Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The cardiac catheterization laboratory (CCL) has evolved from a purely diagnostic facility to a therapeutic one in which many facets of cardiovascular disease can be effectively modified or treated. Despite improvements in equipment, the quality of the procedure depends on well-trained and experienced physicians with proper certification, adequate procedural volume, and personnel committed to the continuous quality improvement process.
Guidelines for diagnostic cardiac catheterization have established indications, contraindications, and criteria to identify high-risk patients. Careful evaluation of the patient before the procedure is critical to minimize risks.
Interventional cardiology began in the late 1970s as balloon angioplasty, with a success rate of 80% and emergent coronary artery bypass graft surgery (CABG) rates of 3% to 5%. Although current success rates exceed 95%, with CABG rates of less than 1%, failed percutaneous coronary intervention (PCI) presents a challenge for the anesthesiologist because of hemodynamic problems, concomitant medications, and the underlying cardiac disease.
Since the introduction of stents, acute closure due to coronary dissection has diminished significantly, and restenosis rates have fallen precipitously. Initially, bare metal stents (BMSs) were developed for this purpose. Later, more advanced stents, named drug-eluting stents (DESs) improved markedly the outcomes of the procedure.
The first-generation DESs (Cypher, Cordis, Miami Lakes, FL, and Taxus, Boston Scientific, Marlborough, MA) were extremely effective at reducing in-stent restenosis when compared with BMSs. However, these DESs have demonstrated higher rates of late stent thrombosis (LST), especially in the setting of premature discontinuation of dual antiplatelet therapy. Second-generation DESs (Xience, Abbott Vascular, Abbott Park, IL, and Resolute, Medtronic, Minneapolis, MN) have LST rates comparable to those of BMSs. Last-generation DESs (i.e., Orsiro, Biotronik; Synergy 2, Boston; Onyx, Medtronic), designed with thinner struts displayed better deliverability and results in complex settings. Addittionally, their improvements favored shorter dual antiplatelet treatments.
As a treatment strategy for patients with acute myocardial infarction, primary PCI is preferred to the administration of thrombolytic therapy because of its higher rates of infarct artery patency and Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow and lower rates of recurrent ischemia, reinfarction, intracranial hemorrhage, and death.
In the world, increasing numbers of diagnostic coronary angiograms and PCIs are performed from a transradial approach because of lower vascular complication rates and patient preference for this approach compared with the more traditional transfemoral approach. In fact, radial artery access is associated with lower mortality in patients undergoing primary PCI.
In multivessel coronary artery disease (CAD), an angiographic SYNTAX score could be calculated to assist with decision-making regarding percutaneous versus surgical revascularization. A multidisciplinary heart team meeting (including a cardiologist, a cardiovascular surgeon, and, occasionally, an anesthesiologist) should then convene to discuss and optimize patient care by providing an individualized treatment recommendation.
Extensive thrombus, heavy calcification, degenerated saphenous vein graft, and chronic total occlusion present specific challenges in PCI. Various specialty devices have been developed to address these problems and have had varying degrees of success.
Acute thrombotic PCI complications can usually be overcome with more aggressive antithrombotic and antiplatelet pharmacotherapy. These medications can complicate the management of an unstable patient who requires transfer for bailout CABG. Appropriate understanding of the pharmacokinetics is essential for the cardiac anesthesiologist.
The reach of the interventional cardiologist has extended beyond coronary vessels to include closure of congenital defects, percutaneous treatment of valve disease, and renal denervation. These complex procedures are more likely to require general anesthesia, but also can be effectively managed with monitored anesthesia care or regional anesthesia techniques.
The first cardiac catheterization was performed in 1929 by Dr. Werner Forssmann, a surgical resident in the Auguste Viktoria Hospital at Eberswalde, near Berlin, in an attempt to identify a safer route to administer epinephrine directly into the heart during intraoperative cardiac arrests. Forssmann asked a colleague to place a catheter in his arm. The catheter was successfully passed to his axilla, at which time Forssmann, under radioscopic guidance and using a mirror, advanced the catheter into his own right atrium (RA). Forssmann subsequently practiced in a small town in the Rhine Valley, but eventually he shared the Nobel Prize in 1956 for this procedure.
The world quickly acknowledged Forssmann’s accomplishments with right-sided heart catheterization (RHC); in 1930, Klein employed RHC to measure cardiac output (CO) using the Fick method. In 1941, André Cournand published his work on RHC in the Proceedings of the Society of Experimental Biology and Medicine. Dexter and colleagues first reported cardiac catheterization in the pediatric population in 1947, and they were the first to document correlation between the pulmonary capillary wedge pressure (PCWP) and left atrial pressure (LAP). Zimmerman and Limon-Lason and Bouchard first performed arterial retrograde heart catheterization in 1950, and Seldinger developed his percutaneous approach in 1953. Ross and Cope developed transseptal catheterization in 1959. The first coronary angiogram was inadvertently performed by Mason Sones in 1958. While performing angiography of the aorta, the catheter moved during placement of the x-ray equipment, and Dr. Sones injected 50 mL of contrast into the patient’s right coronary artery (RCA). Expecting cardiac arrest from this amount of contrast and with no external defibrillator available, Sones jumped to his feet and grabbed a scalpel to perform a thoracotomy. Fortunately, asystole lasted only 5 seconds, the patient awoke perplexed by the commotion, and this incident led to the birth of selective coronary angiography.
Diagnostic catheterization led to interventional therapy in 1977, when Andreas Gruentzig performed the first percutaneous transluminal coronary angioplasty (PTCA). Refinements in both diagnostic and interventional equipment occurred over the next 15 to 20 years, but the focus remained on coronary artery disease (CAD). More recently, cardiologists have expanded into the diagnosis and treatment of peripheral vascular disease and treatment of structural heart disease. Currently, several transcatheter heart valves have been approved by the US Food and Drug Administration (FDA) and are available for the treatment of aortic stenosis. Transapically inserted heart valves are being developed for the treatment of mitral valve (MV) disease and percutaneously deliverable clips for mitral regurgitation (MR).
Hybrid coronary artery bypass procedures are performed in some institutions, with internal mammary artery grafting to the left anterior descending (LAD) coronary artery via a limited incision, with or without robotic guidance, and with percutaneous treatment of other vessels.
This brief historical background serves as an introduction to the discussion of diagnostic and therapeutic procedures in the adult cardiac catheterization laboratory (CCL). The reader must realize the dynamic nature of this field. In the past, up to 5% of PCIs failed, but most centers now report procedural failure rates of less than 1%. Simultaneously, the impact on the anesthesiologist has changed. The high complication rates of years past required holding an operating room (OR) open for all PCIs, but complication rates are now so low that several procedures are performed at hospitals without on-site surgical backup. Despite the lower rate of adverse events, the anesthesiologist is occasionally confronted with a patient who is in need of emergent surgical revascularization. However, a most common requirement for the attending anesthesiologist, in the CCL, are the cardiac structural procedures (i.e., closures, transcatheter aortic valve replacement [TAVR], transcatheter MV repair or replacement, paravalvular leak closure, left atrial appendage closure, renal denervation). When anesthesia is required for procedures in the hybrid laboratory or the CCL, this chapter will help the anesthesiologist, in collaboration with the cardiology and cardiac surgery team, to provide safe anesthesia care for these challenging patients.
This section reviews the importance of radiation safety to prevent the adverse effects of radiation, including dermal necrosis and cellular mutation, that lead to brain, skin, and thyroid cancers; birth defects; and infertility. For the individual laboratory, the monitoring suite is separated from the x-ray imaging equipment by leaded glass and lead-lined walls. Voice communication from the central area is maintained to coordinate tasks performed centrally (e.g., monitoring and recording data, determining the activated coagulation time [ACT]), thereby minimizing staff radiation exposure. A representative CCL is shown in Fig. 2.1 .
Radiation safety must be considered in all aspects of the CCL, from room design to everyday practice. Lead-lined walls, lead-glass partitions, and mobile lead shielding, including aprons and thyroid shields, are useful in limiting the daily exposure of personnel.
A thermoluminescent film badge, also known as a dosimeter, must be worn at all times by any persons exposed to the x-ray equipment, and levels must be monitored regularly. In the past, anesthesiologists responding to emergencies in the CCL were exposed to radiation only briefly and infrequently, if at all. With the requirement for the presence of an anesthesiologist in many of the newer multidisciplinary procedures, the inclusion of anesthesiologists in formal monitoring programs may be appropriate. Radiation levels should not exceed 5 rem (0.05 Sv) per calendar year, 1.25 rem (0.0125 Sv) per calendar quarter, or approximately 100 mrem (0.001 Sv) per week. Operator and staff radiation exposures have been assessed for years, but only recently has the issue of radiation toxicity to the patient gained attention. With long PCI and electrophysiology procedures, radiation injury to the patient and the need to monitor dose delivery to the patient are now appreciated. Contemporary equipment estimates and records patient radiation doses. Lead aprons and thyroid shields are mandatory for all personnel in the procedure suite. Although they are cumbersome, these shields protect the reproductive glands and about 80% of the active bone marrow. Eye shielding also should be considered for those working in close proximity to the x-ray source.
It is not within the scope of this chapter to cover all aspects of radiation safety in cardiovascular imaging. For a more complete review of this topic, the reader is referred to a consensus document published in 2014 by the American College of Cardiology (ACC), American Heart Association (AHA), Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions (SCAI), and the International Commission on Radiological Protection (ICRP).
Nevertheless, several features of radiation safety require a brief review. The duration of the procedure increases the exposure. Cine imaging (ie making a permanent recording) requires about 10 times the radiation exposure of fluoroscopy. Although newer equipment may narrow this ratio and provide a permanent recording of the fluoroscopic images, limiting cine imaging is a way to decrease exposure. Proximity to the x-ray tube, which is usually situated below the patient, is directly related to exposure. The bulk of the radiation exposure to medical personnel is the result of scattered x-rays coming from the patient. When working in an environment where x-rays are in use, clinicians should always remember a simple rule: The amount of radiation exposure is related to the square of the distance from the source. No body part should ever be placed in the imaging field when fluoroscopy or cine imaging is being performed. Finally, the cardiologist can decrease x-ray scatter, and thereby decrease personnel exposure, by placing the imaging equipment as close to the patient as possible.
The anesthesiologist should recognize x-ray use in the CCL and take appropriate precautions. For multidisciplinary procedures, this requires some attention to the location of equipment and the use of portable shields. Most lead aprons have openings in the back and protect best when the wearer is facing the source of the x-rays. Emergent situations, such as when the anesthesiologist is asked to resuscitate a critically ill patient during a procedure, may require the cardiologist to use fluoroscopic imaging while the anesthesiologist is within feet of the x-ray tube or even straddling it. Aprons and thyroid shields are clearly necessary to protect the anesthesiologist while at the head of the patient, and a lead thickness of 0.5 mm will stop 96% of the x-ray beam scatter. The use of x-rays can almost always be interrupted to protect personnel, although needed patient care may require the interruptions to be brief. A collaborative effort between the cardiologist and the anesthesiologist is necessary, and communication is essential. The goal of the anesthesiologist should be to treat the patient while protecting oneself and others from excessive radiation exposure.
Essentially all modern laboratories use filmless or digital recording. Radiation is needed to generate an image, and recordings are made at various frequencies (frames per second). The best image quality for film is obtained with frame rates greater than 30 per second. Digital imaging decreases radiation exposure in the CCL by allowing for image acquisition at lower frame rates (i.e., 15 frames/second) while still maintaining excellent image quality. Cost savings have been achieved by elimination of the need to purchase, process, and store film. Film imaging was an analog technique, and a single recording was made. Copies rarely were made because of the cost and degradation of image quality. If films were loaned, lost, or misplaced, the study could not be reviewed. With the current digital technologies, images are archived on a central server and can be viewed at remote workstations. An unlimited number of copies can be made at low cost and with no loss of image quality.
The evolution of angiographic recording has extended beyond recording formats. Charged-couple device cameras and flat-panel detectors are now ubiquitous in modern laboratories. X-rays are generated from below the patient by the x-ray tube, pass through the patient, and are captured by the detector. In this system, the x-rays are both acquired and digitally processed by the flat panel. The flat panel is above the patient (analogous to the image intensifier). This current generation of imaging in the CCL delivers improved image quality because the dynamic range of the image (i.e., the number of shades of gray) is improved. This type of system has the potential to decrease radiation exposure by providing immediate feedback to the x-ray generator. In laboratories designed for peripheral vascular work, including many of the hybrid ones, the flat panel above the patient can be quite large and may limit access to the patient’s face.
All catheterization facilities must maintain appropriate patient volume to ensure competence. ACC/AHA guidelines previously recommended that a minimum of 300 adult diagnostic cases be performed to provide adequate care, including a minimum of 200 PCIs. However, a 2012 update set no diagnostic minimums and recommended at least 75 PCIs/year. If the individual physician’s annual volume is less than 75 cases, a quality assurance committee should perform an annual review of 15% of cases (randomly chosen) to ensure continued quality. This volume-outcome relationship for revascularization procedures has been also pointed out in the recent European Society of Cardiology (ESC) revascularization guidelines. Among patients with acute coronary syndrome (ACS), particularly ST segment elevated myocardial infarction (STEMI), operator and hospital volumes would play important roles. A large American study reported that for 36,535 patients undergoing primary PCI, in-hospital mortality was lower in institutions with higher primary PCI volumes (5.7% in hospitals performing >33 primary PCIs/year vs 7.7% in hospitals performing <12 primary PCIs/year).
Similarly, operator volume has also been shown to impact outcomes in unprotected left main (LM) PCI. In this procedure, a high-volume operator (defined as ≥15 LM PCI/year; mean 25/year) vs a low-volume operator (<15 LM PCI/year) showed reduced mortality in a single-center study of 1948 patients who underwent LM PCI. This relation has also been depicted for other interventional procedures, for example TAVR.
Facilities performing PCIs without in-house surgical backup are becoming more prevalent. , National guidelines now support the performance of both elective and emergent PCI in centers without cardiac surgical capabilities, giving elective PCI a class IIb rating and primary PCI a class IIa rating. Although emergent coronary artery bypass graft surgery (CABG) is infrequent in the stent era, a well-established agreement must be in place with an on-site surgical hospital to minimize treatment delays when emergent CABG is required.
Primary PCI for acute myocardial infarction (AMI) is the accepted standard treatment for patients who are in cardiogenic shock, those who have contraindications to thrombolytic therapy, and those who have not responded to thrombolytic therapy. It is the preferred therapy for patients who present late in the course of an infarction, and it is probably the optimal treatment for all myocardial infarctions (MIs), provided that it can be performed in a timely manner.
Although minimum volumes are recommended, no regulatory controls currently exist. In a study of volume-outcome relationships published for New York state, a clear inverse relation was identified between CCL caseload and rates of procedural mortality and primary angioplasty. In a nationwide study of Medicare patients, low-volume centers had a 30-day mortality rate of 4.2%, whereas in high-volume centers the mortality rate was 2.7%. However, other reports suggest that activity level is an incomplete surrogate for quality. In other words, high-volume operators and hospitals are not necessarily of high quality, and low-volume operators and hospitals are not necessarily of low quality. Centers of excellence, based on physician and facility quality and the spectrum of overall services provided, may well be the model for cardiovascular care in the future.
Box 2.1 lists indications for cardiac catheterization. The major indication is for the detection of CAD; the remaining indications are focused on hemodynamic assessment to evaluate valvular heart disease (VHD), pulmonary hypertension, and cardiomyopathies. With respect to CAD, approximately 20% of the adult population studied will be found to have nonobstructive lesions in the coronary tree. Nowadays, several invasive tools have been developed to further assess coronary microcirculation and vascular mobility. This reflects limitations in the specificity of the clinical criteria and noninvasive tests used to select patients for catheterization (see Chapters 1 and 2). Despite continued improvements in noninvasive assessment, coronary angiography is currently considered the gold standard for diagnosing and defining the extent of CAD. With advances in MRI and multislice computed tomography (CT) scanning, the next decade may well see a further evolution of the CCL to an interventional suite with fewer diagnostic responsibilities.
Unstable angina
Postinfarction angina
Angina refractory to medications
Typical chest pain with negative diagnostic testing
History of sudden death
Strongly positive exercise tolerance test
Early positive, ischemia in ≥5 leads, hypotension, ischemia present for ≥6 min of recovery
Positive exercise testing after myocardial infarction
Strongly positive nuclear myocardial perfusion test
Increased lung uptake or ventricular dilation after stress
Large single or multiple areas of ischemic myocardium
Strongly positive stress echocardiographic study
Decrease in overall ejection fraction or ventricular dilation with stress
Large single area or multiple or large areas of new wall motion abnormalities
Aortic stenosis with syncope, chest pain, or congestive heart failure
Aortic insufficiency with progressive heart failure
Mitral insufficiency or stenosis with progressive congestive heart failure symptoms
Acute orthopnea/pulmonary edema after infarction with suspected acute mitral insufficiency
Progressive resting left ventricular dysfunction with regurgitant lesion
Decreasing left ventricular function and/or chamber dilation with exercise
Age >50 y with evidence of coronary artery disease
Septum primum or sinus venosus defect
Catheterization for definition of coronary anatomy
Coarctation of the aorta
Detection of collaterals
Coronary arteriography if increased age and/or risk factors are present
Acute myocardial infarction therapy—consider primary percutaneous coronary intervention
Mechanical complication after infarction
Malignant cardiac arrhythmias
Cardiac transplantation
Pretransplantation donor evaluation
Posttransplantation annual coronary artery graft rejection evaluation
Unexplained congestive heart failure
Research studies with institutional board review and patient consent
Diagnostic cardiac catheterization in the 21st century universally is considered an outpatient procedure except for the high-risk patient. Therefore, the precatheterization evaluation is essential for quality patient care. Evaluation before cardiac catheterization includes diagnostic tests that are necessary to identify high-risk patients. An electrocardiogram (ECG) must be obtained for all patients shortly before catheterization. Necessary laboratory studies before catheterization include an appropriate coagulation profile (prothrombin time [PT], partial thromboplastin time [PTT], and platelet count), hemoglobin, and hematocrit. Electrolytes are obtained together with baseline blood urea nitrogen and creatinine (Cr) values to assess renal function. Recent guidelines express a preference for estimation of the glomerular filtration rate (GFR) using accepted formulas, and many clinical laboratories now report this value routinely. Urinalysis and chest radiography may provide useful information but are no longer routinely obtained by all operators. Prior catheterization reports should be available. If the patient had prior PCI or CABG surgery, anatomic information concerning stent or bypass placement also must be available.
The precatheterization history is important to determine the risk profile for the patient, including previous exposure to contrast dye and the reaction to it, if any. If a true contrast reaction (e.g., rash, breathing difficulties, angioedema) occurred with prior contrast exposure, premedication with glucocorticoids and diphenhydramine is required before the patient arrives at the CCL. Nonionic contrast dyes can be administered to patients with a history of anaphylactoid reactions to the ionic contrast dyes. Diabetes mellitus (DM), preexisting chronic kidney disease (CKD), and heart failure are widely accepted risk factors for contrast-induced nephropathy (CIN). A Cr level greater than 1.5 mg/dL, particularly in a patient with DM, or a GFR of less than 60 mL/min should prompt special precautions. If the patient has stage IV CKD with a GFR of less than 30 mL/min, the study may need to be performed very cautiously, minimizing the contrast use and possibly considering the use of protective measures.
Nephrotoxicity is dependent on the amount of dye reaching the renal arteries and can be reduced by hydrating the patient before the procedure, avoiding high-osmolar contrast dyes and nephrotoxic medications (e.g., nonsteroidal antiinflammatory drugs), limiting the amount of dye administered, and maintaining an interval of 72 hours between exposures if multiple studies are required. These measures may reduce the risk of transient worsening of renal dysfunction and the need for permanent renal replacement therapy. In patients at risk for nephrotoxicity, validated equations are used to calculate the maximal allowable contrast dose for any given procedure. Prevention of contrast acute kidney injury can be accomplished by furosemide with matched hydration (Renal Guard system). Another option, if needed, could be the use of continuous veno-venous hemofiltration to achieve an extrarenal depuration of the contrast.
A review of the noninvasive cardiac evaluation before cardiac catheterization allows the cardiologist to formulate objectives for the procedure. In patients with hypotension and severely impaired functional capacity on exercise stress testing, LM coronary artery lesions or high-grade proximal LAD stenoses should be suspected. Knowing the location of perfusion or wall motion abnormalities in a particular coronary distribution, the cardiologist must specifically identify or exclude coronary lesions in these areas during the procedure. In patients with echocardiographic evidence of a left ventricular (LV) thrombus or left-sided endocarditis, left ventriculography may not be performed.
Patient medications may need to be altered in preparation for a heart catheterization. On the morning of the catheterization, antianginal and antihypertensive medications are routinely continued, whereas diuretic therapy is withheld. Diabetic patients are scheduled early, if possible, because the procedure requires NPO status. No short-acting insulin is given, and half of the long-acting insulin dose is usually administered. Patients on oral anticoagulation should stop warfarin (Coumadin) for 48 to 72 hours before catheterization to target an international normalized ratio (INR) of 1.8 or less if, femoral artery access is used. Radial artery access is considered an option without discontinuation of warfarin. For patients who are managed with non–vitamin K antagonist novel oral anticoagulant (NOAC) therapy, the dose may need to be withheld for 24 to 48 hours depending on renal function and the bleeding risk of the procedure. In patients who are anticoagulated because of mechanical prosthetic valves, the best management may be intravenous (IV) heparin before and after the procedure, when the warfarin effect is not therapeutic. Low-molecular-weight heparins (LMWHs) are used in this setting, but this use is controversial. LMWHs vary in their duration of action, and their effect cannot be monitored through use of the activated PTT. Special dosing is required for certain patient groups including the elderly, those with an elevated body mass index, and those with CKD. The differing pharmacokinetics need to be considered, particularly with regard to hemostasis at the vascular access site. IV heparin is routinely discontinued 1 to 2 hours before catheterization, except in patients with unstable angina. Therapy with aspirin or P2Y12 platelet inhibitors or both is almost always continued for patients with angina for those with prior CABG.
Despite advances in facilities, equipment, techniques, and personnel, the precatheterization evaluation must identify those patients who are at increased risk for complications. In a modern facility with an experienced staff, the only absolute contraindication is refusal by a competent patient or an incompetent patient’s inability to provide informed consent. Relative contraindications are listed in Box 2.2 ; the primary operator is responsible for this assessment.
Uncontrolled ventricular irritability: the risk for ventricular tachycardia/fibrillation during catheterization is increased if ventricular irritability is uncontrolled, excepting when the suspected cause is acute coronary ischemia.
Uncorrected hypokalemia or digitalis toxicity.
Intercurrent febrile illness.
Decompensated heart failure, especially acute pulmonary edema, excepting when ongoing ischemia is suspected. If so, an emergent catheterization could be considered.
Anticoagulation state; international normalized ratio (INR) >1.8, femoral approach. Radial approach is strongly warranted for diagnostic and therapeutic coronary procedures.
Severe allergy to radiographic contrast agent.
Severe renal insufficiency and/or anuria, unless dialysis is planned to remove fluid and radiographic contrast load.
Box 2.3 lists criteria for identifying high-risk patients before catheterization. Procedural alterations may be based on this assessment, such as avoidance of crossing an aortic valve or performing ventriculography. In any case, the determination as to whether a patient is a candidate for catheterization must be based on risk versus benefit for each individual patient.
Age
Infant: <1 y old
Elderly: >70 y old
Functional class
Mortality ↑ 10-fold for class IV patients compared with I and II
Severity of coronary obstruction
Mortality ↑ 10-fold for left main disease compared with one- or two-vessel disease
Valvular heart disease as an independent lesion
Greater risk when associated with coronary artery disease
Left ventricular dysfunction
Mortality ↑ 10-fold in patients with low ejection fraction (<30%)
Severe noncardiac disease
Renal insufficiency
Insulin-requiring diabetes
Advanced peripheral and cerebral vascular disease
Severe pulmonary insufficiency
With the increased emphasis on outpatient procedures in medicine today, outpatient diagnostic catheterization is becoming the standard of care for stable patients. Unstable and postinfarction patients are already hospitalized, and catheterization usually is performed before discharge. Planned PCI usually requires hospital admission; however, same-day discharge in carefully selected patients is gaining momentum because it appears to be safe, especially when radial artery access has been used. Even when outpatient catheterization is planned, assessment of the patient after catheterization is required. Some patients, particularly those with LM CAD, critical aortic stenosis, uncontrolled hypertension (HT), significant LV dysfunction with congestive heart failure, or significant postprocedural complications such as a large femoral access site hematoma, will require hospital admission.
In addition to the high-risk cardiac patient, patients with renal insufficiency may require overnight hydration before and after catheterization. Patients on chronic anticoagulation with warfarin require measurement of their coagulation status and may require heparinization before and/or after the procedure. Day-of-procedure ambulation and discharge are planned for patients undergoing outpatient catheterization. Radial artery catheterization is increasing in popularity and is associated with a reduction of vascular complications. , For a variety of reasons, the sheaths used for radial access are not suitable for long-term monitoring purposes and should be removed at the conclusion of the procedure. For patients undergoing catheterization via the percutaneous femoral approach, the use of smaller catheters (4 Fr) for the arterial puncture may hasten ambulation. Alternatively, a variety of vascular closure devices are approved for use. Vascular closure devices differ in the material that is used and are classified as active or passive approximators of the arteriotomy. The most commonly used device, Angio-Seal (St. Jude Medical, Plymouth, MN), uses an intraluminal anchor made of bioabsorbable material. However, it is recommended that the treated vessel not be used for repeat arterial access for up to 3 months, to permit absorption of the anchor and limit the risk for embolization. Protocols for early ambulation may permit the patient to be out of bed 2 to 4 hours after hemostasis, or even earlier, if a closure device is used.
Whether the procedure is elective or emergent, diagnostic or interventional, coronary or peripheral, certain basic components are relatively constant in all circumstances. Variations depend on the specific situation and are discussed later in this chapter.
All patients receive a thorough explanation of the procedure, often including pamphlets and videos. A full explanation of the technique and potential risks minimizes patient anxiety and is similar to the preoperative anesthesia visit. It is important for the cardiologist to meet the patient before the study. This relaxes the patient while allowing the physician to become better acquainted with the patient, aiding in the decision-making process. Although some laboratories allow the patient to have a clear liquid breakfast up to 2 to 3 hours before the procedure, outpatients are routinely asked to have no oral intake for 8 hours before the procedure, except for oral medications. It is also important to ask the patients to avoid the intake of coffee or any caffeinated beverages, since they could artifact some intracoronary studies (interaction with adenosine, producing false negative results).
Patients with previous allergic reactions to iodinated contrast agents require adequate prophylaxis. In a study of 857 patients who had a prior allergic reaction to contrast media, 50 mg of prednisone was administered 13 hours, 7 hours, and 1 hour before the procedure. Diphenhydramine (50 mg intramuscularly) also was administered 1 hour before the procedure. No severe anaphylactic reactions occurred, and the overall incidence of urticarial reactions in known high-risk patients was 10%. The use of nonionic contrast agents may further decrease reactions in patients with known contrast allergies. The administration of histamine H 2 blockers (e.g., 300 mg cimetidine) is less well studied. For patients undergoing emergent cardiac catheterization who have known contrast allergies, 200 mg of hydrocortisone is administered intravenously immediately and repeated every 4 hours until the procedure is completed. Diphenhydramine (50 mg IV) is recommended 1 hour before the procedure.
CIN is defined as an increase in serum Cr concentration of more than 0.5 mg/dL, or 25% above baseline level, within 48 hours. Although it is infrequent, occurring in fewer than 5% of PCIs, when it does occur its impact on patient morbidity and mortality is significant. Total contrast doses of less than 4 mL/kg are recommended for patients with normal renal function, and lower doses are recommended for those with preexisting renal dysfunction (Cr >1.5), particularly diabetic patients. A study of more than 8000 patients undergoing PCI identified eight risk factors for CIN: hypotension, intraaortic balloon pump (IABP), congestive heart failure, CKD, DM, age older than 75 years, anemia, and contrast volume. It is essential that the patient at high risk be identified and properly treated. In addition, renal function should be monitored for at least 48 hours in patients at high risk for CIN, particularly if surgery or other interventions are planned.
Several methods have been used to decrease renal toxicity from contrast agents. The two most important measures are minimizing contrast dose and providing adequate hydration, either with 0.9% saline at a rate of 1 mL/kg per hour for 12 hours before and after the procedure, if tolerated, or with isotonic sodium bicarbonate. Currently, there are various protocols for the infusion of isotonic sodium bicarbonate, which can be prepared by combining 150 mL of NaHCO 3 with 850 mL of sterile H 2 O. However, no prophylaxis was noninferior and cost-saving in preventing CIN compared with routine IV hydration, in patients with estimated glomerular filtration rate (eGFR) >30 mL.
Low-osmolar contrast agents are the standard of care, and despite initial interest in iso-osmolar contrast agents, findings in later studies have been mixed. N -Acetylcysteine (Mucomyst) showed initial promise, but extensive subsequent studies failed to show reductions in CIN, and it is not currently recommended. , As mentioned earlier, furosemide with matched hydration (Renal Guard system), and the use of ultrafiltration dialysis has been beneficial in small studies. , In some studies, the use of high doses of statins showed benefit in preventing CIN.
Standard limb leads with one chest lead are used for ECG monitoring during cardiac catheterization. One inferior and one anterior ECG lead are monitored during diagnostic catheterization. During an interventional procedure, two ECG leads are monitored in the same coronary artery distribution as the vessel undergoing PCI. Radiolucent ECG leads permit monitoring without interfering with angiographic data.
Sedation in the CCL, from preprocedural administration or from IV administration during the procedure, may lead to hypoventilation and hypoxemia. The administration of midazolam, 1 to 5 mg intravenously, with fentanyl, 25 to 100 μg, is common practice. For older patients, or if there is a risk of respiratory compromise, a dexmedetomidine infusion starting at 0.2 to 1 μg/kg per hour after a loading dose of 0.25 to 0.5 μg/kg per hour over 10 minutes can be helpful because it provides sedation and analgesia without affecting respirations. Institutional guidelines for conscious sedation typically govern these practices, although frequently, only local anesthesia is required for uneventful coronary procedures. Light-to-moderate sedation could be beneficial to some patients, particularly for selected complex angiographic imaging and interventional procedures and to reduce the radial artery spasm. Deep sedation, in addition to its widely recognized potential to cause respiratory difficulties, poses distinct problems in the CCL. Deep sedation often requires supplemental oxygen, which complicates the interpretation of oximetry data and may alter hemodynamics.
Sparse data exist regarding the effect of sedation on hemodynamic variables and respiratory parameters in the CCL. One study examined the cardiorespiratory effects of diazepam sedation and flumazenil reversal of sedation in patients in the CCL. A sleep-inducing dose of diazepam that was administered intravenously in the CCL produced only slight decreases in mean arterial pressure, PCWP, and LV end-diastolic pressure (LVEDP), with no significant changes in intermittently sampled arterial blood gases. Flumazenil rapidly reversed the sedative effects without significant alterations in hemodynamic or respiratory variables.
More complex interventions have resulted in longer procedures. Although hospitals require conscious sedation policies, individual variation in the type and degree of sedation is common. General anesthesia is rarely required for coronary procedures, but it is frequently used for percutaneous valve procedures (eg transcatheter aortic valve, MV replacement), atrial septal defect (ASD) closure, and aortic endografts. Advancements in intracardiac echocardiography have decreased the need for intubation and transesophageal echocardiography (TEE) in certain patients and procedures. Pediatric procedures require general anesthesia more commonly than those in adults. As the frequency of noncoronary procedures increases, the presence of an anesthesiologist in the CCL will be required more often.
Left-sided heart catheterization (LHC) traditionally has been performed by means of a brachial or femoral artery approach. In the 1950s, the brachial approach was first introduced, using a cutdown with brachial arteriotomy. The brachial arteriotomy is often time-consuming, can seldom be performed more than three times in the same patient, and has greater complication rates. This led operators to adopt the femoral approach, which became almost universally accepted. The percutaneous radial artery approach was later developed to improve patient comfort and reduce vascular complications, but its use remained relatively stagnant for more than 10 years. The percentage of procedures performed via the radial approach in the United States is increasing rapidly. Transulnar access has been reported to be safe and have comparable outcomes to transradial. Another recent alternative to the regular transradial access is the dorsal distal radial artery. Over the most recently reported 6-year time period, there was a 13-fold increase in radial artery PCI, with wide geographic variation. This approach may be preferred in patients with significant lower extremity peripheral artery disease, recent (<6 months) femoral or abdominal aortic surgery, significant HT, coagulopathy, morbid obesity, advanced age, female sex, or an initial cardiac presentation of an ACS.
The placement of an intraarterial sheath into the radial artery is similar to the placement of devices into any other artery except that the artery has a much smaller caliber and is prone to spasm. Some operators recommend performance of an Allen or a Barbeau test before the procedure to assess for adequate contralateral blood flow into the hand from the ulnar artery. This is an area of controversy because the fundamental question is whether the result of either test is predictive of hand ischemia when radial artery occlusion occurs. Case series of patients with abnormal collateral flow from an absent or diminutive ulnar artery who underwent radial artery harvesting for use as bypass conduits have reported no postoperative hand ischemia. Therefore, because of the lack of outcome data demonstrating the predictive value of testing for dual circulation, some operators have moved away from the routine use of such tests.
Because of the unique characteristics of the radial artery, special dedicated hydrophilic sheaths and access kits are routinely used when gaining access into this arterial bed. Once access has been achieved, to reduce the incidence of radial artery occlusion, intraarterial medications including some combination of nitroglycerin (NTG), a calcium channel blocker (verapamil, diltiazem, or nicardipine), and lidocaine are administered. Most of the time, this combination is physician specific, but at times, the patient’s hemodynamic status dictates the medical regimen. For example, verapamil and diltiazem should be avoided in the setting of bradycardia, and NTG and nicardipine may be avoided in the setting of hypotension. In addition, the patient who is undergoing a diagnostic heart catheterization from the radial approach requires the use of parenteral anticoagulation with either unfractionated heparin (UFH) or bivalirudin, which have been shown to decrease the rate of radial artery occlusion. The recommended regimen is intraarterial or IV UFH at a dose of at least 50 units/kg or 4000 to 5000 units. In those patients with heparin-induced thrombocytopenia, the recommendation is to use a bivalirudin bolus dose of 0.75 mg/kg. ,
The development of dedicated catheters for engagement of the coronary arteries when using a radial artery approach has greatly reduced the procedure time. In addition, advances in the management of sheath removal have improved the rates of radial artery occlusion. The current recommendation is to remove the sheath immediately after completion of the procedure and to place a hemostatic compression device, which prevents hematoma formation at the access site but allows for patency of the vessel. Patency of the radial artery can be assessed by evaluating for a radial artery pulse just distal to the compression device. This technique has been called patent hemostasis. When it is used properly, the rates of radial artery occlusion are on the order of 1% to 5%.
There are relatively few contraindications to performing a radial artery procedure: patients who require support devices or other devices that are not compatible with sheaths smaller than 7 Fr, patients with known congenital or noncongenital vascular anomalies of the upper limb, patients who require dialysis fistulas, patients who require use of the radial artery as a conduit for CABG surgery, and patients with known peripheral vasoocclusive disease, including thromboangiitis obliterans (Buerger disease) and Raynaud disease.
The main advantage of the radial artery approach is that it minimizes vascular complications in patients presenting with ACS, because many of these patients are treated with aggressive anticoagulant and antiplatelet therapy before vascular access is attempted. The RIVAL and RIFLE-STEACS trials both demonstrated lower vascular complication rates and evidence to suggest an overall mortality benefit for the radial approach compared with a transfemoral approach. , As the adoption of the radial approach continues to increase worldwide, it is important for anesthesiologists to appreciate the unique features of access and hemostasis with this approach compared with the more traditional transfemoral approach.
The percutaneous femoral artery approach is performed using catheters that allow for operator ease and speed of performance. The landmarks for the percutaneous femoral approach are illustrated in Fig. 2.2 . Recently, the use of ultrasound-guided punctures has become an important resource to minimize complications.
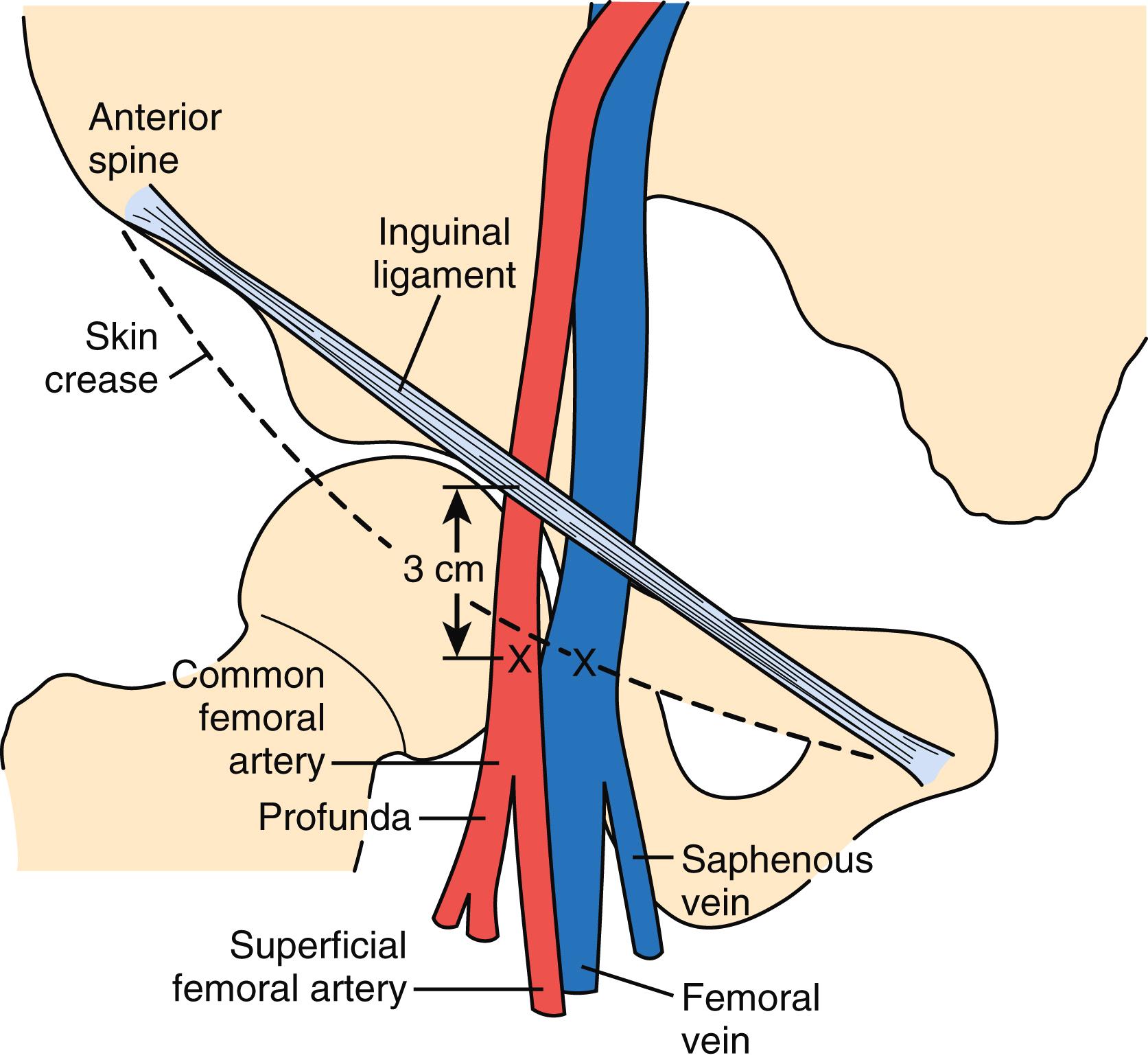
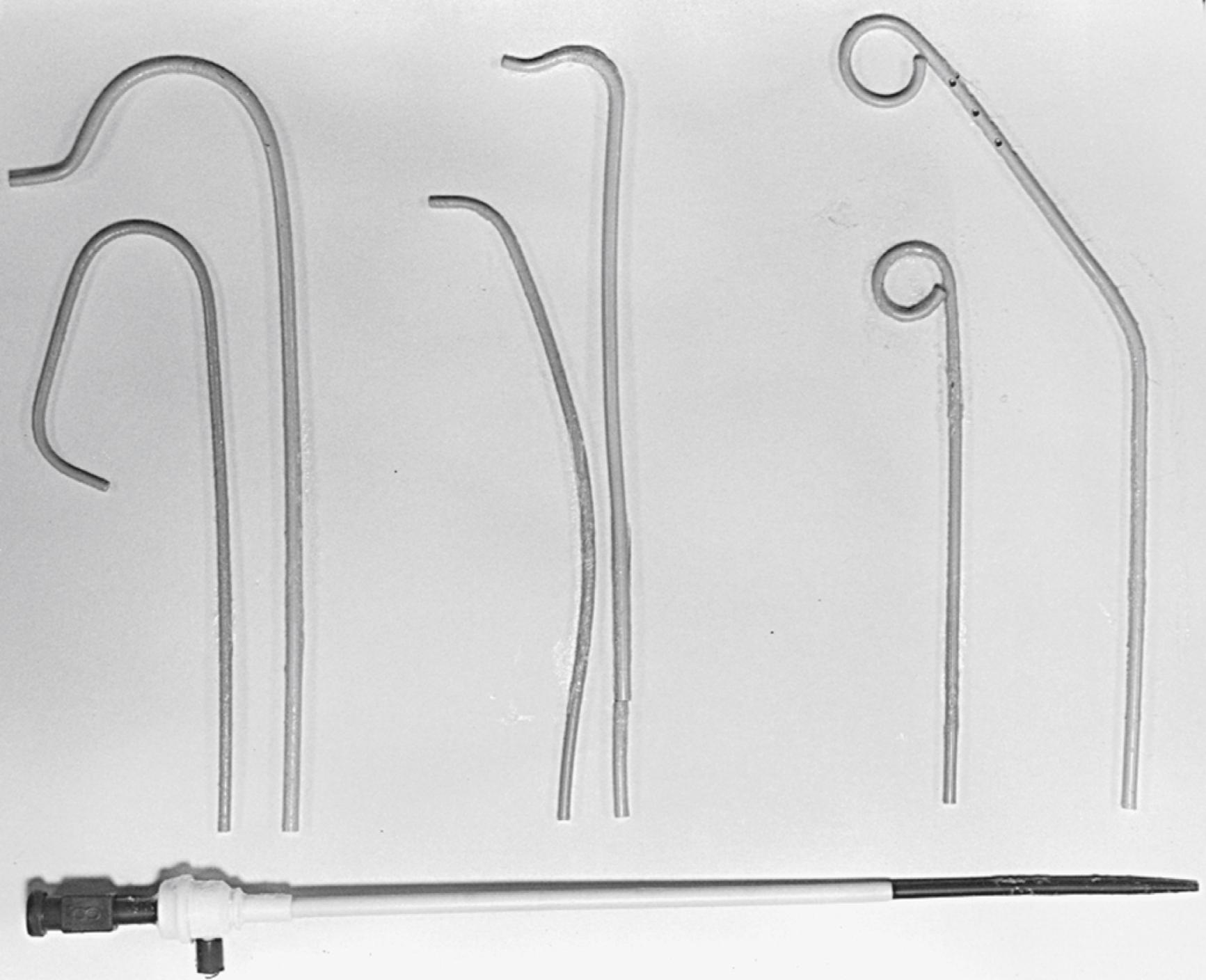
The percutaneous approach uses the Seldinger technique or modifications thereof with a Cook needle, which does not have an internal obturator. Once the wire is successfully inserted into the vessel, standard sheaths (4 to 8 Fr) are placed in the femoral artery. Through these sheaths, separate coronary artery catheters are inserted to perform left and then right coronary cineangiography, and left ventriculography is performed with the use of a pigtail catheter. These standard catheters and a sheath are illustrated in Fig. 2.3 .
In patients with synthetic grafts in the femoral area, arterial access is possible after the grafts are a few months old, and complication rates are similar to those seen in patients with native vessels. An additional problem may be encountered with aortofemoral grafts. If the native iliac system or distal aorta is occluded, it can be a challenge to advance the catheters through the bypass conduit, and a radial approach should be strongly considered.
At the completion of catheterization via the femoral approach, a closure device may be inserted. If so, femoral arteriography typically is performed via the sheath to assess the adequacy for use of the device. If hemostasis will be obtained with manual compression, the patient is returned to the preprocedure/postprocedure holding area for sheath removal. If an RHC is performed, arterial and venous sheaths should be removed separately to avoid the formation of an atrioventricular (AV) fistula. Pressure is then applied manually or by a compression device. The duration of bed rest depends on the size of the sheath. Closure devices provide for more rapid hemostasis after the procedure, allowing for earlier ambulation and discharge. However, complication rates have not decreased with use of these devices. Closure devices include collagen plugs placed within the artery, which require avoidance of the site for repeat puncture for 3 months, external arterial or subcutaneous (SC) plugs that do not hinder repeat access, and suture devices that perform percutaneous arteriotomy closure.
Once hemostasis has been achieved, access site checks and distal pulse assessments should be performed on a regular basis. Sandbag placement is seldom used. In most outpatient diagnostic studies, patients are ambulatory and ready to be discharged 2 to 4 hours after the procedure.
In contemporary practice, routine anticoagulation for diagnostic procedures from the femoral approach often is omitted because of the limited arterial access times, unproven need for anticoagulation, and risks for reversing anticoagulation and/or potential delay in sheath removal. If a sheath is to be left in place for longer than 30 to 60 minutes (i.e., to confer about management or to transfer a patient), then anticoagulation is recommended. Heparinization is used routinely during brachial or radial catheterization to prevent thrombosis of the smaller arm arteries that may be obstructed by the sheath, as discussed earlier.
Adverse reactions have been the major disadvantage of the ionic contrast agents since their introduction for urinary tract visualization in 1923. Contrast agents used for cardiovascular imaging are divided into two groups according to whether they dissociate into ionic particles in solution (ionic media) or do not dissociate (nonionic media). The ionic agents were the first group developed, with sodium diatrizoate and iothalamate anions as the iodine carriers. Commercially available agents using meglumine and sodium salts of diatrizoic acid include Renografin, Hypaque, and Angiovist. In 1975, a prospective survey of 30 university hospitals in the United States, Canada, Europe, and Australia involving 112,003 patients was reported, in which ionic contrast agents were used for cardiovascular diagnosis. The overall rate of adverse reactions was 5.65%, with 0.02% of patients having severe reactions, including death in eight patients.
The next generation of contrast agents began to impact clinical practice in the 1980s. These agents, listed in Table 2.1 , are predominantly monomeric, nonionic agents with the exception of the two dimers: ioxaglate (ionic) and iodixanol (nonionic). These agents, particularly the nonionic dimer iodixanol, have lower osmolarity and potentially lower systemic toxicity.
| Product a | Type of Contrast Agent | Concentration (mg/mL) | Osmolality (mOsm/kg water) |
|---|---|---|---|
| Monomers | |||
| Iohexol (Omnipaque) | Nonionic LOCM | 350 | 844 |
| Iopamidol (Isovue) | Nonionic LOCM | 370 | 796 |
| Ioxilan (Oxilan) | Nonionic LOCM | 350 | 695 |
| Iopromide (Ultravist) | Nonionic LOCM | 370 | 774 |
| Ioversol (Optiray) | Nonionic LOCM | 350 | 792 |
| Dimers | |||
| Iodixanol (Visipaque) | Nonionic IOCM | 320 | 290 |
| Ioxaglate (Hexabrix) | Ionic LOCM | 320 | 600 |
a Omnipaque and Visipaque are registered trademarks of Nycomed, Zurich, Switzerland. Isovue is a registered trademark of Bracco Diagnostics, Princeton, NJ. Oxilan and Hexabrix are registered trademarks of Guerbet, Villepinte, France. Ultravist is a registered trademark of Berlex Laboratories, Wayne, NJ. Optiray is a registered trademark of Mallinckrodt Medical, St. Louis, MO.
Several areas must be discussed when comparing ionic and nonionic contrast agents. First, the ECG effects (transient heart block, QT and QRS prolongation), depression of LV contractility, and systemic hypotension from peripheral vasodilation are more pronounced with the ionic agents but statistically only marginally different from the effects of the nonionic compounds. The hemodynamic effects of the nonionic dimer, iodixanol, were compared with those of the nonionic monomer, iohexol, in 48 patients; although both agents caused an increase in LVEDP, the increase was significantly less in the iodixanol group. In addition, iodine content may vary among agents, resulting in variations in opacification. In patients who have had previous anaphylactoid reactions to iodinated contrast, nonionic contrast decreases the incidence of an anaphylactoid reaction with repeat contrast exposure. Finally, the nonionic agents and dimers are more expensive than the ionic agents. When these agents were first introduced, this difference was large and slowed the adoption of the newer agents. Current price differences are less dramatic, and nonionic agents are used in most laboratories.
Both ionic and nonionic agents have anticoagulant and antiplatelet effects, these being more pronounced with ionic agents. A comparison of the nonionic agents iohexol (monomer) and iodixanol (dimer) with the ionic dimer, ioxaglate, demonstrated a clear distinction, with the in vivo antiplatelet effect of the ionic agent 65% greater than that of the nonionic agents. Regardless of the agent used, these differences are unlikely to be important for diagnostic procedures. Although minute thrombi may form when blood and nonionic contrast remain in a syringe, clinical sequelae have not been reported.
Patients with impaired renal function (Cr >1.5 mg/dL; GFR <60 mL/minute), particularly if they are diabetic, have an increased risk of renal impairment after contrast administration. The effects of contrast agents on the kidneys are more pronounced when larger volumes are delivered near the renal arteries. Therefore, the choice of contrast is most important with arteriography of the renal arteries or the abdominal aorta. Abdominal arteriography can be done with digital subtraction techniques and the intraarterial injection of gaseous carbon dioxide, avoiding the use of any iodinated contrast.
Two large, multicenter trials have compared ionic and nonionic agents in patients undergoing cardiovascular diagnostic imaging. One involved 109,546 patients in Australia, and the other involved 337,647 patients in Japan. These studies demonstrated severe adverse reactions in 0.9% and 0.25% in those patients exposed to ionic agents, respectively, whereas the rates of severe adverse reactions in patients exposed to nonionic agents were 0.02% and 0.04%, respectively. For intervention procedures, one trial compared the iso-osmolar nonionic dimer, iodixanol, with the ionic dimer, ioxaglate, in 856 PCI patients at high risk and reported a 45% reduction in major adverse cardiac events (MACEs) in the iodixanol group. Iodixanol (Visipaque) has also been compared with low-osmolar contrast agents in attempts to limit nephrotoxicity, with mixed results.
Minimizing the use of contrast is the surest way to limit nephrotoxicity. For patients at greatest risk, this might require that procedures be staged; for instance, a diagnostic study may be performed on one day and an interventional procedure at a later date. An additional concern is that iodinated contrast is administered frequently for other purposes, such as CT. If staging of procedures or repeat contrast administration is required, delaying these additional studies for 72 hours or until renal dysfunction has recovered is recommended.
The Cournand catheter initially was used to measure right-sided heart pressures but required fluoroscopic guidance for placement. The Cournand catheter permitted the measurement of CO by the Fick method. Clinical applications of right-sided hemodynamic monitoring changed greatly in 1970 with the development of the flow-directed, balloon-tipped, pulmonary artery catheter (PAC) by Swan and Ganz. This balloon flotation catheter allowed the clinician to measure pulmonary artery (PA) and wedge pressures without fluoroscopic guidance. It also incorporated a thermistor, making repeated measurements of CO feasible. With this development, the PAC left the CCL and entered both the OR and the intensive care unit.
In the CCL, RHC is performed for diagnostic purposes. The routine use of RHC during standard LHC was studied by Hill and associates. Two hundred patients referred for LHC for suspected CAD also underwent RHC. This resulted in an additional 6 minutes of procedure time and 90 seconds of fluoroscopy time. Abnormalities were detected in 35% of the patients; however, management was altered in only 1.5% of the patients. With this in mind, routine RHC cannot be recommended. Box 2.4 outlines acceptable indications for RHC during LHC.
Significant valvular pathology
Suspected intracardiac shunting
Evaluation of right- and/or left-sided heart failure
Evaluation of pulmonary hypertension
Severe pulmonary disease
Evaluation of pericardial disease
Constrictive pericarditis
Restrictive cardiomyopathy
Pretransplantation assessment of pulmonary vascular resistance and response to vasodilators
Some structural procedures (i.e., Triclip, Tric valve)
CO measurements during RHC using the thermodilution technique allow for a further assessment of ventricular function. This obviously is helpful in the setting of an AMI to identify high-risk groups and to measure the effects of cardiac medications. Measurement of CO can differentiate high-output failure states (e.g., hyperthyroidism, Paget disease, beriberi, anemia, AV malformations, AV fistula) from those occurring secondary to a low CO. In patients with congenital heart disease, RHC allows for measurement of oxygen saturation in various cardiac chambers and calculation of intracardiac shunting. In patients with ASD, the right-sided heart catheter passes through the defect into the left atrium (LA), allowing for complete saturation and pressure measurements. The thermodilution technique cannot be used to measure CO in the setting of intracardiac shunting; in such cases, the Fick method must be used. With significant tricuspid regurgitation or a very low CO, the Fick method provides a more accurate measurement of CO and is preferred. Pharmacologic therapy for pulmonary HT has become more effective, and RHC is used to confirm the diagnosis of pulmonary arterial hypertension (PAH) and differentiate it from pulmonary venous HT. Knowledge of the response of PAH to nitric oxide, adenosine, or a vasodilator is helpful for the cardiologist to determine optimal therapy, so these agents are occasionally administered during an RHC. , The widespread use of echocardiography has limited the need for RHC.
The brachial, femoral, and internal jugular venous approaches are the approaches most commonly used for RHC in the CCL. The brachial approach for RHC may be done percutaneously or by means of a venotomy. One pitfall in the brachial approach is identification of the proper vein for insertion. The basilic and brachial veins are preferable, whereas the cephalic vein on the radial aspect of the arm is tortuous in the axilla and should be avoided for catheter insertion. When the left brachial (or left internal jugular) approach is considered, the operator must be aware of the possibility of an anomalous left-sided superior vena cava (SVC) that empties into the coronary sinus, hindering catheter passage into the right ventricle (RV). Whenever the peripheral arm veins are entered, the catheter or sheath must be moist and inserted quickly to decrease venous spasm.
The femoral approach for PAC insertion is performed under fluoroscopic guidance using one of two approaches. The catheter can be advanced against the lateral wall of the atrium, creating a loop in the RA, after which the balloon is inflated and advanced across the tricuspid and pulmonic valves to the PCWP position. Or the catheter can be passed from the RA into the RV; with clockwise rotation and balloon inflation, the catheter then enters the pulmonary outflow tract and is advanced into the PA and PCWP positions.
Although it is common to obtain oxygen saturation values from the PA during RHC, a complete oxygen saturation assessment is required in patients with suspected left-to-right shunts. In the adult population, ASDs and postinfarction ventricular septal defects (VSDs) are the most common left-to-right shunts requiring identification. In these patients, 0.5 to 1.0 mL of blood is obtained from the following locations: the SVC and the inferior vena cava (IVC); high, middle, and low portions of the RA; the right ventricular apex and outflow tract; and the main PA (rarely, right and left PA). These saturations are obtained on entry with the PAC, and repeat sampling is done during pullback if the data are ambiguous. The samples must be obtained in close temporal proximity to avoid systemic factors affecting oxygen saturation (eg hypoventilation). A step-up in saturation identifies the level at which the shunt is occurring. Right-to-left shunts are suspected when the arterial blood is not fully saturated even with maximal oxygen supplementation; this must be differentiated from intrapulmonary shunting.
Pulmonic and systemic flows are calculated as modifications of the Fick equation for CO determination. The Fick equation states that the CO can be calculated as the oxygen consumption divided by the arteriovenous oxygen difference. It is important that measurements be made during steady-state conditions. The
ratio is calculated for patients with left-to-right shunting by the following equation:
= (Sa o 2 –
)/(Spv o 2 – Spa o 2 ) where
is pulmonary flow,
is systemic flow, Sa o 2 is systemic arterial oxygen saturation,
is mixed venous oxygen saturation, Spv o 2 is pulmonary venous oxygen saturation, and Spa o 2 is PA oxygen saturation. The pulmonary and systemic flows are measured in L/minute and the oxygen saturations in mL/L.
In the presence of an RA step-up, an estimated resting
sample is obtained by the following weighted average:
Saturation values are measured in high and low regions of the SVC and IVC and are normally the same. If anomalous pulmonary venous drainage is present, regional differences in saturation in either the SVC or the IVC may occur. Calculation of the
ratio does not require measurement of oxygen consumption (mL/minute), and it can be calculated with any stable level of oxygen supplementation. However, calculation of the absolute values of pulmonary and systemic flow does require this measurement, which can be complicated if supplemental oxygen is required.
Correction of the defect is required if the
ratio is greater than 2 but is unnecessary if it is less than 1.5. Ratios between 1.5 and 2.0 require additional confirmatory evidence and clinical assessment before a decision to intervene can be made.
The following example demonstrates the calculation of left-to-right shunting in a patient with an ASD given the following oxygen saturation values: IVC = 68%; SVC = 60%; mid-RA = 77%; mid-RV = 77%; Spa o 2 = 77%; and Sa o 2 = 92%.
Significant bidirectional or right-to-left shunting is unusual in adult patients. These shunts occur in the setting of congenital heart disease, typically after the development of pulmonary arterial disease. As more children with corrected or partially corrected congenital heart disease reach adulthood, the likelihood of encountering an adult with a complicated shunt will increase. These encounters may be additionally complicated by the development of adult cardiology problems, mainly CAD. However, about 25% of the population has a patent foramen ovale (PFO), and right-to-left shunting through the PFO with systemic oxygen desaturation can occur if the RA pressures become increased. This may occur after pulmonary embolism or after an RV infarction, among other causes.
Calculation of bidirectional shunting involves determination of the effective blood flow (
).
represents the flow if no right-to-left or left-to-right shunting existed. Right-to-left shunting is equal to
, and left-to-right shunting is equal to
. The following formulas are derived from the Fick equation for CO:
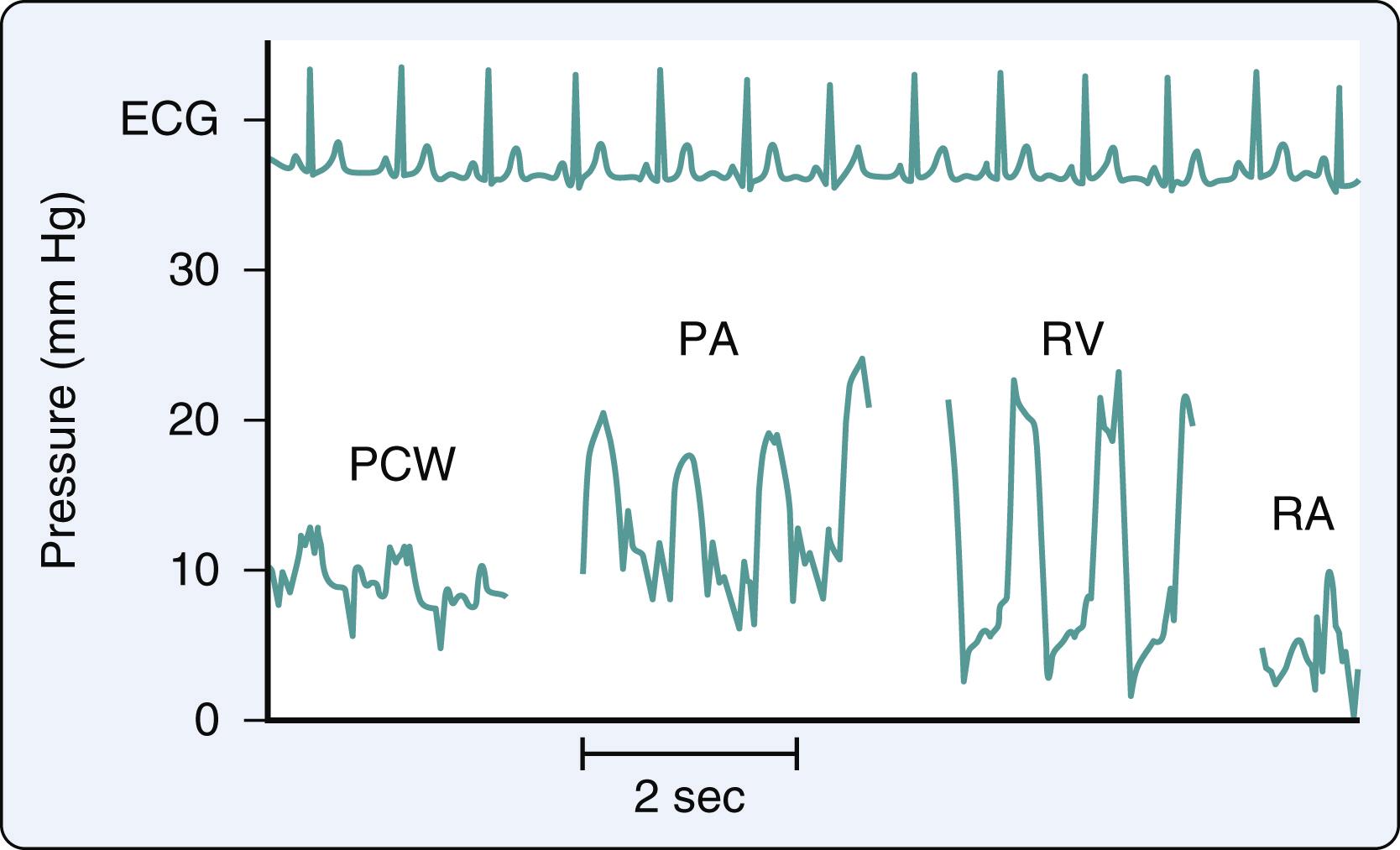
Right-sided heart pressure may be obtained either on entry or on pullback ( Fig. 2.4 ). Catheter placement via the femoral approach may be time-consuming, with expedited passage necessary to prevent catheter softening. For this reason, pressure measurements often are obtained during catheter pullback to ensure temporal proximity. As with all invasive procedures, complications can occur with RHC, so risks and benefits must be assessed in advance. If general anesthesia is required, the fraction of inspired oxygen (F io 2 ) is reduced to approximately 25% or lower when checking the saturations in different chambers of the heart. Higher F io 2 during the procedure may change the pulmonary vascular resistance measurements.
Endomyocardial biopsy is the most reliable method to detect rejection in the transplanted heart. However, its role in the management of other cardiovascular diseases in adult and pediatric patients remains controversial. In 2007, the ACC/AHA/ESC published recommendations on endomyocardial biopsy. The preferred approach is through the internal jugular vein (in the United States) or the femoral vein (in Europe), with subclavian and even brachial approaches also used. Complications are infrequent and are related to the access site (in 2% of patients), arrhythmia or conduction abnormalities (1–2%), or perforation (0.5%). Death, a rare event, is related to perforation. Histologic evaluation of the tissue is the purpose of the procedure, and it must be done by experienced pathologists to justify the risks.
Indications are controversial, but most groups agree that important information can be obtained in the setting of new-onset heart failure (<2 weeks) and also for those patients who have had heart failure for 2 weeks to 3 months without response to therapy. Other potential indications include unexplained restrictive cardiomyopathy, anthracycline cardiomyopathy, suspected cardiac tumor, unexplained arrhythmias, and heart failure associated with hypertrophic cardiomyopathy (HCM), but these are less clear.
Although adult diagnostic catheterization with selective coronary cineangiography has been performed since the late 1950s, complication rates were not monitored until 1979, when the SCAI established the first registry to prospectively follow the performance of participating laboratories. In 1982, the first publication from this registry reported complication rates from a study population of more than 50,000 patients. This was updated in 1989 with a report on 222,553 patients who underwent selective coronary arteriography between 1984 and 1987. Similar complication rates were noted in the two reports. Complications are related to multiple factors, but severity of disease is important. Mortality rates are low. Complications are specific for RHC and LHC ( Box 2.5 ). The registry reported incidences of major complications as follows: death, 0.1%; MI, 0.06%; cerebrovascular accident, 0.07%; arrhythmia, 0.47%; contrast reaction, 0.23%; and vascular complications, 0.46%. Infectious complications are infrequent, although they may be underreported. Guidelines for infection control are based more on extrapolation from OR studies than on randomized control data from the CCL. Although advances in technology have been made, similar complication rates persist, most likely because of the higher risk status of patients undergoing catheterization today. The current registries for identifying complications are focused primarily on percutaneous interventions. In addition to institutional and regional databases such as those of the Cleveland Clinic and Northern New England, the ACC maintains the National Cardiovascular Data Registry (NCDR).
Death
Myocardial infarction
Ventricular fibrillation
Ventricular tachycardia
Cardiac perforation
Left bundle brunch block (LBBB)
Complete heart block
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here