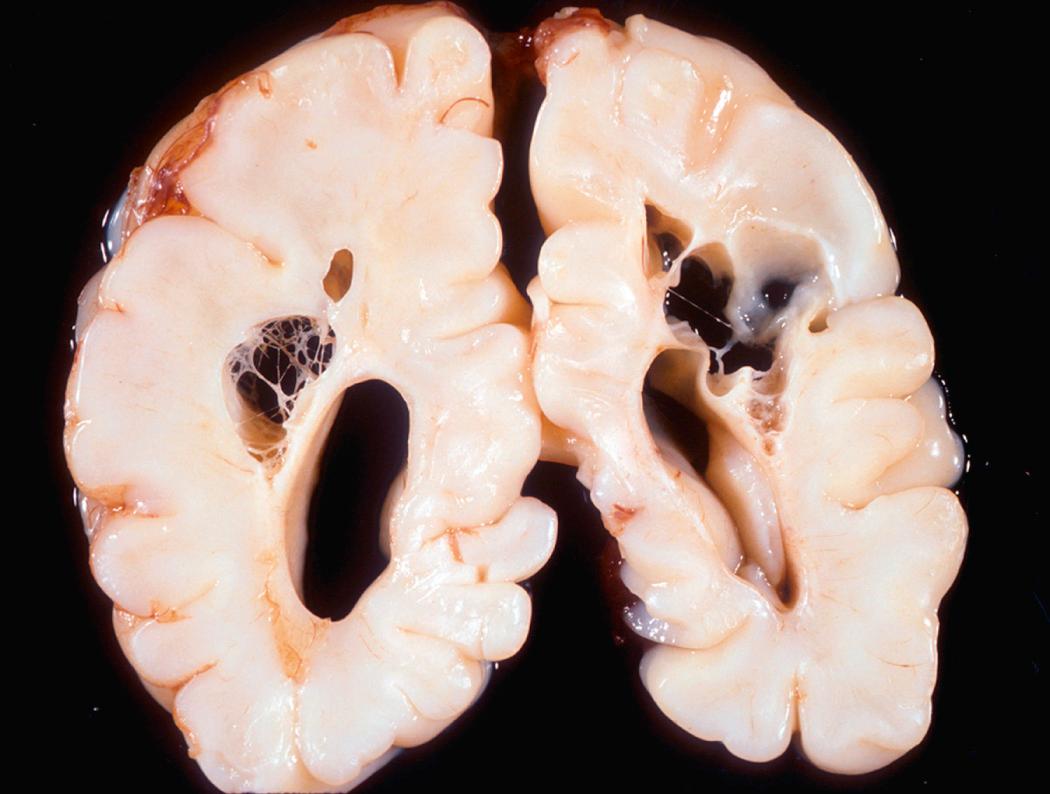
Infarcts may occur in the supratentorial periventricular white matter (periventricular leukomalacia), especially in premature infants; they take the form of chalky yellow plaques that consist of discrete white matter necrosis, often with dystrophic calcification. Ultimately, the infarcted areas develop into large cystic lesions ( Fig. 28.8 ); when damage is extensive and involves both gray and white matter, the condition is termed multicystic encephalopathy .
In perinatal ischemic lesions of the cerebral cortex, the depths of sulci bear the brunt of injury and result in mushroom-shaped gyri with thinned-out, gliotic stalks (ulegyria) . The basal ganglia and thalamus may also suffer ischemic injury, with patchy neuronal loss and reactive gliosis. Later, aberrant and irregular myelinization gives rise to a marble-like appearance of the deep nuclei (status marmoratus). Because the lesions are in the caudate, putamen, and thalamus, movement disorders such as choreoathetosis are common clinical sequelae.
The anatomic location of the lesion and the limited capacity of the brain for functional repair are major determinants of the consequences of CNS trauma. Injury of several cubic centimeters of brain parenchyma may be clinically silent (e.g., in the frontal lobe), severely disabling (e.g., in the spinal cord), or fatal (e.g., in the brainstem).
The physical forces associated with head injury may result in skull fractures, parenchymal injury, and vascular injury; all three can coexist. The magnitude and distribution of a traumatic brain lesion depends on the shape of the object causing the trauma, the force of impact, and whether the head is in motion at the time of injury. A blow to the head may be penetrating or blunt; it may cause either an open or a closed injury.
A fracture in which bone is displaced into the cranial cavity by a distance greater than the thickness of the bone is called a displaced skull fracture . The thickness of the cranial bones varies; therefore, their resistance to fracture differs greatly. Also, the relative incidence of fractures among skull bones is related to the pattern of falls. For example, when an individual falls while awake (such as might occur when stepping off a ladder), the site of impact is often the occipital portion of the skull; in contrast, a fall that follows loss of consciousness (as might follow a syncopal attack) can result in either frontal or occipital impact. Symptoms referable to the lower cranial nerves or the cervicomedullary region, and the presence of orbital or mastoid hematomas distant from the point of impact, raise the suspicion of a basal skull fracture, which typically follows impact to the occiput or sides of the head; CSF leakage from the nose or ear and infection (meningitis) may follow.
The structural consequences and clinical manifestations of the parenchymal injury depend on the nature and severity of the causative head trauma and range from mild (concussion) to severe (contusions and lacerations).
Concussion is a clinical syndrome of altered consciousness secondary to head injury, typically brought about by a change in the momentum of the head (e.g., following impact with a rigid object). The characteristic clinical picture includes the sudden onset of transient neurologic dysfunction, including loss of consciousness, temporary respiratory arrest, and loss of reflexes. Although neurologic recovery is usually complete, amnesia for the event often persists. The pathogenesis of the disruption of neurologic function is unknown, but likely involves dysregulation of the reticular activating system in the brainstem. Postconcussive neuropsychiatric syndromes, typically associated with repetitive injuries, are well recognized, and there is increasing evidence that significant cognitive impairment can emerge along with distinct pathologic findings termed chronic traumatic encephalopathy (discussed later).
Contusions and lacerations are brain injuries caused by transmission of kinetic energy to the brain. A contusion is analogous to the familiar bruise caused by blunt trauma, and a laceration is an injury caused by penetration of an object and tearing of tissue. As with any other organ, a blow to the surface of the brain, transmitted through the skull, leads to rapid tissue displacement, disruption of vascular channels, and subsequent hemorrhage, tissue injury, and edema. Hemorrhage can extend into the subarachnoid space from these lesions. The crests of gyri are most susceptible because this is where the direct force is greatest. The most common locations for contusions correspond to the most frequent sites of direct impact and to regions of the brain that overlie a rough and irregular inner skull surface, such as the frontal lobes along the orbital ridges and the temporal lobes. Contusions are less frequent over the occipital lobes, brainstem, and cerebellum, but may be seen when there is an adjacent skull fracture (fracture contusions).
A person who suffers a blow to the head may develop a contusion at the point of contact (a coup injury) or on the brain surface diametrically opposite to it (a contrecoup injury). Their macroscopic and microscopic appearances are indistinguishable, and the distinction between them is based on identification of the point of impact. In general, if the head is immobile at the time of trauma, only a coup injury is found. If the head is mobile, both coup and contrecoup lesions may be found, though the latter predominate and are thought to develop when the brain strikes the opposite inner surface of the skull after sudden deceleration.
Sudden impacts that result in violent posterior or lateral hyperextension of the neck (as occurs when a pedestrian is struck from the rear by a vehicle) may avulse the pons from the medulla or the medulla from the cervical cord, causing instant death.
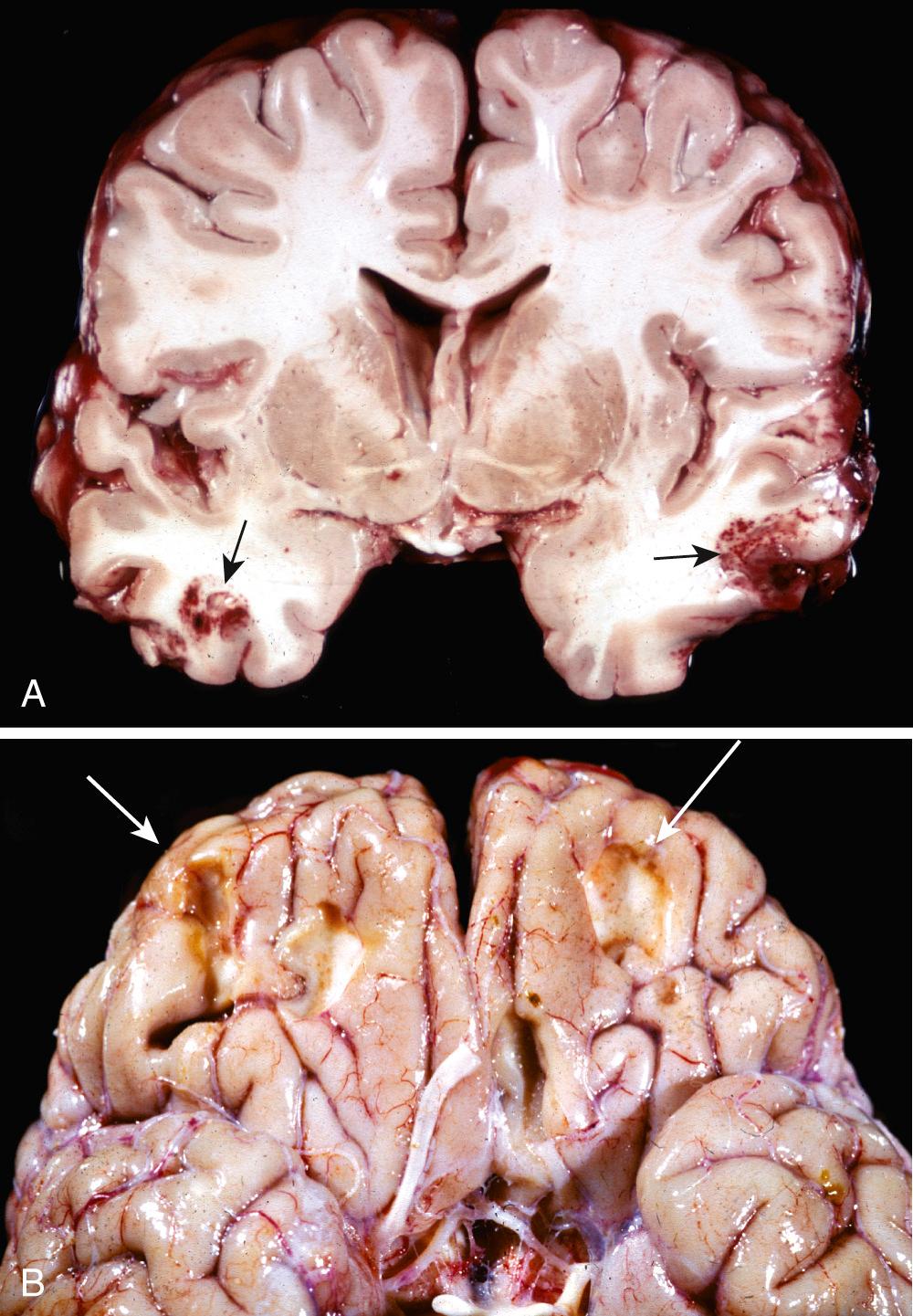
When seen on cross-section, contusions are wedge shaped, with the broad base lying along the surface at the point of impact ( Fig. 28.9A ). The appearance of contusions is similar regardless of the source of the trauma. In the earliest stages, there is edema and hemorrhage, which is often pericapillary. During the next few hours, the extravasation of blood extends throughout the involved tissue, across the width of the cerebral cortex, and into the white matter and subarachnoid space. Morphologic evidence of neuronal injury (pyknosis of the nucleus, eosinophilia of the cytoplasm, and disintegration of the cell) takes 12 to 24 hours to appear, although functional deficits generally occur earlier. Axonal swellings develop along the full length of the damaged neurons. The inflammatory response to the injured tissue follows its usual course, with the appearance of sparse neutrophils followed by abundant macrophages. Old traumatic lesions on the surface of the brain have a characteristic gross appearance. They are depressed, retracted, yellowish brown patches involving the crests of gyri, most commonly those that are located at the sites of contrecoup injuries (inferior frontal cortex, temporal and occipital poles); these lesions, called plaque jaune ( Fig. 28.9B ), can become epileptic foci. More extensive hemorrhagic regions of brain trauma give rise to larger cavitated lesions, which resemble remote infarcts. In old contusions, gliosis and residual hemosiderin-laden macrophages predominate.
Traumatic brain injuries may also damage the deep white matter regions, cerebral peduncles, superior colliculi, and deep reticular formation in the brainstem. The microscopic findings include axonal swelling, indicative of diffuse axonal injury, and focal hemorrhagic lesions. As many as 50% of individuals who develop coma shortly after trauma, even without cerebral contusions, are believed to have diffuse axonal injury. Axons are injured directly by mechanical forces, with subsequent alterations in axoplasmic flow. Such injuries may occur from marked changes in angular acceleration (e.g., from blast injuries), even in the absence of physical impacts involving the skull.
Diffuse axonal injury is characterized by widespread, often asymmetric axonal swellings that appear within hours of the injury and may persist for much longer. The swelling is best demonstrated with silver impregnation techniques or with immunoperoxidase stains for axonally transported proteins, such as amyloid precursor protein and α-synuclein. Later, increased numbers of microglia are seen in damaged areas of the cerebral cortex, and subsequently there is degeneration of the involved fiber tracts.
Vascular injury is a frequent component of CNS trauma. It results from disruption of the vessel wall and leads to hemorrhage in different anatomic sites ( Table 28.1 ). Depending on the position of the ruptured vessel, hemorrhage may occur in the epidural, subdural, subarachnoid, and intraparenchymal compartments, sometimes in combination ( Fig. 28.10 ). Epidural and subdural hemorrhages rarely occur outside of the setting of trauma; in some settings, such as coagulopathy or significant cerebral atrophy, subdural hemorrhages can follow even minor trauma. Subarachnoid hemorrhage almost always accompanies parenchymal trauma, but can also develop spontaneously secondary to vascular anomalies (discussed later).
| Location | Etiology | Additional Features |
|---|---|---|
| Epidural space | Trauma | Usually associated with skull fracture (in adults); rapidly evolving neurologic symptoms (often after a short lucid period) that require intervention |
| Subdural space | Trauma | May follow minor trauma; slowly evolving neurologic symptoms, often with a delay from the time of injury |
| Subarachnoid space | Trauma | Typically associated with underlying parenchymal injury |
| Vascular abnormality (arteriovenous malformation or aneurysm) | Sudden onset of severe headache, often with rapid neurologic deterioration; secondary injury may emerge and is associated with vasospasm | |
| Intraparenchymal space | Trauma (contusions) | Selective involvement of the crests of gyri, where the brain is in contact with the inner surface of the skull (frontal and temporal tips, orbitofrontal surface) |
| Ischemia (hemorrhagic conversion of an ischemic infarct) | Petechial hemorrhages in an area of previously ischemic brain, usually following the cortical ribbon | |
| Cerebral amyloid angiopathy | “Lobar” hemorrhage, involving subcortical white matter and often with extension into the subarachnoid space | |
| Hypertension | Centered in the deep white matter, thalamus, basal ganglia, or brainstem; may extend into the ventricular system | |
| Tumors (primary or metastatic) | Associated with high-grade gliomas or certain metastases (melanoma, choriocarcinoma, renal cell carcinoma) |
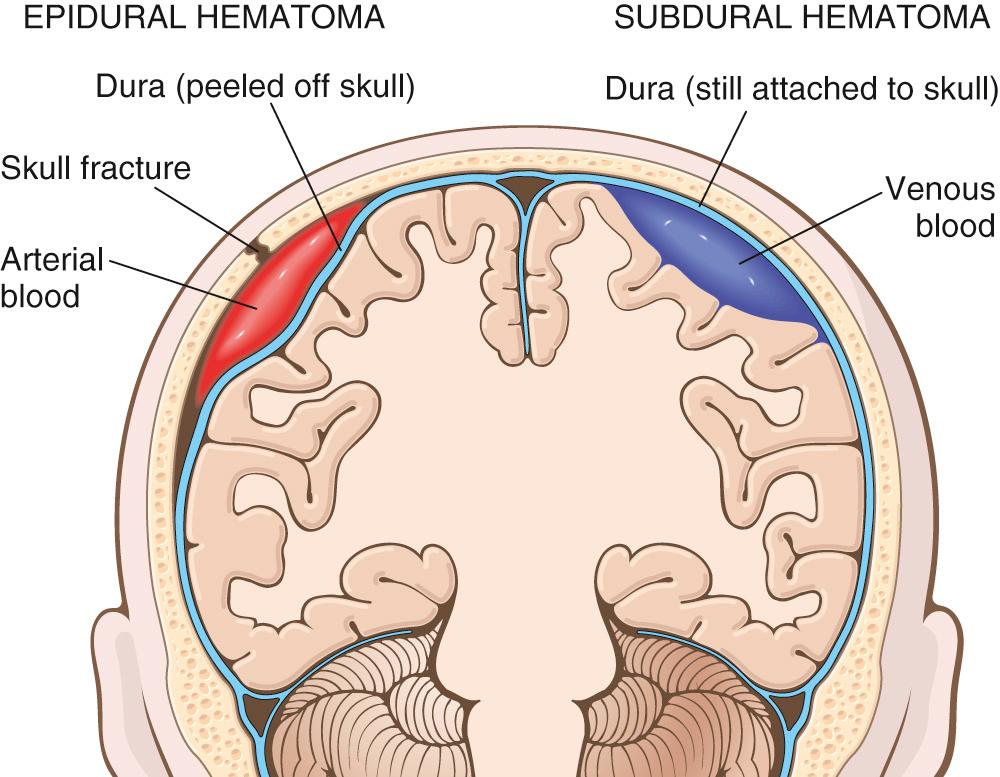
The dura is fused with the periosteum and is supplied by a number of dural arteries. These arteries, most notably the middle meningeal artery, are vulnerable to traumatic injury. In adults, this most often occurs with temporal skull fractures in which the fracture crosses the course of the vessel. In children, in whom the skull is deformable, a temporary displacement of the skull bones leading to laceration of a vessel can occur in the absence of a skull fracture.
Once a vessel has been torn, the extravasation of blood under arterial pressure can cause the dura to separate from the periosteum, creating a space ( Fig. 28.11 ). The expanding hematoma compresses the underlying brain. When blood accumulates slowly, patients may experience a lucid period before the onset of neurologic signs. A symptomatic epidural hematoma is a neurosurgical emergency; without prompt diagnosis and drainage, fatal brain herniation may occur within a few hours.
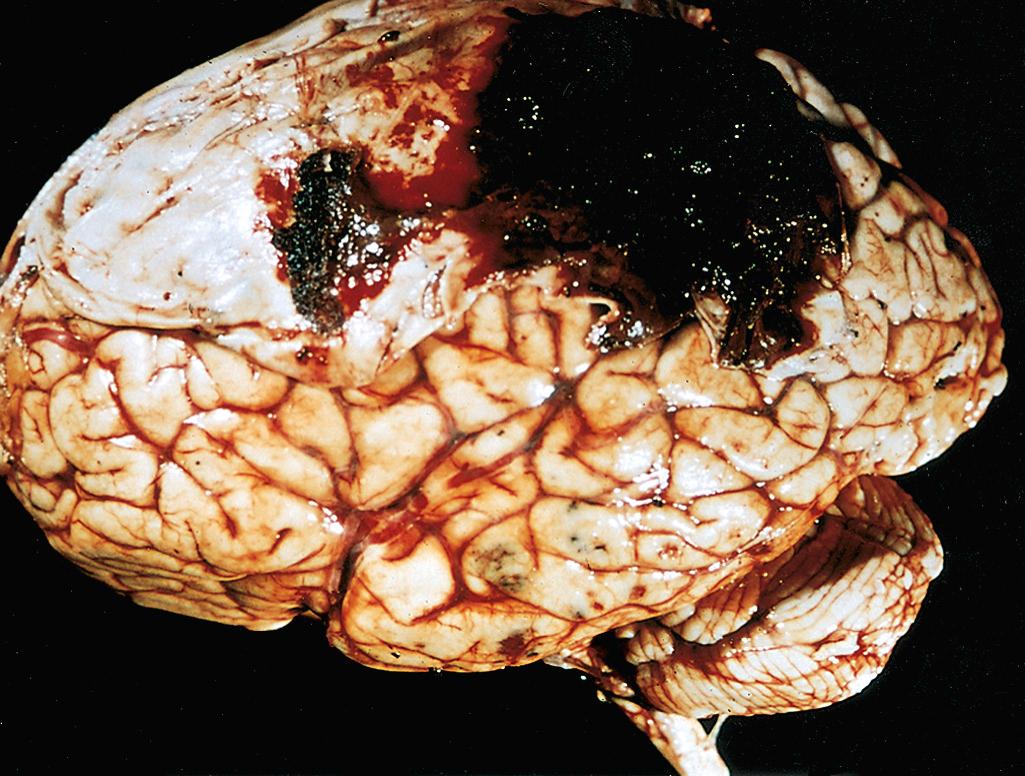
The dura is composed of two layers—an external collagenous layer and an inner more cellular layer containing fibroblasts. Bridging veins travel from the convexities of the cerebral hemispheres through the subarachnoid space and dura to empty into the dural sinuses. The brain is suspended in CSF, but the venous sinuses are fixed relative to the dura; as a result, traumatic displacement of the brain can tear the veins at the point where they penetrate the dura. The extravasated blood dissects through the two layers of the dura, producing a subdural hematoma. In older individuals with brain atrophy, the bridging veins are stretched, hence the increasing incidence of subdural hematoma with aging. Infants are also very susceptible to subdural hematomas because their bridging veins are thin-walled.
Grossly, acute subdural hematomas appear as a collection of freshly clotted blood along the brain surface, without extension into the depths of sulci ( Fig. 28.12 ). The underlying brain is flattened, and the subarachnoid space is often clear. Usually, venous bleeding is self-limited and the resulting hematoma is broken down and organized over time; this most often occurs in the following sequence:
Lysis of the clot (about 1 week)
Growth of fibroblasts from the dural surface into the hematoma (2 weeks)
Early development of hyalinized connective tissue (1 to 3 months)
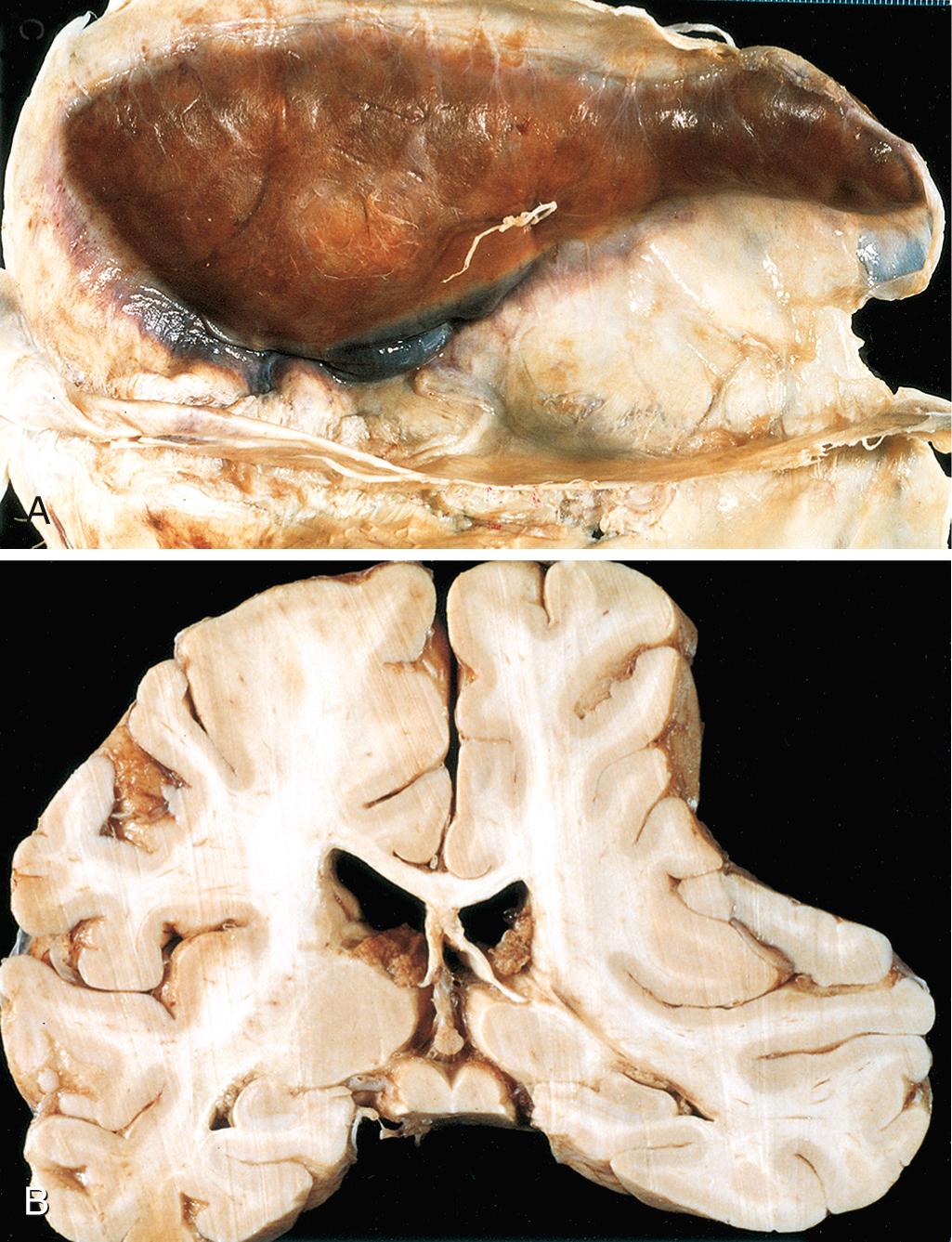
Typically, the organized hematoma is firmly attached to the inner surface of the dura by ingrowing fibrous tissue and is free of the underlying arachnoid, which does not contribute to healing. The lesion can eventually retract as the granulation tissue matures until only a thin layer of reactive connective tissue remains (“subdural membranes”). In other cases, however, multiple recurrent episodes of bleeding occur (chronic subdural hematoma), presumably from the thin-walled vessels of the granulation tissue.
Symptomatic subdural hematomas most often manifest within 48 hours of injury. They are most common over the lateral aspects of the cerebral hemispheres and are bilateral in about 10% of cases. Neurologic signs are attributable to the pressure exerted on the adjacent brain. There may be focal signs, but often the clinical manifestations are nonlocalizing and include headache and confusion. Slowly progressive neurologic deterioration is typical, but acute decompensation may also occur. The treatment of subdural hematomas is to remove the blood and associated organizing tissue. The risk of repeat bleeding is greatest in the first few months after the initial hemorrhage.
A broad range of neurologic syndromes may become manifest months or years after brain trauma of any cause. These have gained increasing notice in the context of litigation involving issues of compensation for those in the civilian work force, professional athletes, and the military services.
Posttraumatic hydrocephalus is largely due to obstruction of CSF resorption from hemorrhage into the subarachnoid space.
Chronic traumatic encephalopathy (CTE, previously referred to as “dementia pugilistica”) is a dementing illness that develops after repeated head trauma. Affected brains are atrophic, with enlarged ventricles, and show accumulation of tau-containing neurofibrillary tangles in a characteristic pattern involving gyral depths and perivascular regions in the frontal and temporal lobe cortices. Although concussion is thought of as having no structural consequences, it is clear that repeated events are antecedents to CTE; however, it remains uncertain which factors determine whether encephalopathy will ultimately develop (the number, frequency, and/or severity of individual traumatic events, or some combination of these).
Other important sequelae of brain trauma include posttraumatic epilepsy, risk of infection, and psychiatric disorders.
The spinal cord is vulnerable to trauma from its skeletal encasement. Most injuries that damage the cord are associated with the transient or permanent displacement of the vertebral column. The level of cord injury determines the extent of the neurologic manifestations: lesions involving the thoracic vertebrae or below can lead to paraplegia; cervical lesions result in quadriplegia; those above C4 can, in addition, lead to respiratory compromise from paralysis of the diaphragm. Damage at the region of impact to descending and ascending white matter tracts isolates the distal spinal cord from the rest of the brain. This interruption, paired with the localized gray matter damage at the level of the impact, is the principal cause of neurologic deficits.
The histologic features of traumatic injury of the spinal cord are similar to those found at other sites in the CNS. At the level of injury the acute phase consists of hemorrhage, necrosis, and axonal swelling in the surrounding white matter. The lesion tapers above and below the level of injury. In time, central areas of neuronal destruction become cystic and gliotic; cord sections above and below the lesion show secondary ascending and descending Wallerian degeneration, respectively, involving the long white-matter tracts at the site of trauma.
Timing of injury is critical, with earlier events resulting in greater damage and deficits.
Cerebral palsy is the term used for nonprogressive deficits associated with injury during the prenatal and perinatal periods.
Physical injury to the brain can occur when the inside of the skull comes into forceful contact with the brain.
In blunt trauma, if the head is mobile there may be brain injury both at the original point of contact (coup injury) and on the opposite side of the brain (contrecoup injury).
Parenchymal injuries take the form of contusions, with hemorrhage extending into the subarachnoid space.
Rapid displacement of the head and brain can tear axons (diffuse axonal injury), often immediately causing severe, irreversible neurologic deficits.
Traumatic tearing of blood vessels leads to epidural or subdural hematoma.
Cerebrovascular disease—injury to the brain as a consequence of altered blood flow—can be grouped into ischemic and hemorrhagic etiologies, with tissue infarction the ultimate consequence of both. “Stroke” is the clinical designation applied to these conditions and is defined as neurologic signs and symptoms that can be explained by a vascular mechanism, have an acute onset, and persist beyond 24 hours. (If symptoms disappear within 24 hours, the event is termed “transient ischemic event.”) Cerebrovascular disease is the third leading cause of death (after heart disease and cancer) in the United States, and the most prevalent cause of morbidity and mortality from neurologic disease. Stroke stems from two major mechanisms:
Ischemia and/or hypoxia resulting from impairment of blood supply and oxygenation of CNS tissue. This can be either a global or focal process, with the clinical manifestations determined by the region of brain affected. In the brain, embolism is a more common cause of vascular occlusion than thrombosis.
Hemorrhage resulting from rupture of CNS vessels. Common etiologies include hypertension and vascular anomalies (aneurysms and malformations; see Table 28.1 ).
Although the brain accounts for only 1% to 2% of body weight, it receives approximately 15% of the resting cardiac output and accounts for 20% of the body's oxygen consumption. Cerebral blood flow remains relatively constant over a wide range of blood pressure and intracranial pressure because of autoregulation of vascular resistance. The brain is strictly dependent on aerobic metabolism to meet its constant energy demands, and it may be deprived of oxygen by either hypoxemia (low blood oxygen content) or ischemia (inadequate blood flow). Inadequate blood flow may result from a reduction in perfusion pressure (as in hypotension), small- or large-vessel obstruction, or both.
When blood flow to a portion of the brain is reduced, the survival of the tissue at risk depends on the presence of collateral circulation, the duration of ischemia, and the magnitude and rapidity of the reduction of flow. These factors determine, in turn, the precise anatomic site and size of the lesion and, consequently, the clinical deficit.
The general biochemical changes in cells resulting from ischemia are discussed in Chapter 2 . In addition to processes shared with ischemia in other parts of the body, ischemia in the CNS can result in inappropriate release of excitatory amino acid neurotransmitters such as glutamate, which can damage neurons by allowing excessive influx of calcium ions through N-methyl-D-aspartate (NMDA)-type glutamate receptors; this phenomenon is termed excitotoxicity . In the region of transition between necrotic tissue and the normal brain, there is an area of “at-risk” brain, referred to as the penumbra; this region can be rescued from cell death in many animal models with a variety of anti-apoptotic interventions, implying that ischemic neurons may die by apoptosis as well as necrosis.
Focal cerebral ischemia follows reduction or cessation of blood flow to a localized area of the brain due to partial or complete arterial obstruction. When the ischemia is sustained, infarction follows in the territory of the compromised vessel. The size, location, and shape of the infarct and the extent of tissue damage that results are influenced by the duration of the ischemia and the adequacy of collateral flow. The major source of collateral flow is the circle of Willis (supplemented by external carotid-ophthalmic artery collaterals). Inconstant collateral leptomeningeal vessels from the surface of the brain may also supply the distal branches of the anterior, middle, and posterior cerebral arteries through cortical-leptomeningeal anastomoses; in contrast, there is little if any collateral flow for the deep penetrating vessels of the thalamus, basal ganglia, and deep white matter.
Occlusive vascular disease of severity sufficient to lead to cerebral infarction may be due to embolization from a distant source, in situ thrombosis, or various vasculitides; the pathology of these conditions is also discussed in Chapters 4 and 11 .
Embolism to the brain occurs from a variety of sources. Cardiac mural thrombi are among the most common culprits; myocardial infarct, valvular disease, and atrial fibrillation are important predisposing factors. Next in frequency are thromboemboli originating from arteries, most often atheromatous plaques within the carotids. Other sources of emboli include paradoxical thromboemboli, particularly in children with cardiac anomalies; thromboemboli associated with cardiac surgery; and emboli of other types (tumor, fat, or air). The territory supplied by the middle cerebral artery—the direct extension of the internal carotid artery—is most frequently affected by embolic infarction; the incidence is about equal in the two hemispheres. Emboli tend to lodge where blood vessels branch or in areas of preexisting luminal stenosis. “Shower embolization,” as in fat embolism, may occur after fractures; affected individuals manifest generalized cerebral dysfunction with disturbances of higher cortical function and consciousness, often without localizing signs. Widespread hemorrhagic lesions involving the white matter are characteristic of embolization of bone marrow after trauma.
Thrombotic occlusion of the cerebral arteries is most commonly caused by acute change of vulnerable atherosclerotic plaques, as in coronary artery disease ( Chapter 12 ). The most common sites are the carotid bifurcation, the origin of the middle cerebral artery, and either end of the basilar artery. Thrombi cause progressive narrowing of the lumen, may be accompanied by anterograde extension, and may progress to fragmentation and distal embolization. Atherosclerotic cerebrovascular disease is frequently associated with systemic diseases such as hypertension and diabetes.
Inflammatory processes that involve blood vessels may also lead to luminal narrowing, occlusion, and, hence, cerebral infarcts. Although infectious vasculitis of small and large vessels occurs with syphilis and tuberculosis, it is now more common in the setting of immunosuppression and opportunistic infection (e.g., aspergillosis). Polyarteritis nodosa and other noninfectious vasculitides may involve cerebral vessels and cause single or multiple infarcts throughout the brain. Primary angiitis of the CNS can also develop in the absence of systemic vasculitis.
Other conditions that may cause thrombosis (and intracranial hemorrhage) include hypercoagulable states, dissecting aneurysm of extracranial arteries in the neck that supply the brain, and drug abuse (amphetamines, heroin, cocaine).
Brain infarcts are subdivided into two broad groups based on the presence of secondary hemorrhage. Because the brain has end-organ circulation with limited collateral supply, occlusive brain infarcts generally start as nonhemorrhagic (pale/anemic; Fig. 28.13A ); clinically, these nonhemorrhagic infarcts are called ischemic, a confusing term as every infarct, not just this type, is caused by tissue ischemia. Secondary hemorrhage can occur from ischemia-reperfusion injury following spontaneous or therapeutic dissolution or fragmentation of the intravascular occlusive material. This process (termed secondary hemorrhagic transformation and leading to a hemorrhagic infarct) develops if the causative ischemic event lasts long enough to damage small blood vessels in the affected area; the resulting reperfusion hemorrhages are largely petechial in nature, but may be multiple or even confluent (see Fig. 28.13B ). The clinical management of patients with nonhemorrhagic and hemorrhagic infarcts differs greatly, although the underlying causes are the same (for instance, thrombolytic therapy is contraindicated in a patient with brain hemorrhage of any etiology).
Both the gross and microscopic appearance of a nonhemorrhagic infarct changes over time. Grossly, there is little change in appearance during the first 6 hours of irreversible injury. By 48 hours, however, the tissue becomes pale, soft, and swollen, and the gray-white matter junction becomes indistinct. From 2 to 10 days, the brain becomes gelatinous and friable, and the previously ill-defined boundary between normal and infarcted tissue becomes more distinct as edema resolves in the viable adjacent tissue. From 10 days to 3 weeks, the tissue liquefies, eventually leaving a fluid-filled cavity that continues to expand until all of the dead tissue has been removed ( Fig. 28.14 ).
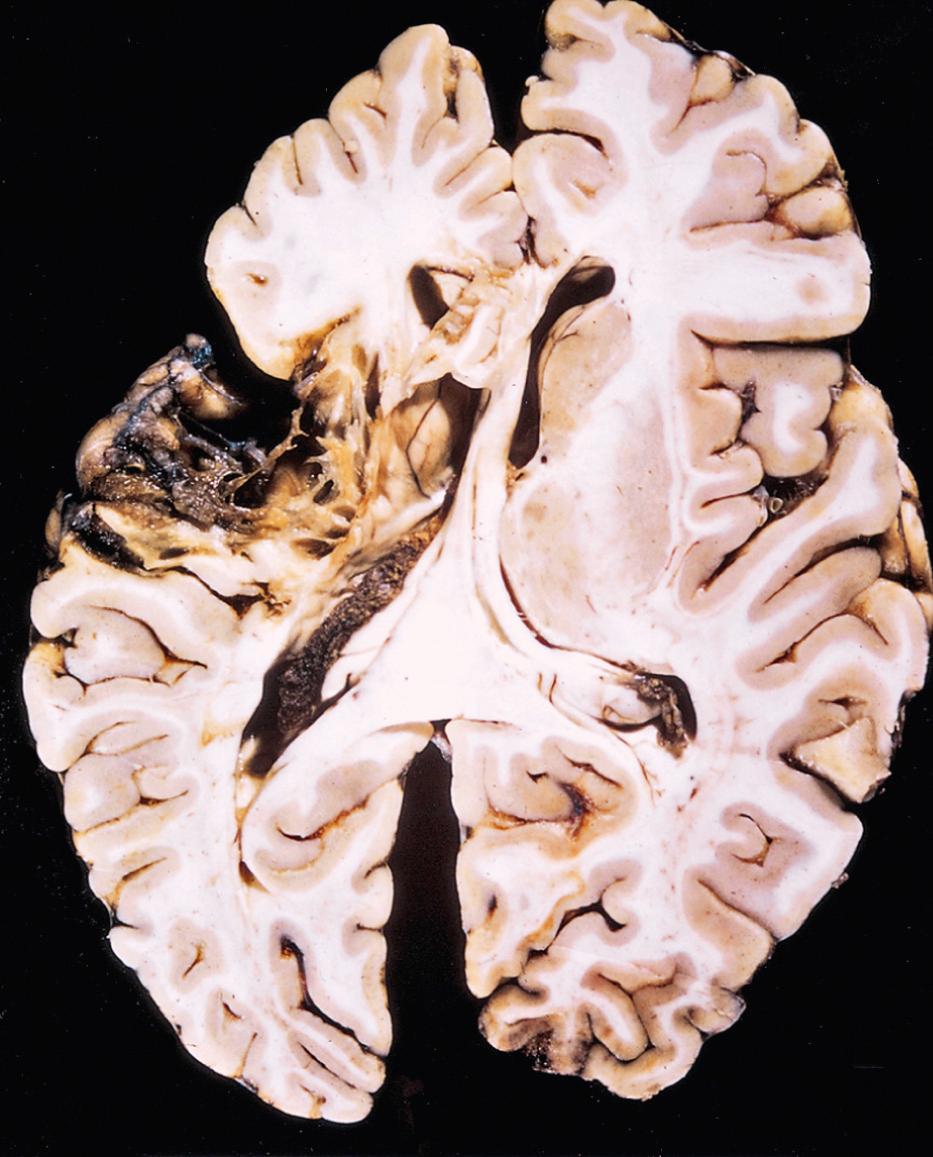
Microscopically, the tissue reaction evolves along the following sequence:
Acute infarct ( Fig. 28.15A ). After the first 6 to 12 hours , neurons in the affected area show eosinophilic neuronal necrosis (increased eosinophilia of the cytoplasm followed by nuclear pyknosis and karyorrhexis; “dead red neurons”); both cytotoxic and vasogenic edema are present. There is loss of the usual tinctorial characteristics of white- and gray-matter structures. Endothelial and glial cells, mainly astrocytes, swell, and myelinated fibers begin to disintegrate. Up to 48 hours , neutrophilic emigration progressively increases and then falls off (but is never as prominent as in myocardial infarction).
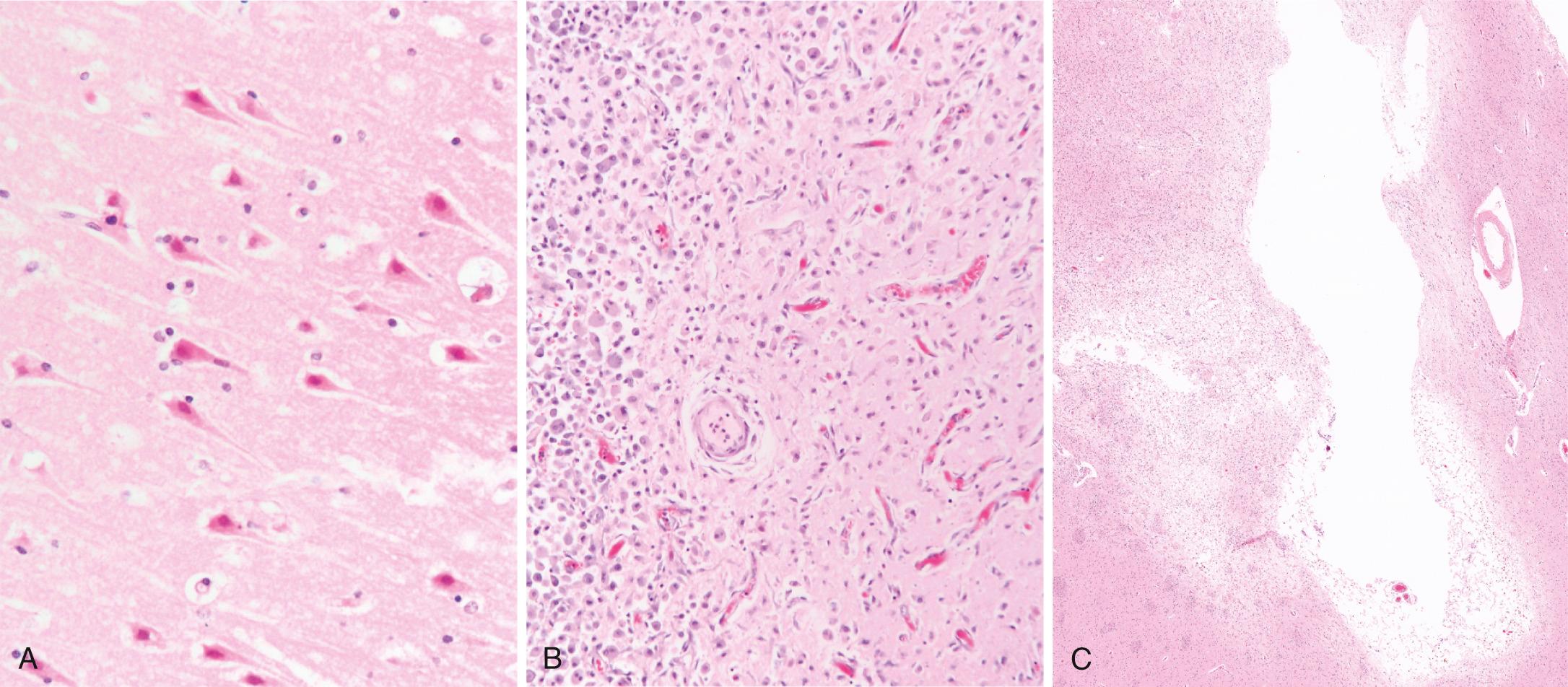
Subacute (evolving) infarct ( Fig. 28.15B ). Phagocytic cells, derived from circulating monocytes and activated microglia, are evident at 48 to 72 hours and become the predominant cell type in the ensuing 2 to 3 weeks. The macrophages become stuffed with the products of myelin breakdown or blood and may persist in the lesion for months to years. Reactive astrocytes and newly formed vessels can be seen at the periphery of the lesion as early as 1 week after the insult. As the process of liquefaction and phagocytosis proceeds, astrocytes at the edges of the lesion progressively enlarge, divide, and develop a prominent network of cytoplasmic extensions.
Healed infarct ( Fig. 28.15C ). After several months , the astrocytic response recedes, leaving behind a dense meshwork of glial fibers admixed with new capillaries and some perivascular connective tissue. In the cerebral cortex, the cavity is separated from the meninges and subarachnoid space by a gliotic layer of tissue, which is derived from the molecular layer of the cortex. The pia and arachnoid are not affected. Infarcts undergo these reactive and reparative stages from the edges inward; thus, different areas of a lesion may appear chronologically divergent, revealing the natural progression of the response.
The features and temporal evolution of hemorrhagic infarctions parallel ischemic infarctions, with the addition of blood extravasation and resorption. In individuals receiving anticoagulant treatment, hemorrhagic infarcts may be associated with extensive intracerebral hematomas. Venous infarcts are often hemorrhagic and may occur after thrombotic occlusion of the superior sagittal sinus or other sinuses, or after occlusion of the deep cerebral veins. Neoplasms, localized infections, and other conditions leading to a hypercoagulable state increase the risk for venous thrombosis.
Hypertension affects the deep penetrating arteries and arterioles that supply the basal ganglia and hemispheric white matter as well as the brainstem; these cerebral vessels develop arteriolosclerosis, also known as small vessel disease because it affects arteries 40 to 900 µm in diameter, described in more detail later. If this disease process progresses to thrombosis and complete vessel occlusion, the end-result is development of small cavitary infarcts known as lacunes or lacunar infarcts ( Fig. 28.16 ). These lakelike spaces, arbitrarily defined as less than 15 mm wide, can be single or multiple and involve the putamen, globus pallidus, thalamus, internal capsule, deep white matter, caudate nucleus, and pons, in descending order of frequency. On microscopic examination, there is tissue loss surrounded by gliosis. Depending on their location in the CNS, lacunar infarcts can be clinically silent or cause severe neurologic impairment. Affected vessels may also be associated with widening of the perivascular spaces without tissue infarction ( état criblé ).

Deficits produced by infarction are determined by the anatomic distribution of the damage, rather than the underlying cause. Neurologic symptoms referable to the area of injury often develop rapidly, over minutes, and may continue to evolve over hours. There can be improvement in severity of symptoms that is associated with reversal of injury in the ischemic penumbra and resolution of associated local edema. In general, there is often slow improvement during a period of months. Because strokes are frequently associated with cardiovascular disease, many of the genetic and lifestyle risk factors are shared. Early diagnosis is of critical importance, as rapid treatment of nonhemorrhagic strokes with thrombolytic agents can often limit or entirely prevent the development of permanent neurologic deficits.
Global cerebral hypoxia or ischemia occurs when there is a generalized reduction of cerebral perfusion (as in cardiac arrest, shock, and severe hypotension) or decreased oxygen carrying capacity of the blood (e.g., in carbon monoxide poisoning). The clinical outcome varies with the severity and length of the insult. In mild cases, there may only be a transient postischemic confusional state, followed by complete recovery. Nevertheless, irreversible CNS damage may occur in individuals who experience global hypoxic and/or ischemic insults (diffuse hypoxic/ischemic encephalopathy) . Among CNS cells, there is a hierarchy of sensitivity to hypoxia/ischemia: neurons are the most sensitive, although glial cells (oligodendrocytes and astrocytes) are also vulnerable. The most sensitive neurons in the brain are pyramidal neurons in the hippocampus (especially area CA1, also referred to as Sommer sector), cerebellar Purkinje cells, and pyramidal neurons in the cerebral cortex (especially layers III and V); the molecular mechanisms underlying this selective vulnerability are not understood. With severe global cerebral hypoxia/ischemia, widespread neuronal death occurs, irrespective of regional vulnerability; patients who survive this injury often remain in a persistent vegetative state. Other patients meet the current clinical criteria for “brain death,” including evidence of irreversible diffuse cortical injury (isoelectric, or “flat,” electroencephalogram), brainstem damage (such as absent reflexes and respiratory drive), and absent cerebral perfusion. When individuals with this pervasive form of injury are maintained on mechanical ventilation, the brain gradually undergoes widespread liquefaction, producing so-called “respirator brain.”
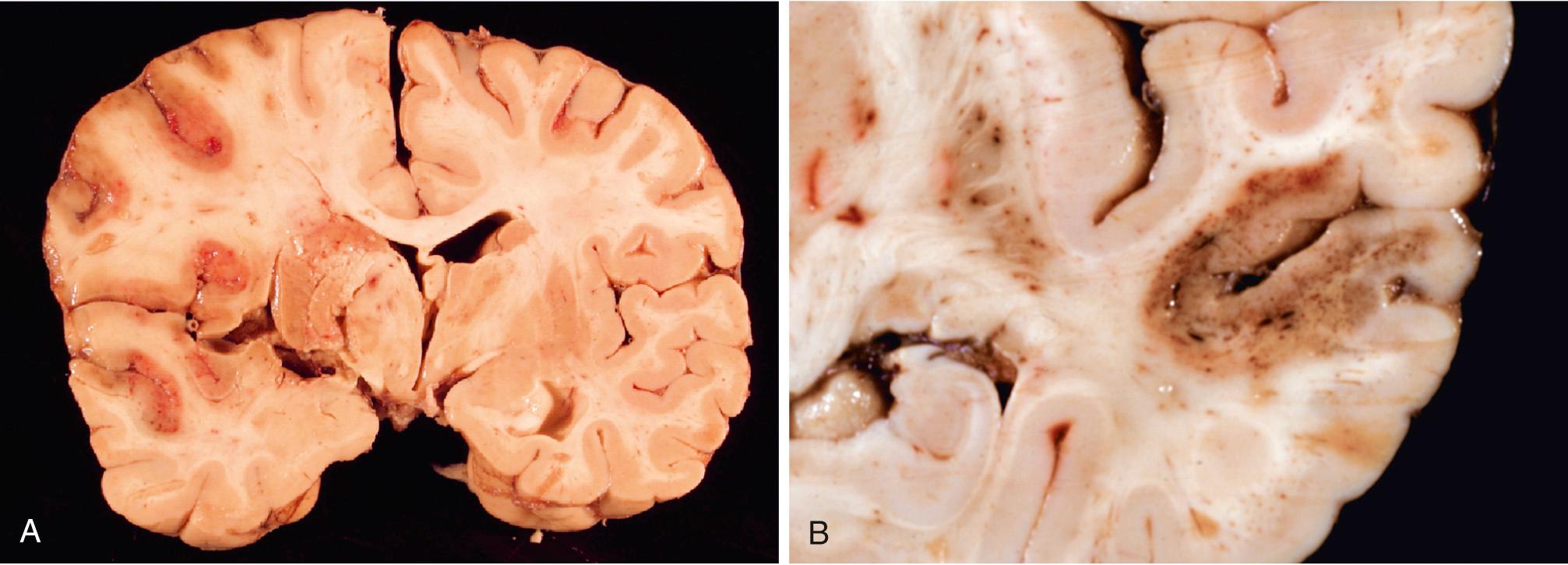
Border zone (“watershed”) infarcts occur in the regions of the brain or spinal cord that lie at the most distal reaches of the arterial blood supply (i.e., the border zones between arterial territories). In the cerebral hemispheres, the border zone between the anterior and the middle cerebral artery distributions is at greatest risk. Damage to this region, located a few centimeters lateral to the interhemispheric fissure, results in a cortical wedge-shaped infarct that usually shows secondary hemorrhagic transformation ( Fig. 28.17 ) and is often bilateral. Border zone infarcts usually develop after severe hypotensive episodes and are most commonly seen in patients resuscitated after cardiac arrest.
In the setting of global ischemia, the brain becomes edematous and swollen, producing widening of the gyri and narrowing of the sulci. The cut surface shows poor demarcation between gray and white matter. The microscopic features of irreversible ischemic injury evolve over time and mimic the changes seen in infarcts; the distinction between global and focal ischemic injury is based not on the nature of cellular pathology but on the overall pattern of brain involvement. Early changes, occurring 6 to 12 hours after the insult, are seen in neurons (dead red neurons described earlier); similar acute changes occur somewhat later in astrocytes and oligodendroglia. Subacute changes, occurring at 24 hours to 2 weeks, include tissue necrosis, influx of macrophages, vascular proliferation, and reactive gliosis. Repair, robust after approximately 2 weeks, is characterized by removal of necrotic tissue, loss of normal CNS architecture, and gliosis. In the cerebral neocortex, the neuronal loss and gliosis are uneven, with preservation of some layers and destruction of others, producing a pattern of injury termed laminar necrosis.
Hemorrhages may occur at any site within the cranium—outside the brain or within it (intraparenchymal). Hemorrhages in the epidural or subdural space are typically associated with trauma and were discussed earlier; hemorrhages within the brain parenchyma and in the subarachnoid space, in contrast, are more often a manifestation of underlying cerebrovascular disease and are discussed in the following sections (see Table 28.1 ).
Rupture of a small intraparenchymal vessel can result in a primary hemorrhage within the brain, often associated with sudden onset of neurologic symptoms (stroke); this should not be confused with the secondary hemorrhagic transformation of an occlusive infarct (described earlier). Spontaneous (nontraumatic) intraparenchymal hemorrhages occur most commonly in middle to late adult life, with a peak incidence at about 60 years of age. Hemorrhages in the basal ganglia and thalamus are commonly designated “ganglionic hemorrhages,” whereas those that occur in the lobes of the cerebral hemispheres are called “lobar hemorrhages”; the two major causes of these patterns of hemorrhage are hypertension and cerebral amyloid angiopathy, respectively. In addition, other local and systemic factors may cause or contribute to nontraumatic hemorrhage, including systemic coagulation disorders, neoplasms, vasculitis, aneurysms, and vascular malformations.
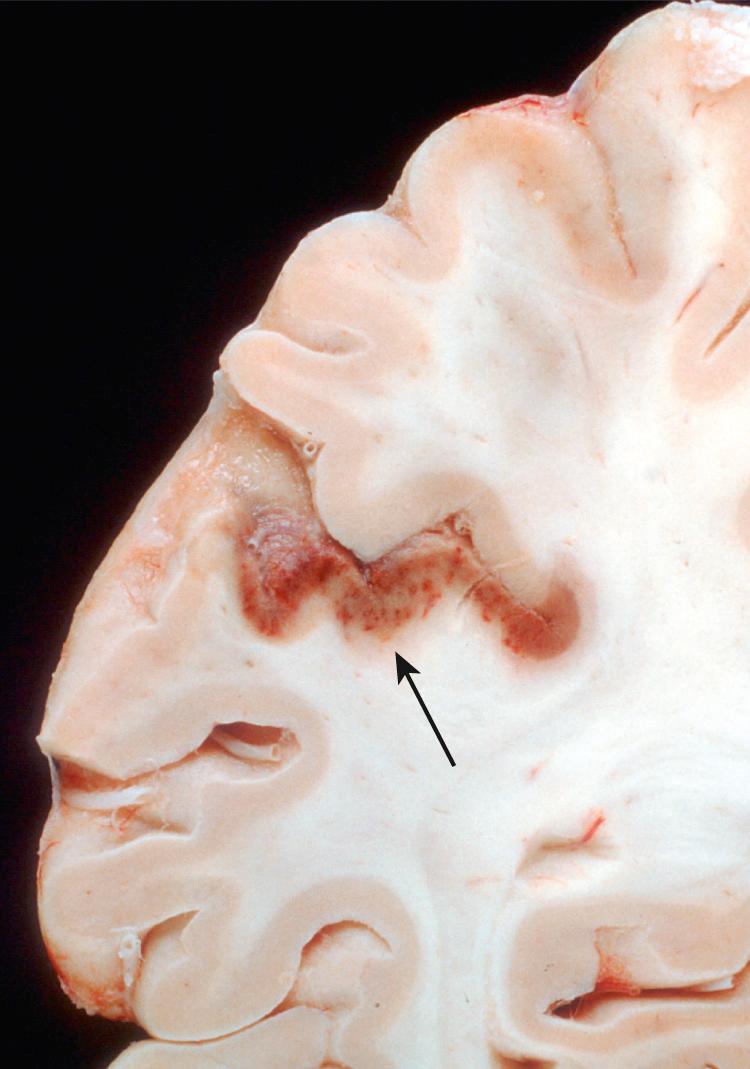
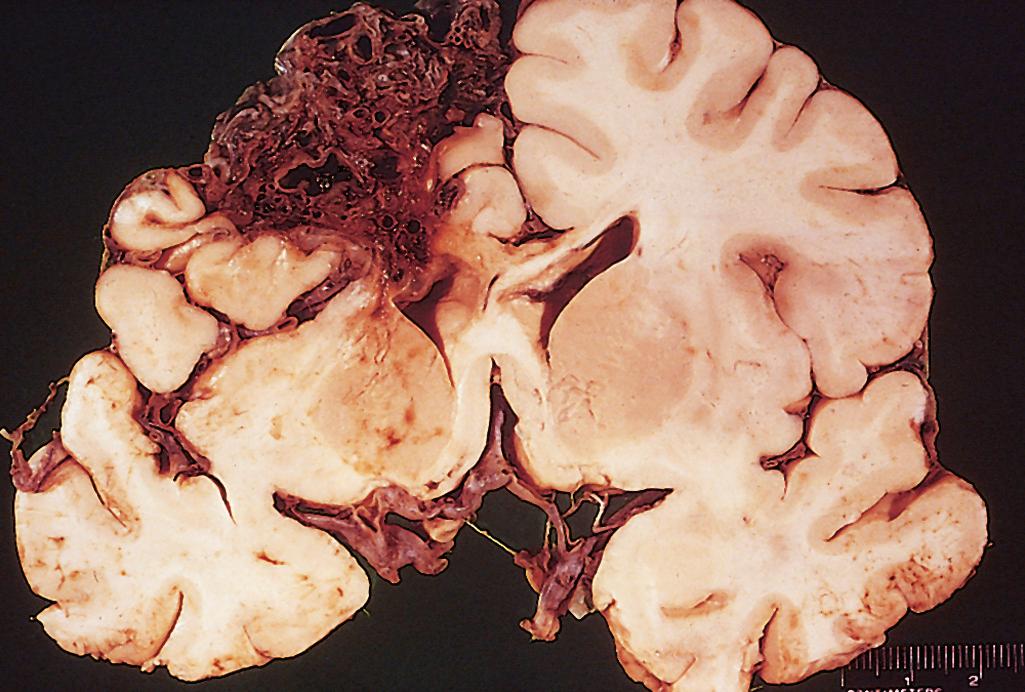
Hypertension is the risk factor most commonly associated with deep brain parenchymal hemorrhages, accounting for more than 50% of clinically significant hemorrhages and for roughly 15% of deaths among individuals with chronic hypertension. Hypertensive intraparenchymal hemorrhage may originate in the putamen (50% to 60% of cases), thalamus, pons, cerebellar hemispheres (rarely), and other regions of the brain ( Fig. 28.18A ). Hypertension leads to a number of vessel wall abnormalities, including accelerated atherosclerosis in larger arteries, hyaline arteriolosclerosis in smaller arteries, and (in severe cases) proliferative changes and frank necrosis of arterioles. Arteriolar walls affected by hyaline change ( Fig. 28.18B ) are thickened but more vulnerable to rupture than normal vessels; these changes are most prominent in the basal ganglia and the subcortical white matter. As described earlier, if small arteries affected by hyaline arteriolosclerosis do not rupture but are occluded, the result is lacunar infarction.
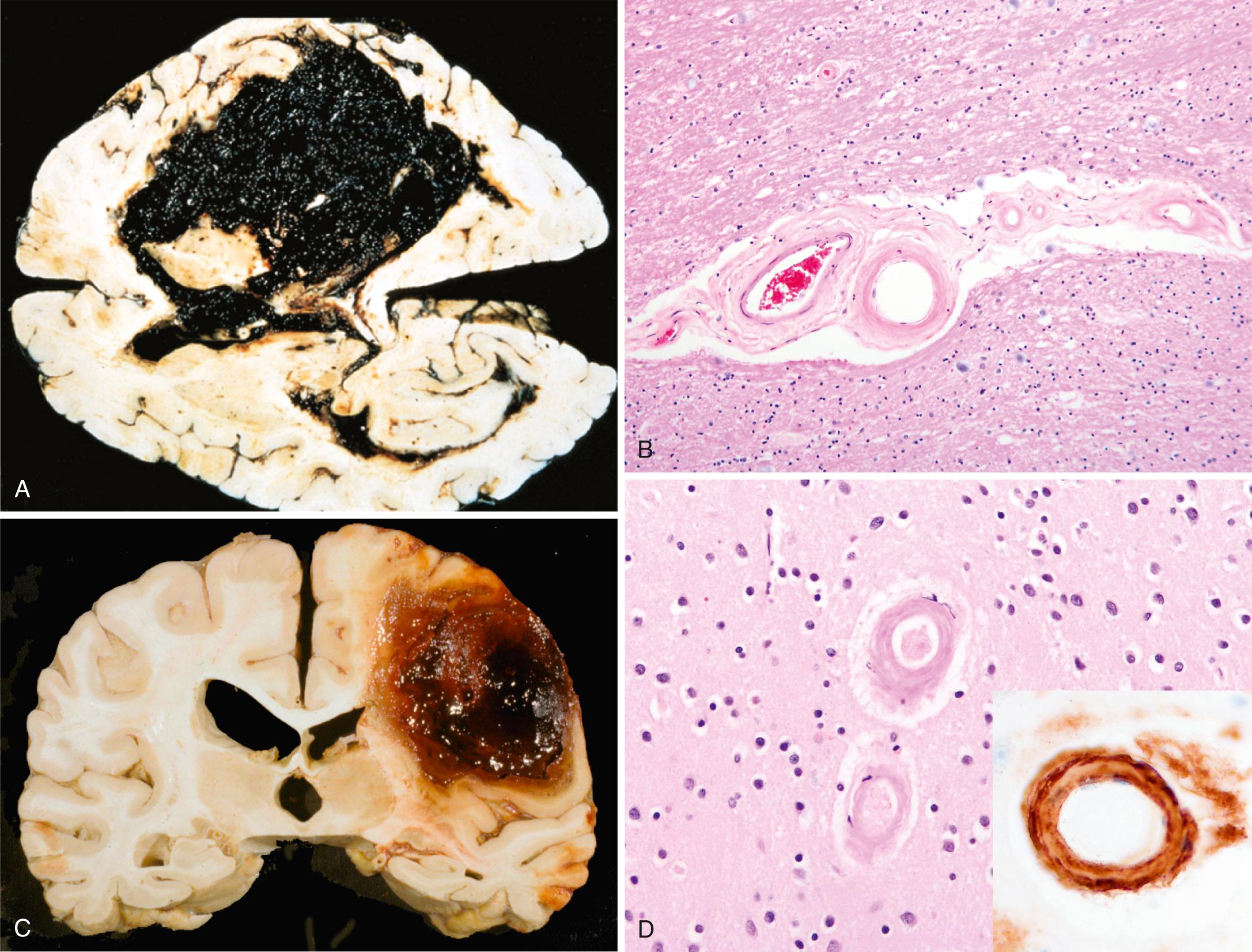
Cerebral amyloid angiopathy (CAA) is the risk factor most commonly associated with lobar hemorrhages ( Fig. 28.18C ). In CAA, amyloidogenic peptides, usually the same ones found in Alzheimer disease (Aβ; see later), are deposited in the walls of medium- and small-caliber meningeal, cortical, and cerebellar vessels; involved vessels are rigid, and as a result fail to collapse during tissue processing and sectioning. Although similar to hyaline arteriolosclerosis on routine hematoxylin and eosin (H&E) stain, the hyaline material in CAA consists of β amyloid rather than collagen ( Fig. 28.18D ) and is primarily seen in the leptomeningeal and cortical (rather than basal ganglia and white matter) vessels. Amyloid deposition can weaken the vessel wall and lead to hemorrhage; as a result, many individuals with CAA have evidence of numerous small hemorrhages within the brain (“microbleeds”) that can be visualized by various imaging methods. As with Alzheimer disease (discussed later), there is a relationship between polymorphisms in the gene that encodes apolipoprotein E (ApoE) and risk of disease; specifically, the presence of either an ε2 or ε4 allele increases the risk of bleeding. Autosomal dominant forms of CAA are associated with certain mutations in the APP gene, which encodes the precursor for the Aβ peptides that are prone to deposit as amyloid.
Other forms of hereditary small-vessel diseases of the CNS have been identified. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an autosomal dominant disorder caused by mutations in the NOTCH3 gene that lead to misfolding of the extracellular domain of the NOTCH3 receptor. The disease is characterized clinically by recurrent small vessel strokes (usually infarcts, less often hemorrhages) and dementia. Imaging studies show that the first detectable changes are in white matter, usually presenting around 35 years of age and then progressing further over time. Other forms of the heritable small vessel disease include a disorder associated with mutations in the gene for COL4A1, a component of the vascular basement membrane.
Acute primary intraparenchymal hemorrhages are characterized by a central core of clotted blood that compresses the adjacent parenchyma; this compression leads to secondary infarction of the affected brain tissue, with anoxic neuronal and glial changes as well as edema. Eventually the edema resolves, hemosiderin- and lipid-laden macrophages appear, and proliferation of reactive astrocytes is seen at the periphery of the lesion; the cellular events then follow the same time course that is observed after cerebral infarction. Old hemorrhages show areas of parenchymal cavitary destruction with a rim of brownish discoloration.
Intracerebral hemorrhage can be clinically devastating if it involves a large part of the brain or extends into the ventricular system. When hemorrhage affects smaller regions, it is either clinically silent or evolves like an infarct; over weeks or months, there is a gradual removal of the hematoma, sometimes with considerable clinical improvement. Again, the location of the hemorrhage determines the clinical manifestations.
The most frequent cause of spontaneous subarachnoid hemorrhage is rupture of a saccular (“berry”) aneurysm in a cerebral artery. (As noted earlier, brain trauma is the most common cause of subarachnoid hemorrhage overall.) Nontraumatic subarachnoid hemorrhage may also result from rupture of a primary intracerebral hemorrhage into the ventricular system, vascular malformation, hematologic disturbances, and tumors.
Saccular aneurysm is the most common type of intracranial aneurysm; other aneurysm types include atherosclerotic (fusiform; mostly of the basilar artery), mycotic, traumatic, and dissecting. These latter three, like saccular aneurysms, are most often found in the anterior circulation, but more often cause cerebral infarction rather than subarachnoid hemorrhage.
Saccular aneurysms are found in about 2% of the population according to recent data from community-based radiologic studies. About 90% of saccular aneurysms are found near major arterial branch points in the anterior circulation ( Fig. 28.19 ); multiple aneurysms exist in 20% to 30% of cases based on autopsy series.
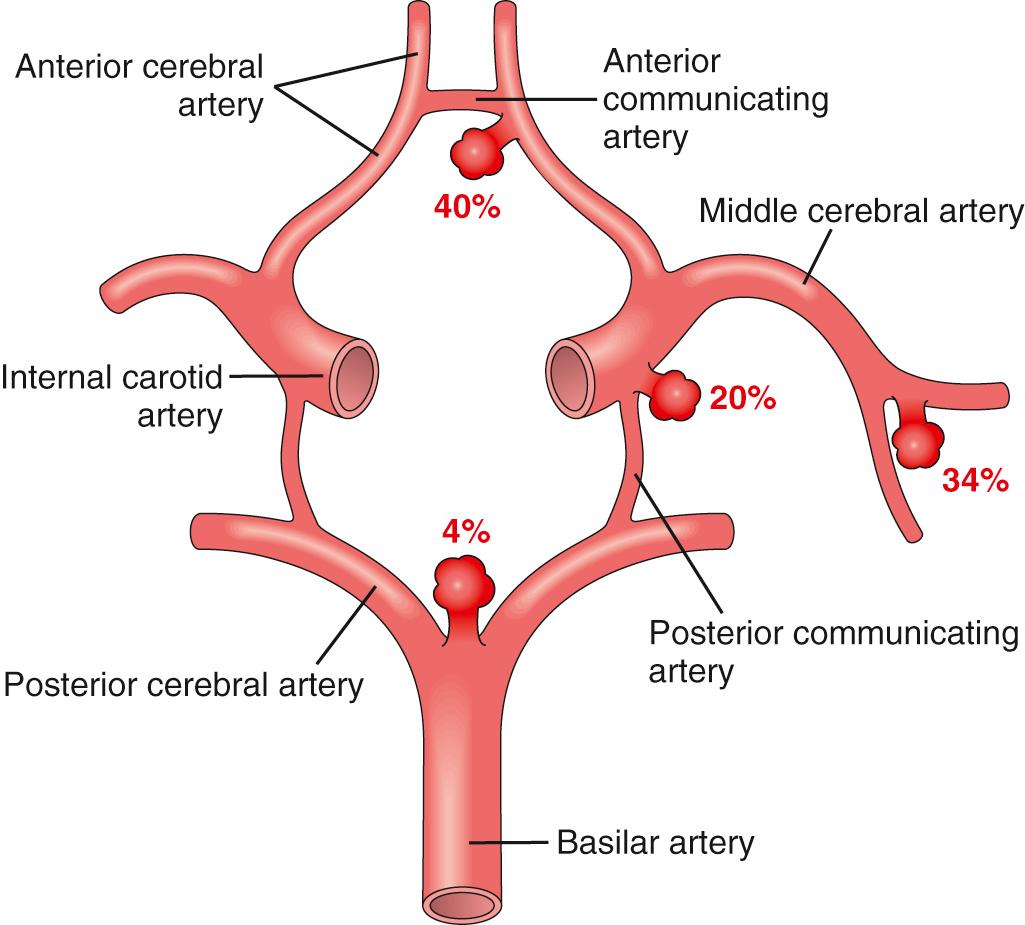
Although the etiology of saccular aneurysms remains obscure, the structural abnormality of the involved vessel (absence of smooth muscle and intimal elastic lamina) suggests that they are developmental anomalies. The majority occur sporadically, but genetic factors may be important in their pathogenesis because there is an increased incidence of aneurysms in first-degree relatives of those affected. There is also an increased incidence in individuals with certain Mendelian disorders (e.g., autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome type IV, neurofibromatosis type 1 [NF1], and Marfan syndrome), fibromuscular dysplasia of extracranial arteries, and coarctation of the aorta. Other predisposing factors include cigarette smoking and hypertension (estimated to be present in about half of affected individuals). Although they are sometimes referred to as “congenital,” the aneurysms are not present at birth but develop over time because of an underlying defect in the media of the vessel wall.
An unruptured saccular aneurysm is a thin-walled outpouching, usually at an arterial branch point along the circle of Willis or a major vessel just beyond. Saccular aneurysms measure from a few millimeters to 2 or 3 cm in diameter and have a bright red, shiny surface and a thin, translucent wall ( Fig. 28.20 ). Atheromatous plaques, calcification, or thrombi may be found in the wall or lumen of the aneurysm. Sometimes there is evidence of prior hemorrhage, in the form of brownish discoloration of the adjacent brain and meninges. The neck of the aneurysm may be wide or narrow. Rupture usually occurs at the apex of the sac and leads to extravasation of blood into the subarachnoid space, the substance of the brain, or both. The arterial wall adjacent to the neck of the aneurysm often shows some intimal thickening and attenuation of the media. Smooth muscle and intimal elastic lamina do not extend into the neck and are absent from the aneurysm sac itself, which is made up of thickened hyalinized intima and a covering of adventitia.
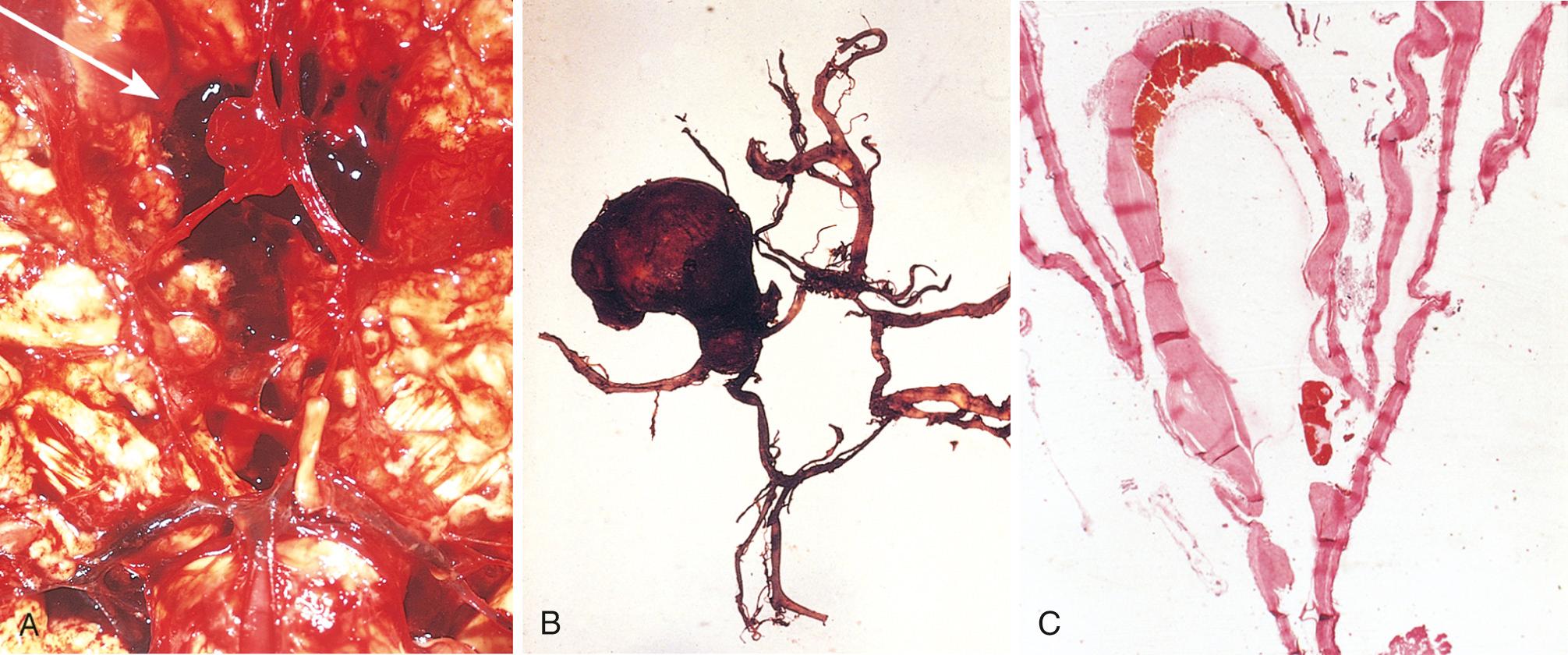
Rupture of an aneurysm leading to subarachnoid hemorrhage is most frequent in the fifth decade and is slightly more frequent in women. Overall, aneurysms rupture at a rate of 1.3% per year, but the risk is higher for larger aneurysms; for example, aneurysms greater than 10 mm in diameter have a roughly 50% risk of bleeding per year. Rupture may occur at any time, but in about one-third of cases it is associated with acute increases in intracranial pressure, such as with straining at stool or sexual orgasm. Blood under arterial pressure is forced into the subarachnoid space, and affected individuals are stricken with a sudden, excruciating headache (“the worst headache I've ever had”) and rapidly lose consciousness. Between 25% and 50% of patients die with the first rupture, but patients who survive often improve and recover consciousness in minutes. Repeat bleeding is common in survivors and unpredictable in timing. With each episode of bleeding, the prognosis is worse.
The clinical consequences of blood in the subarachnoid space can be separated into acute events (occurring within hours to days after the hemorrhage) and late sequelae associated with the healing process. In the first few days after a subarachnoid hemorrhage, regardless of the etiology, there is an increased risk of additional ischemic injury from vasospasm affecting vessels bathed in the extravasated blood. This problem is of greatest significance in cases of basal subarachnoid hemorrhage, in which vasospasm can involve major vessels of the circle of Willis. Various mediators have been proposed to have a role in this process, including endothelins, nitric oxide, and arachidonic acid metabolites. In the healing phase of subarachnoid hemorrhage, meningeal fibrosis and scarring occur, sometimes leading to obstruction of CSF flow as well as interruption of the normal pathways of CSF resorption.
Vascular malformations of the brain are classified into four principal groups: arteriovenous malformations, cavernous malformations, capillary telangiectasias, and venous angiomas . Of these, the first two are the types associated with risk of hemorrhage and development of neurologic symptoms. Although lacking morphologic features that typify neoplasms, arteriovenous malformations are frequently associated with activating somatic mutations in the KRAS oncogene within the endothelial cells that line the malformed vessels, suggesting that dysregulated RAS signaling has a central role in their pathogenesis.
Arteriovenous malformations (tangled networks of wormlike vascular channels with high blood flow due to prominent, pulsatile arteriovenous shunting) may involve vessels in the subarachnoid space, the brain, or both ( Fig. 28.21 ). They are composed of greatly enlarged blood vessels separated by gliotic tissue, often with evidence of prior hemorrhage. Some vessels can be recognized as arteries with duplication and fragmentation of the internal elastic lamina, while others show marked thickening or partial replacement of the media by hyalinized connective tissue.
Cavernous malformations consist of distended, loosely organized vascular channels arranged back to back with collagenized walls of variable thickness; there is usually no brain parenchyma between vessels in this type of malformation. They occur most often in the cerebellum, pons, and subcortical regions, in decreasing order of frequency, and are “low-flow” channels that do not participate in arteriovenous shunting. Foci of old hemorrhage, infarction, and calcification frequently surround the abnormal vessels.
Arteriovenous malformations are the most common clinically significant vascular malformation. Males are affected twice as frequently as females. The lesion often presents between 10 and 30 years of age as a seizure disorder, an intracerebral hemorrhage, or a subarachnoid hemorrhage. The most common site is the territory of the middle cerebral artery, particularly its posterior branches. Large arteriovenous malformations occurring in the newborn period can lead to congestive heart failure because of shunt effects, especially if the malformation involves the vein of Galen. Cavernous malformations are unique among this group of disorders in that familial forms are relatively common. Multiplicity of lesions is a hallmark of familial cases, which are inherited as a highly penetrant autosomal dominant trait.
Individuals who, over the course of many months and years, suffer multiple, bilateral, gray matter (cortex, thalamus, basal ganglia) and white matter (centrum semiovale) infarcts may develop a distinctive clinical syndrome characterized by dementia, gait abnormalities, and pseudobulbar signs, often with superimposed focal neurologic deficits. The syndrome, generally referred to as vascular dementia, is caused by multifocal vascular disease of several types, including (1) cerebral atherosclerosis, (2) vessel thrombosis or embolization from carotid vessels or from the heart, and (3) cerebral arteriolosclerosis from chronic hypertension. When the pattern of injury preferentially involves large areas of the subcortical white matter with myelin and axon loss, the disorder is referred to as Binswanger disease (subcortical white matter dementia); this distribution of vascular white-matter injury must be distinguished clinically and radiologically from other diseases that affect the hemispheral white matter. In addition, many individuals with neurodegenerative diseases resulting in cognitive impairment or dementia also have evidence of cerebrovascular disease. The presence of significant cerebrovascular disease increases risk of neurologic impairment for a given level of lesions associated with the degenerative diseases, suggesting that it is an independent contributing factor to disruption of normal brain function.
Stroke is the clinical term for acute-onset neurologic deficits that last longer than 24 hours and that are a consequence of vessel occlusion or vessel rupture.
Cerebral infarction follows loss of blood supply and can be focal, widespread, or restricted to regions with the least robust vascular supply (boundary zones).
Large artery infarcts are most commonly embolic; with subsequent dissolution of the embolus and reperfusion, a nonhemorrhagic infarct can become hemorrhagic.
Primary intraparenchymal hemorrhages lead to infarction of the adjacent brain parenchyma and are typically either ganglionic (most commonly due to hypertension) or lobar (most commonly due to CAA).
Hyaline arteriolosclerosis (small vessel disease), which is caused by a long-standing hypertension, can lead to either lumen occlusion (resulting in a lacunar infarct) or wall rupture (resulting in a ganglionic hemorrhage).
Spontaneous subarachnoid hemorrhage is usually caused by a structural vascular abnormality, such as an aneurysm or arteriovenous malformation.
Infection may damage the nervous system directly through injury of neurons or glia by the infectious agent, or indirectly through microbial toxins, the destructive effects of the inflammatory response, or as a result of immune-mediated mechanisms. There are four principal routes by which microbes enter the nervous system.
Hematogenous spread is the most common; infectious agents ordinarily gain access through the arterial circulation, but retrograde venous spread can occur via anastomoses with veins of the face.
Direct implantation of microorganisms is most often traumatic, but can be sometimes associated with congenital malformations (e.g., meningomyelocele) that provide ready access for microorganisms.
Local extension can originate from infected adjacent structures, such as air sinuses, teeth, skull, or vertebrae.
Viruses also may be transported along the peripheral nervous system, as occurs with rabies and herpes zoster viruses.
General aspects of the pathology of infectious agents are discussed in Chapter 8 ; distinctive forms of CNS infections are described herein ( Table 28.2 ).
| Type of Infection | Clinical Syndrome | Common Causative Organisms |
|---|---|---|
| Bacterial Infections | ||
| Meningitis | Acute pyogenic meningitis | Escherichia coli or group B streptococci (infants) |
| Neisseria meningitidis (young adults) | ||
| Streptococcus pneumoniae or Listeria monocytogenes (older adults) | ||
| Chronic meningitis | Mycobacterium tuberculosis | |
| Localized infections | Abscess | Streptococci and staphylococci |
| Empyema | Polymicrobial (staphylococci, anaerobic gram-negative) | |
| Viral Infections | ||
| Meningitis | Acute aseptic meningitis | Enteroviruses |
| Influenza species | ||
| Lymphocytic choriomeningitis virus | ||
| Encephalitis | Encephalitic syndromes | Herpes simplex (HSV-1, HSV-2) |
| Cytomegalovirus | ||
| Human immunodeficiency virus | ||
| JC polyomavirus (progressive multifocal leukoencephalopathy) | ||
| Arthropod-borne encephalitis | West Nile virus | |
| Eastern equine encephalitis virus | ||
| Western equine encephalitis virus | ||
| St. Louis encephalitis virus | ||
| La Crosse encephalitis virus | ||
| Venezuelan equine encephalitis virus | ||
| Japanese encephalitis virus | ||
| Tick-borne encephalitis virus | ||
| Brainstem and spinal cord syndromes | Rhombencephalitis | Rabies |
| Acute flaccid myelitis/poliomyelitis | Poliovirus | |
| West Nile virus | ||
| Enterovirus D68 | ||
| Spirochetes and Fungi | ||
| Meningoencephalitic syndromes | Neurosyphilis | Treponema pallidum |
| Lyme disease (neuroborreliosis) | Borrelia burgdorferi | |
| Fungal meningitis | Cryptococcus neoformans | |
| Candida albicans | ||
| Protozoa and Metazoa | ||
| Encephalitis | Amebic encephalitis | Balamuthia and Acanthamoeba species |
| Naegleria species | ||
| Localized infections | Toxoplasmosis | Toxoplasma gondii |
| Cysticercosis | Taenia solium | |
Meningitis is an inflammatory process of the leptomeninges and CSF within the subarachnoid space, usually caused by an infection. Meningoencephalitis refers to inflammation of the meninges and brain parenchyma. Although infections are the most common causes of meningitis and meningoencephalitis, this reaction may also occur in response to a nonbacterial irritant introduced into the subarachnoid space (chemical meningitis) or in the context of a systemic autoimmune disease. Based on the etiology and clinical evolution of the illness, infectious meningitis is broadly classified into acute pyogenic (usually bacterial), aseptic (usually acute or subacute viral), and chronic (usually tuberculous, spirochetal, or cryptococcal). Each type is accompanied by characteristic changes in the CSF.
Distinctive microorganisms cause acute pyogenic meningitis in various age groups: Escherichia coli and the group B streptococci in neonates; Streptococcus pneumoniae and Listeria monocytogenes in the elderly; and Neisseria meningitidis in adolescents and young adults, with clusters of cases raising public health concerns. The introduction of immunization against Haemophilus influenzae has markedly reduced the incidence of this infection in the developed world, particularly among infants (who used to be at high risk).
Affected individuals typically show systemic signs of infection superimposed on symptoms related to meningeal irritation and neurologic impairment, including headache, photophobia, irritability, clouding of consciousness, and neck stiffness. A spinal tap yields cloudy or frankly purulent CSF with as many as 90,000 neutrophils per cubic millimeter, increased CSF pressure and increased protein concentration, and markedly reduced glucose content. Untreated pyogenic meningitis can be fatal, while effective treatment with antibiotics markedly reduces mortality. One feared complication is the Waterhouse-Frederichsen syndrome, which results from meningitis-associated septicemia and hemorrhagic infarction of the adrenal glands ( Chapter 24 ). It occurs most often with meningococcal and pneumococcal meningitis. In the immunosuppressed individual, purulent meningitis may be caused by several other infectious agents, such as Klebsiella or anaerobic organisms, and may have an atypical clinical course and uncharacteristic CSF findings, rendering timely diagnosis more difficult.
In acute meningitis, an exudate is evident within the leptomeninges over the surface of the brain ( Fig. 28.22 ). The meningeal vessels are engorged and stand out prominently. The anatomic distribution of the exudate varies; in H. influenzae meningitis, for example, it is usually basal, whereas in pneumococcal meningitis it is often densest over the cerebral convexities near the sagittal sinus. From the areas of greatest accumulation, tracts of pus follow along blood vessels on the surface of the brain. When the meningitis is fulminant, the inflammation may extend to the ventricles, producing ventriculitis. The ventricles may also be the portal for CSF involvement by blood-borne infections because the choroid plexus lacks a blood-brain barrier.
On microscopic examination, neutrophils fill the subarachnoid space in severely affected areas and are found predominantly around the leptomeningeal blood vessels in less severe cases. Particularly in untreated meningitis, Gram stain reveals variable numbers of bacteria. In fulminant meningitis, the inflammatory cells infiltrate the walls of the leptomeningeal veins and may extend focally into the substance of the brain (cerebritis); secondary vasculitis and venous thrombosis may lead to hemorrhagic cerebral infarction.
Leptomeningeal fibrosis may follow pyogenic meningitis and cause hydrocephalus. Particularly in pneumococcal meningitis, large quantities of the capsular polysaccharide of the organism produce a gelatinous exudate that promotes arachnoid fibrosis, a condition referred to as chronic adhesive arachnoiditis .
Aseptic meningitis is a clinical term used for an absence of organisms by bacterial culture in a patient with manifestations of meningitis, including meningeal irritation, fever, and alterations of consciousness of relatively acute onset. The disease is generally of viral etiology, but may be bacterial, rickettsial, or autoimmune in origin. The clinical course is less fulminant than that of pyogenic meningitis, and the CSF findings also differ; in aseptic meningitis, there is a lymphocytic pleocytosis, the protein elevation is only moderate, and the glucose content is nearly always normal. The viral aseptic meningitides are usually self-limited and are treated symptomatically. Remarkably, the etiologic agent is identified in only a minority of cases; however, this may change through use of more sensitive and specific detection techniques (such as next-generation sequencing), which are under development. When pathogens are identified, enteroviruses are the most common etiology, accounting for 80% of cases. The spectrum of pathogens varies seasonally and geographically. An aseptic meningitis-like picture may also develop subsequent to rupture of an epidermoid cyst into the subarachnoid space or the introduction of a chemical irritant (chemical meningitis). The CSF is sterile in these cases, and there is pleocytosis with neutrophils and an increased protein concentration, but the sugar content is usually normal.
Focal suppurative infections are typically caused by pyogenic bacteria or fungi, and can arise in several different compartments including the brain parenchyma, subdural space, and extradural space.
A brain abscess is a localized focus of necrosis of brain tissue with accompanying inflammation, usually caused by a bacterial infection. Brain abscesses may arise by direct implantation of organisms, local extension from adjacent foci (mastoiditis, paranasal sinusitis), or hematogenous spread (usually from a primary site in the heart, lungs, or bones of the extremities, or secondary to bacteremia from dental procedures). Predisposing conditions include acute bacterial endocarditis, which may give rise to multiple brain abscesses; congenital heart disease with right-to-left shunting and loss of pulmonary filtration of organisms; chronic pulmonary sepsis, as in bronchiectasis; and systemic disease with immunosuppression. Streptococci and staphylococci are the most common offending organisms identified in nonimmunosuppressed patients.
Abscesses are discrete lesions with central liquefactive necrosis surrounded by brain swelling ( Fig. 28.23 ). At the outer margin of the necrotic lesion, there is exuberant granulation tissue with neovascularization. The newly formed vessels are abnormally permeable, accounting for marked vasogenic edema in the adjacent brain tissue. In well-established lesions, a collagenous capsule is produced by fibroblasts derived from the walls of blood vessels. Outside the fibrous capsule is a zone of reactive gliosis containing numerous gemistocytic astrocytes.
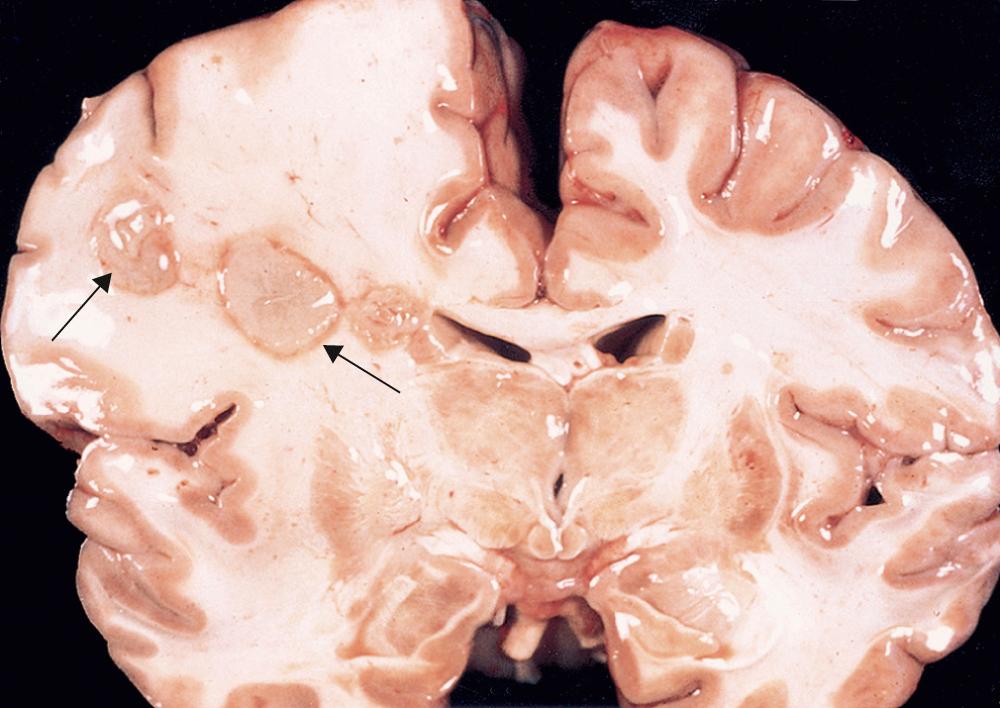
Cerebral abscesses are destructive lesions, and patients often present with progressive focal neurologic deficits; signs and symptoms related to increased intracranial pressure may also develop. Typically, the CSF has a high white cell count and an increased protein concentration, but the glucose content is normal. The source of infection may be apparent or may be traced to a small distant focus that is not symptomatic. The increased intracranial pressure can lead to fatal herniation; other complications include abscess rupture with ventriculitis or meningitis, and venous sinus thrombosis. With surgery and antibiotic treatment, the otherwise high mortality rate can be reduced to less than 10%.
Bacterial, and rarely fungal, infections of the skull bones or air sinuses can spread to the subdural space, producing a subdural empyema. Although the underlying arachnoid and subarachnoid spaces are usually not affected, a large subdural empyema with mass effect and/or thrombophlebitis of the bridging veins can cause venous occlusion and infarction of the brain. In addition to symptoms referable to the source of the infection, most patients are febrile and have headache and neck stiffness. The CSF profile is similar to that seen in brain abscesses because both are parameningeal infectious processes. If untreated, focal neurologic signs, lethargy, and coma may develop. With prompt diagnosis and treatment, including surgical drainage, resolution and full recovery is possible, the only residuum being a thickened dura.
Extradural abscess, commonly associated with osteomyelitis, often arises from an adjacent focus of infection, such as sinusitis, or following a surgical procedure. When the process occurs in the spinal epidural space, it may cause spinal cord compression and constitute a neurosurgical emergency.
Chronic bacterial infection of the meninges and the brain may be caused by Mycobacterium tuberculosis , Treponema pallidum , and Borrelia species.
Tuberculosis of the CNS may be part of active disease elsewhere in the body, or appear in isolation following seeding from silent lesions elsewhere, usually the lungs. It may involve the meninges or the brain.
The most common pattern of tuberculous involvement is a diffuse meningoencephalitis. The subarachnoid space contains a gelatinous or fibrinous exudate that characteristically involves the base of the brain, effacing the cisterns and encasing cranial nerves. There may be discrete, white areas of inflammation scattered over the leptomeninges. On microscopic examination, involved areas contain a mixed inflammatory infiltrate with lymphocytes, plasma cells, and macrophages. Florid cases show well-formed granulomas with caseous necrosis and giant cells. Arteries running through the subarachnoid space may show obliterative endarteritis and marked intimal thickening. Organisms can often be seen with acid-fast stains. The infectious process may spread to the choroid plexus and ependymal surface, traveling through the CSF. In long-standing cases, a dense, fibrous adhesive arachnoiditis may develop, most conspicuous around the base of the brain. Hydrocephalus may result.
CNS involvement may also take the form of one or more well-circumscribed intraparenchymal masses (tuberculomas), which may be associated with meningitis. A tuberculoma may be as large as several centimeters in diameter, causing significant mass effect. These granulomas usually have central caseous necrosis; calcification may occur in inactive lesions.
Patients with tuberculous meningitis usually have headache, malaise, mental confusion, and vomiting. The CSF typically shows a pleocytosis made up of mononuclear cells or a mixture of neutrophils and mononuclear cells, an elevated protein concentration (often strikingly so), and a moderately reduced or normal glucose. The most serious complications of chronic tuberculous meningitis are arachnoid fibrosis producing hydrocephalus and obliterative endarteritis producing arterial occlusion and brain infarction. When the process involves the spinal cord subarachnoid space, nerve roots may also be affected. Tuberculomas produce symptoms typical of space-occupying brain lesions and must be distinguished from CNS tumors.
CNS tuberculosis in patients with acquired immunodeficiency syndrome (AIDS) is pathologically similar, but there may be less host reaction than in immunocompetent individuals.
Neurosyphilis is a manifestation of the tertiary stage of syphilis and occurs in only about 10% of individuals with untreated infection. The major patterns of CNS involvement are meningovascular neurosyphilis, paretic neurosyphilis, and tabes dorsalis. Affected individuals often show an incomplete or mixed picture, most commonly the combination of tabes dorsalis and paretic disease (taboparesis). Because of impaired cell-mediated immunity, individuals infected with HIV are at increased risk for neurosyphilis, particularly acute syphilitic meningitis or meningovascular disease; the rate of disease progression and severity are also accelerated.
Neurosyphilis presents in several distinct forms.
Meningovascular neurosyphilis is chronic meningitis involving the base of the brain and more variably the cerebral convexities and spinal leptomeninges. In addition, there may be an associated obliterative endarteritis (Heubner arteritis) accompanied by a distinctive perivascular inflammatory reaction rich in plasma cells and lymphocytes. Cerebral gummas (plasma cell-rich mass lesions) may also occur in the meninges and extend into the parenchyma.
Paretic neurosyphilis is caused by invasion of the brain by T. pallidum and manifests as insidious but progressive cognitive impairment associated with mood alterations (including delusions of grandeur) that terminate in severe dementia (general paresis of the insane). Parenchymal damage of the cerebral cortex is particularly common in the frontal lobe, but also occurs in other areas of the isocortex. The lesions are characterized by loss of neurons, proliferation of microglia, gliosis, and iron deposits; the latter are demonstrable with the Prussian blue stain perivascularly and in the neuropil, and are presumably the sequelae of small bleeds stemming from microvascular damage. The spirochetes can, at times, be demonstrated in tissue sections.
Tabes dorsalis is the result of damage to the sensory axons in the dorsal roots. This causes impaired joint position sense and ataxia (locomotor ataxia); loss of pain sensation, leading to skin and joint damage (Charcot joints); other sensory disturbances, particularly the characteristic “lightning pains”; and absence of deep tendon reflexes. On microscopic examination, there is loss of both axons and myelin in the dorsal roots, with corresponding pallor and atrophy in the dorsal columns of the spinal cord. Organisms are not demonstrable in the cord lesions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here