Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The autonomic nervous system plays a pivotal role in the control of cardiac function. Cardiac autonomic control occurs through sympathetic, parasympathetic, and sensory neurons that densely innervate the heart and provide tropic responses. The parasympathetic system promotes negative chronotropic (heart rate), inotropic (contractility), and dromotropic (conduction) responses primarily through the release of acetylcholine (ACh). Cholinergic nerves are most abundant in the atria and cardiac conducting system, where stimulation of M2 muscarinic receptors decreases atrial contractility and can slow pacemaking and conduction through the atrioventricular node. Although less abundant in the ventricle, parasympathetic nerves can trigger negative inotropic responses and promote conserved cardiac output. The sympathetic system, on the other hand, initiates rapid increases in cardiac output in response to physiologic stress, that is, the “fight or flight” response. Increased chronotropic and inotropic responses are initiated primarily by the release of norepinephrine (NE) from the sympathetic nerves. NE binds to β-adrenergic receptors (β-ARs) on the cardiac myocyte, which trigger a signaling cascade that affects myocyte calcium (Ca 2+ ) handling and electrophysiology ( Fig. 41.1 ). The spatial and temporal properties of the sympathetic nervous system are key for normal myocyte contractility and electrophysiology, but they may also play a role in arrhythmogenesis. This chapter therefore focuses on the modulation of cardiac electrophysiology via sympathetic mechanisms during normal conditions and in response to sympathetic remodeling.
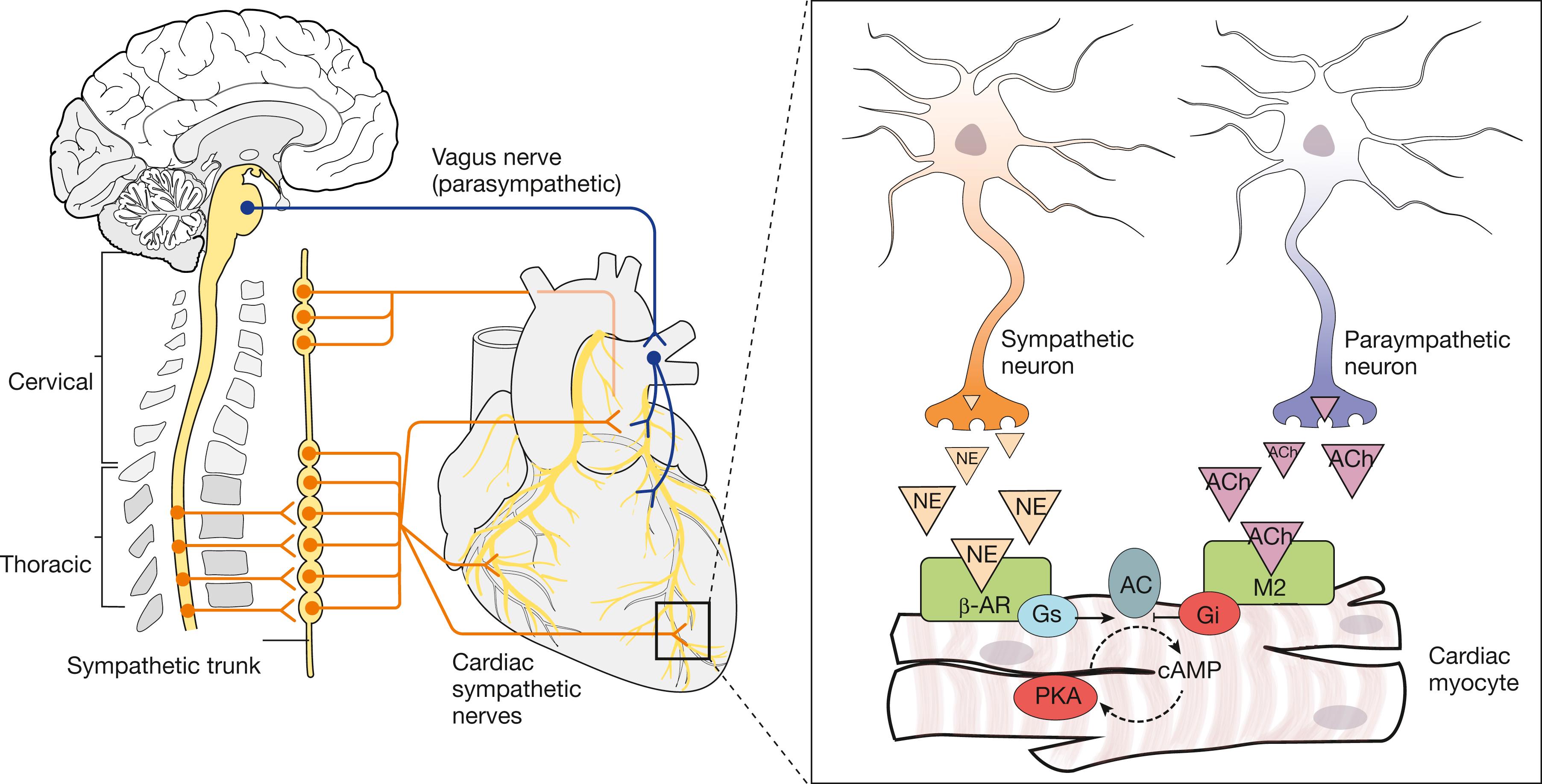
The cardiac nervous system can be divided into two parts, the extrinsic cardiac nervous system (ECNS) and the intrinsic cardiac nervous system (ICNS), and interplay from both components is required for physiologic control. The efferent ECNS consists of peripheral sympathetic and parasympathetic nerves traveling from the central nervous system to the surface of the heart (see Fig. 41.1A ). The extrinsic sympathetic nerves are mostly derived from autonomic ganglia along the cervical and thoracic spinal cord, and parasympathetic innervation originates almost entirely within the vagus nerve. The ICNS is contained within the pericardium and is a complex network composed of ganglionated plexi (GP) and interconnecting neurons. , The GPs are primarily located on the posterior atrial surface and epicardial fat pads and contain cholinergic, adrenergic, and afferent nerve fibers and interconnecting neurons. Intrinsic cardiac nerves extend from GPs to the atria, interatrial septum, and the ventricles. ,
Despite extensive sympathetic innervation, nerve density is not uniform throughout the myocardium. Innervation gradients exist, primarily from base to apex, and from epicardium to endocardium. Sympathetic nerves are most dense in the atria and at the base of the ventricles, with fewer nerves found near the apex of the heart. Greater nerve density in the base of the heart is accompanied by a greater abundance of the sympathetically mediated slowly activating delayed rectifier potassium channels (I Ks ). , Consistent with this innervation and ion channel gradient, we and others have reported that, in the rabbit heart, action potential duration (APD) shortens more at the base compared with the apex during sympathetic stimulation. , Additionally, the epicardial to endocardial innervation gradient accompanies an epicardial to endocardial gradient in APD that is important for normal activation and repolarization of the left ventricle. Experiments in transgenic mouse hearts have demonstrated that disrupting this transmural innervation gradient alters transmural APD distribution and is arrhythmogenic. In the healthy heart, sympathetic neurons closely interact with nearly every cardiac myocyte, and Prando and colleagues have demonstrated, within a single myocyte, selective activation of only those β-AR receptors that were in close contact with nerve varicosities. Thus considering the heterogeneity of nerve density throughout the heart, and within a single myocyte, complex spatiotemporal electrophysiologic and Ca 2+ handling responses may occur.
The sympathetic nerves act on the cardiac myocyte through the release of NE binding to β-AR to modulate repolarization and contractility ( Fig. 41.2 ). Ventricular myocytes express at least two types of β-ARs. β 1 -ARs account for the majority (80%) of receptors expressed in the heart and can initiate a sympathetic response across the myocyte. β 2 -ARs are less densely expressed and are predominately found on the T-tubule membrane where they preferentially colocalize with the L-type Ca 2+ channels, allowing for compartmentalized control and selective phosphorylation. Stimulation of the β-AR signaling cascade facilitates inotropic responses by activation of cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA). PKA phosphorylates several intracellular proteins that mediate excitation-contraction coupling, such as L-type Ca 2+ channels, ryanodine receptors (RyRs), and phospholamban. Phosphorylation contributes to the positive inotropic effects of β-AR stimulation by increased Ca 2+ influx via the L-type Ca 2+ current and increased sarcoplasmic reticulum (SR) content by phospholamban phosphorylation, thus activating sarcoplasmic/endoplasmic reticulum Ca 2+ ATPase (SERCA). Myocyte contraction is relative to the amplitude of the systolic Ca 2+ transient, which is highly dependent on SR Ca 2+ content, thus sympathetic stimulation enables more Ca 2+ release into the cytosol to increase contractility. It has also been suggested that PKA phosphorylation of RyR increases its open probability, , allowing for greater SR Ca 2+ release. This notion is somewhat debated, however, with conflicting results in different experimental conditions. ,
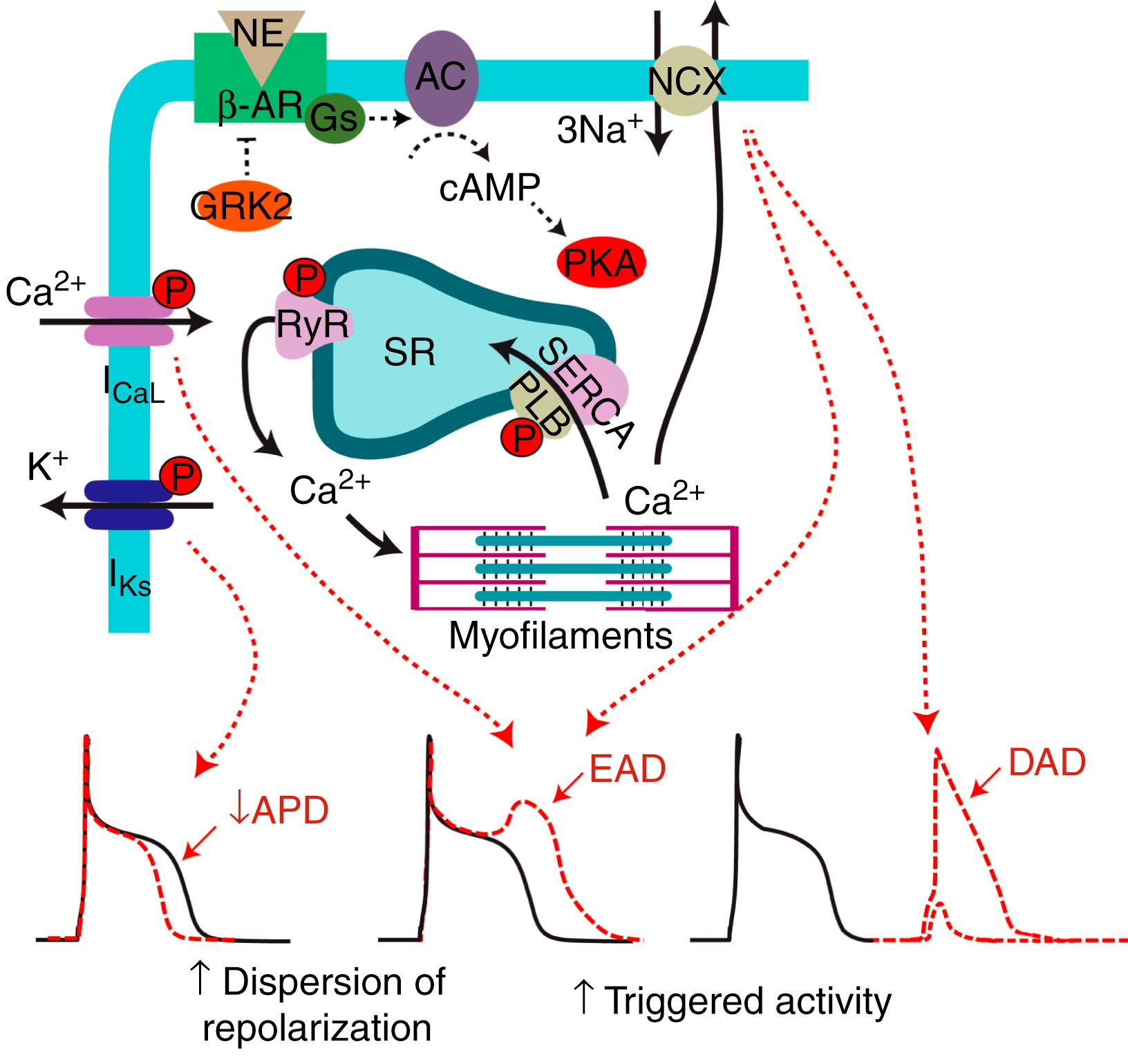
Alongside Ca 2+ handling modulation, adrenergic stimulation also shapes the cardiac action potential (AP) via both α- and β-ARs, the details of which have been reviewed extensively elsewhere. Of these pathways, phosphorylation of the Ca V 1.2 channel by cAMP/PKA signaling causing an increase in L-type Ca 2+ current is best known. Increased Ca 2+ current alone would prolong APD, but as cAMP/PKA signaling also enhances K + currents, AP prolongation is offset and Ca 2+ loading is limited. This counterbalance between currents is achieved by a greater increase in I Ks compared with the rapidly activating potassium channels (I Kr ) after β-AR stimulation. , In larger mammals, increases in K + currents offset increases in L-type Ca 2+ currents to produce APD shortening, which is required for increased heart rates during sympathetic activity. This is not the case in all species, however, and we have recently demonstrated that physiologic sympathetic nerve stimulation in mice results in prolongation of APD, suggesting that increases in the Ca 2+ current dominated. Interestingly, prolongation of APD in mice still led to enhanced Ca 2+ transient kinetics, signifying that different electrophysiologic responses may be required for optimal adrenergic responses across species. Although sympathetic activity exerts positive chronotropic and inotropic responses to increase cardiac output, excessive sympathetic activity can be associated with electrophysiologic abnormalities, triggered activity, and the onset of pathologic ventricular arrhythmias.
Increased risk for cardiac arrhythmia during sympathetic activation can occur via focal (triggered) or reentry mechanisms (see Fig. 41.2 ), and likely a combination of both triggered activity and reentry are involved in initiating and sustaining ventricular arrhythmias. Triggered activity can result from early afterdepolarizations (EADs) or delayed afterdepolarizations (DADs) occurring either during the AP plateau or during diastole, respectively (see Fig. 41.2 ). DADs typically arise in conditions of elevated cellular Ca 2+ where the likelihood of spontaneous SR Ca 2+ release during diastole is increased. Rises in intracellular Ca 2+ activate the electrogenic Na + /Ca 2+ exchanger (NCX) generating a net inward current. If the inward current is large enough, the membrane will depolarize, even during diastole, and a triggered AP occurs. SR Ca 2+ overload can also be associated with EADs, where NCX-driven depolarization leads to afterdepolarizations during phase 3 of the AP. EADs, however, more typically occur when increased L-type Ca 2+ current or reduced K + currents lead to APD prolongation, allowing L-type Ca 2+ current reactivation during the plateau phase of the AP.
Afterdepolarizations in individual myocytes do not necessarily initiate focal arrhythmia in intact tissue, as the resting neighboring myocardium acts as a “sink” for local depolarizing current. This source-sink “mismatch” can be overcome if many cells experience DADs simultaneously to produce sufficient depolarizing current to generate AP propagation. We have shown that localized β-AR stimulation can be one potential mechanism for simultaneous DAD initiation in the healthy heart via synchronization of SR Ca 2+ overload and release, , suggesting that localized release of β-AR agonists can directly induce focal activity. Thus in pathologic conditions where electrophysiologic remodeling is common and sympathetic pathways are heightened, the likelihood of afterdepolarizations and arrhythmia generation is increased. Altered sympathetic signaling can also lead to reentrant arrhythmias via increased dispersion of repolarization caused by nonuniform APD shortening throughout the heart. Heterogeneous remodeling of sympathetic nerves can amplify the dispersion of repolarization, and even in the normal heart, the base-to-apex innervation gradient leads to increased dispersion of repolarization and potential for reentrant arrhythmias during sympathetic stimulation. Therefore in pathologic conditions, such as myocardial infarction (MI), where sympathetic nerve remodeling is more heterogeneous, even greater heterogeneity of APD and repolarization exists, , and therein lies a greater potential for reentrant arrhythmia.
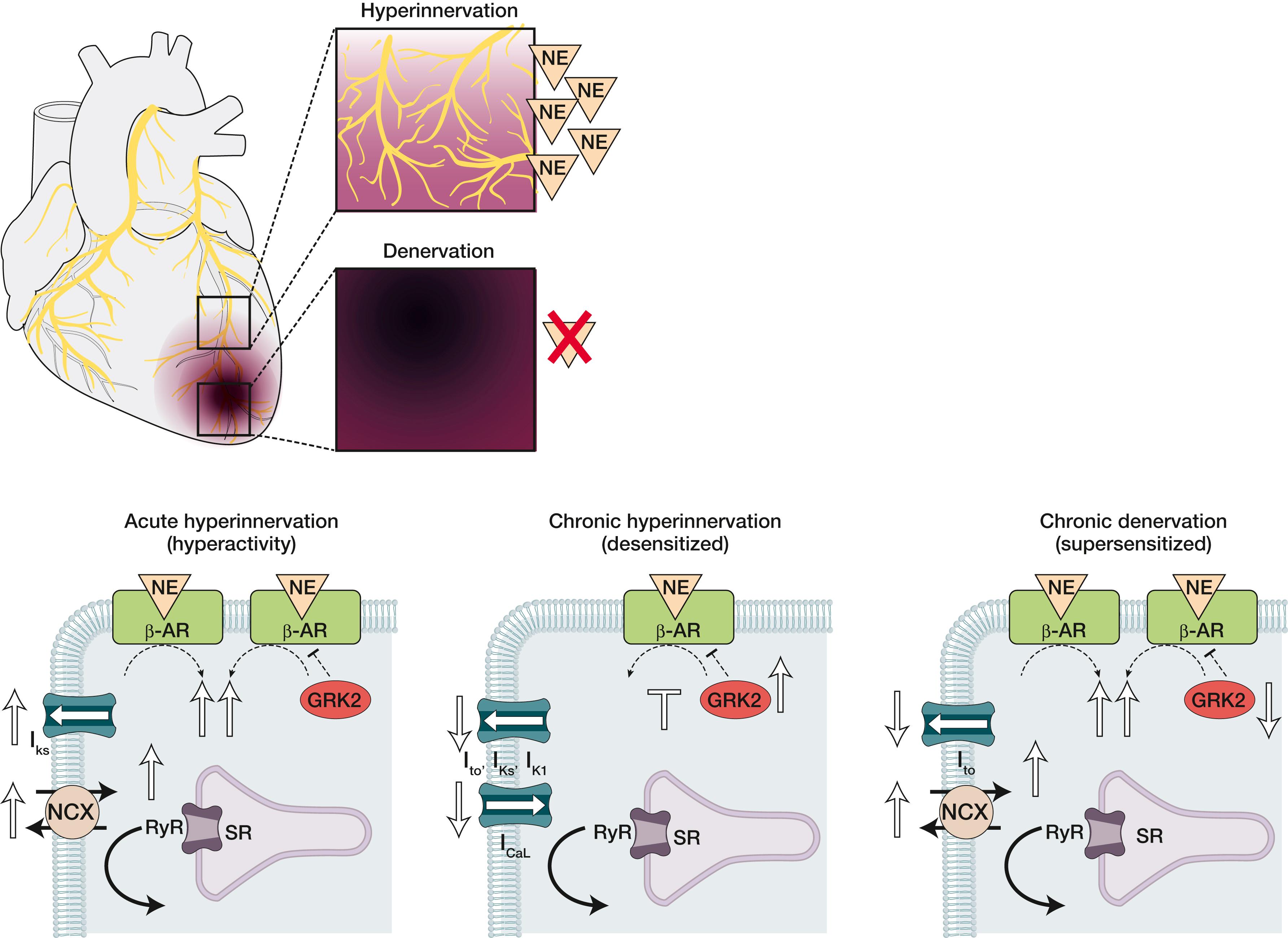
Disruption of the sympathetic nervous system in response to injury and disease increases the risk for the development of ventricular arrhythmia and sudden cardiac death. Increased central sympathetic drive and parasympathetic withdrawal are hallmarks of cardiovascular disease. This sympathetic hyperactivity often occurs alongside disease-related electrophysiologic remodeling, and anatomic and functional nerve remodeling. The next section will take into consideration hyperinnervation, hypoinnervation ( Fig. 41.3 ), and altered neurotransmitter content and release.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here