Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spasticity is defined as a velocity-dependent resistance to passive movement of a joint and its associated musculature and is characterized by hyperexcitability of the stretch reflex. Disorders are related to the failure of descending supraspinal inhibition. Spasticity should not be treated just because it is present, as it may compensate for loss of motor power. Spasticity must be treated only when excess of tone leads to functional impairment, discomfort, pain, and deformities. Functional neurosurgery should be considered when the harmful components of spasticity cannot be controlled by physical therapy, medications, and botulinum toxin injections. The surgical procedures must be performed so that only excess of tone be reduced, without suppressing useful muscular tone or impairing the residual motor/sensory functions. In patients who retain some—often masked—voluntary motility, surgery aims to improve—or restore—voluntary motor function. In patients with poor residual function, the aim is to stop the evolution of orthopedic deformities, reduce pain, and improve comfort.
Neurosurgical methods are classified according to whether their impact is general or focal and whether the effects should be temporary or permanent ( Fig. 113.1 ) . Besides botulinum toxin, methods include intrathecal baclofen (ITB) therapy, as well as lesioning techniques directed to peripheral nerves, dorsal roots (DRs), the spinal cord, or the dorsal root entry zone (DREZ).
Because spasticity features may differ from one patient to another, the first step is to define the objective(s) of the treatment for every patient, in other words what can be and what will not be obtained by surgery. These issues must be discussed within the frame of a multidisciplinary team and explained to the patient, relatives, and caregivers. The final decisions as well as the patient-tailored preoperative chart have to be written down and signed in the informed consent form.
The guidelines given in the present chapter have been developed based on personal surgical experience with more than 1300 adult or children patients operated over the last 30 years.
ITB therapy consists in an intrathecal catheter connected to an implanted pump, which delivers medication in the cerebrospinal fluid (CSF) surrounding the spinal cord ( Fig. 133.2 ) . Baclofen is a γ-aminobutyric acid B analogue, and direct delivery to the intrathecal CSF bypasses the blood–brain barrier. ,
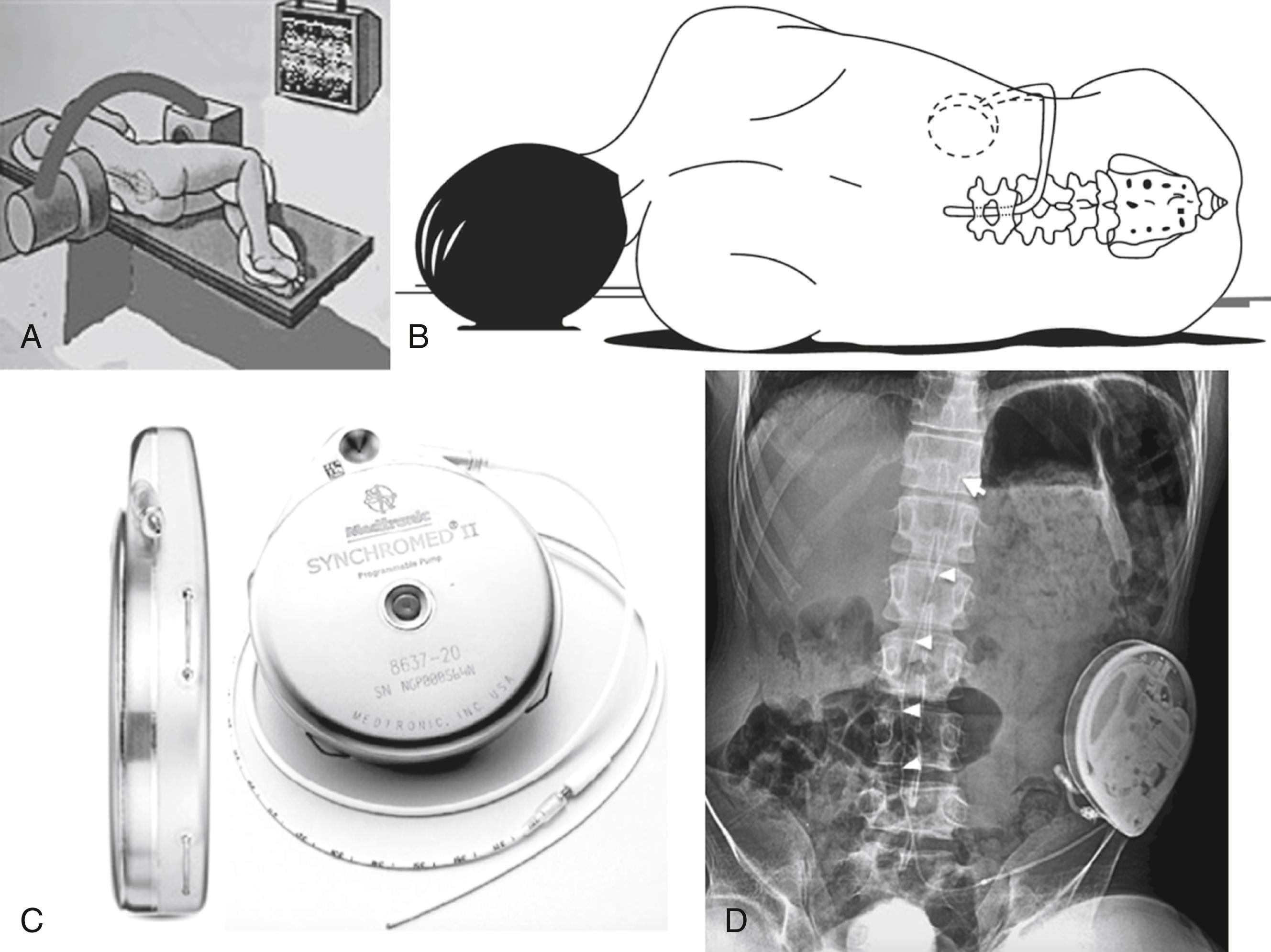
ITB can be preceded by a test to screen for adequate response to the medication. In the standard procedure the patient receives a bolus of 25 to 50 μg of baclofen via lumbar puncture or via a temporary lumbar catheter connected to a subcutaneous access reservoir. In the absence of a positive response, indicated by a two-point reduction in the patient’s Ashworth score 4 to 8 hours following administration, the bolus dose is increased in 25-μg increments up to a maximum bolus of 100 μg. Once a positive response is observed without unacceptable loss of function, the patient is considered to be a candidate for pump implantation. However, the “bolus method” can be misread as “false-negative responses” in the sense that it may produce a brutal or exaggerated loss of motor power, which might be interpreted as a decrease in functional status. This holds especially true for the patients with the ability to walk. Therefore the bolus test should be replaced by a continuous infusion test, using an external automatic injection pump connected to a line implanted into a subcutaneous reservoir of the Ommaya-type. The test should last several days so that functional capabilities can be reliably evaluated. Typically the initial starting dose is double the effective screening dose. The dose is then increased daily by 10% to 30% until the desired effect is achieved. The most useful criterion for dose adjustment is effective suppression of the hyperactive reflexes, such as tendon jerk, clonus, spasms, cramps, and decrease of muscle tone.
For paraplegic or paraparetic patients with clinically typical hyperspasticity or those with handicapping mixed hypertonia in whom the spastic component is predominant, ITB will be effective on tone regulation. A preliminary bolus test of ITB would not be necessary to prove that such a patient will be a “responder.” The patient can undergo implantation directly and doses should be adjusted thereafter. In contrast to that situation, if the indication is not clear, that is, the diagnosis of spasticity is uncertain or involvement of the spastic component in the patient’s handicap is not established, a primary test of ITB should be considered. For patients for whom it is important to evaluate whether a reduction in spasticity will improve function, and to what degree, or on the contrary will weaken functionally useful hypertonia, a preliminary test prior to decision is mandatory. For such an evaluation a continuous infusion test for about one week is much preferable to the bolus method.
Note that whatever the techniques used, lumbar puncture(s) or continuous infusions, CSF depletion or leak may provoke headaches, nausea, and vomiting that confound interpretation until those symptoms disappear.
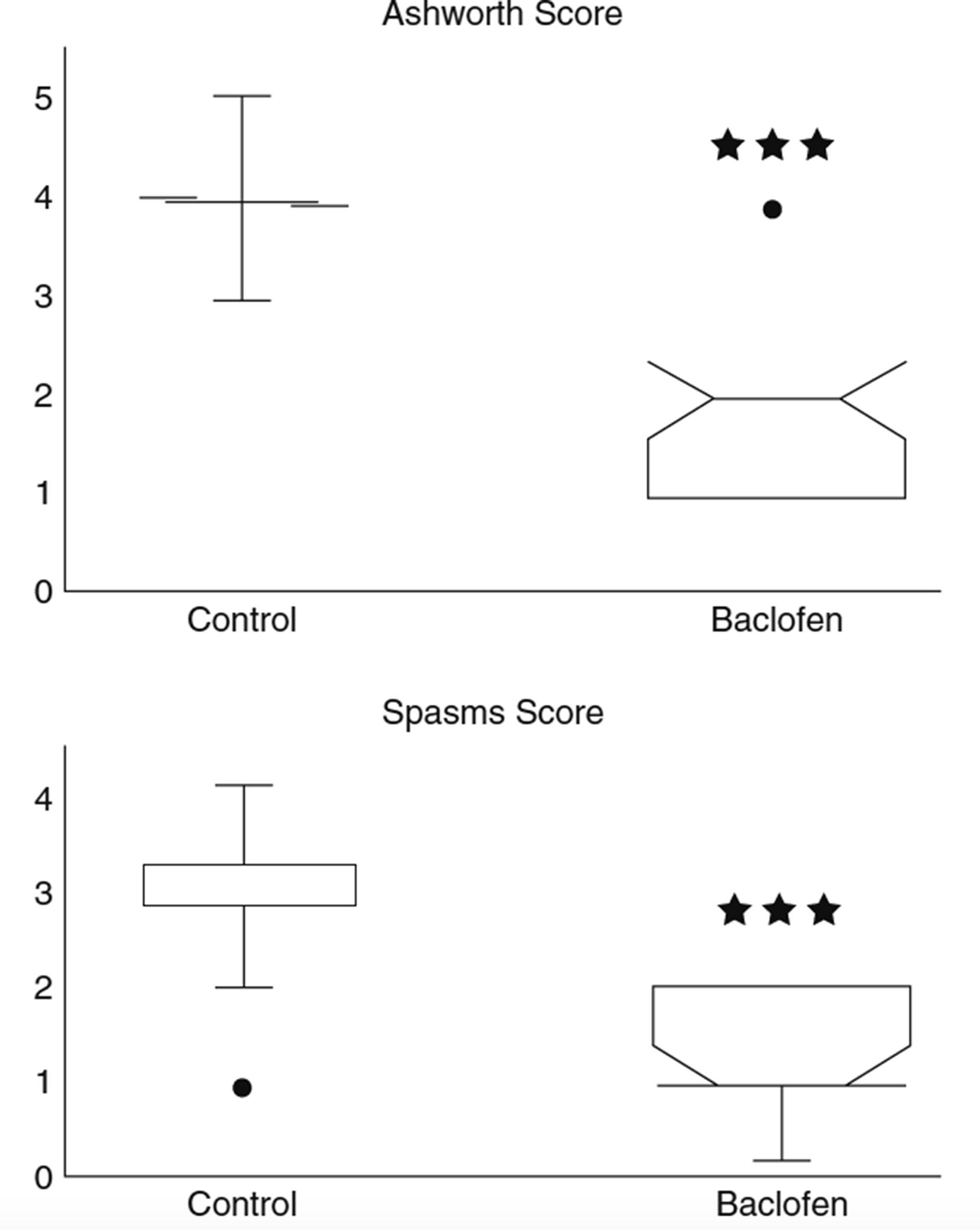
A programmable pump allowing cyclic dose adjustments makes it possible to provide levels that correlate with the daily variability of spastic symptoms. The SynchroMed pump (Medtronic, Minneapolis, MN) is the most frequently used device. According to the largest published series, the ITB dosage varies between 167 and 462 μg/day (average 298 μg/day), a mean Ashworth score decreasing from between 3 and 4 to between 0.5 and 1.8 ( Fig. 113.3 ).
Adverse effects under continuous-infusion mode are frequent but most often transient. They may include drowsiness, dizziness, mental confusion, light-headedness, constipation, urinary retention. These side effects are reversed by decreasing the doses. Muscular hypotonia may also occur, leading to loss of muscular power and capacity to stand or walk in ambulatory patients. Adjusting the doses generally reverses hypotonia to the desirable level. Patients with multiple sclerosis or cerebral lesions are more inclined to present those adverse effects, especially fatigue and confusion.
A potential serious risk of ITB is overdose, which could be irreversible because of lack of true baclofen antagonists. Fortunately, overdosing is infrequent. When it occurs it is rarely due to pump malfunctioning. It may rather be the consequence of inappropriate bolus dose, changes in drug concentration, or misprogramming the pump after reprogramming . Symptoms include weakness with rostral progression, blood pressure changes, respiratory depression, and alteration of consciousness: from somnolence to coma. There is no real antagonizing substance (antidote) of baclofen. However, physostigmine administered intravenously at a dose of 2 mg can reverse respiratory depression and lethargy. In the exceptional situation of respiratory distress, assisted ventilation should be performed on emergency.
Baclofen withdrawal syndrome may happen if the pump is not refilled properly or at the scheduled intervals or in a case of pump or catheter malfunction. Symptoms include rebound motor spasticity and spasms, dysesthetic or itching sensations, headaches, drowsiness, confusion or even hallucinations, seizures, tachycardia, labile blood pressure, and fever. Treatment is readministration of ITB. In life-threatening situations baclofen should be given by lumbar puncture or external catheter. To avoid a withdrawal syndrome even in a mild form, after ITB has been initiated, oral baclofen should be withdrawn gradually over a period of several weeks.
Catheter problems and CSF leaks are the most common complications. Pump malfunction occurs rarely, the latter approximately at 1% per year. CSF leaks may happen; their appearance is more commonly observed in children than in adults, 12% versus 3%, respectively. Infection of the pump pocket and/or of the CSF may develop, in 3% of the patients. Clinical manifestations of infection may not become obvious until weeks or months after implantation.
ITB is particularly indicated for patients with severe spasticity from a spinal cord origin, especially if painful contractions are present, as in advanced multiple sclerosis or after severe spinal cord injury. ITB can also be indicated for spastic quadriplegia due to brain-stem lesions. ITB has also been included in the neurosurgical armamentarium for cerebral palsy (CP) patients.
Recently several studies have reported the use of intraventricular baclofen (IVB) in refractory spasticity or dystonia: IVB may also be the first option in patients with generalized dystonia . , The rationale for the use of IVB therapy is that for the treatment of dystonia the site of baclofen activity may be at the cortical level. As a matter of fact, intraventricular infusion results in a baclofen concentration over the cortex greater than that resulting from intrathecal infusion. When treating generalized dystonia, baclofen would act by inhibiting the stimulation of the premotor and supplementary motor cortex.
Peripheral neurotomy (PN) was introduced as early as 1912 by Stoffel for the treatment of the spastic foot. Then PN was made more selective by using microsurgical dissection and mapping with electric stimulation to better identify the corresponding muscular “response functions” of individual nerve branches or fascicles. PN consists in partial sectioning of one or several motor fascicles corresponding to the muscle(s) harboring spasticity considered excessive, and acts by interrupting the segmental reflex arch at both its afferent and efferent pathways. PN must not involve sensory fibers, as even their partial sectioning could result in paresthesias and neuropathic pain. Empirically it is agreed upon that 50% to 80% of all motor fascicles to the targeted muscle(s) have to be sectioned for PN to be effective. PN aims not only to reduce spasticity but also to improve function by re-equilibrating the tonic balance between agonist and antagonist muscles ( Fig. 113.4 ).
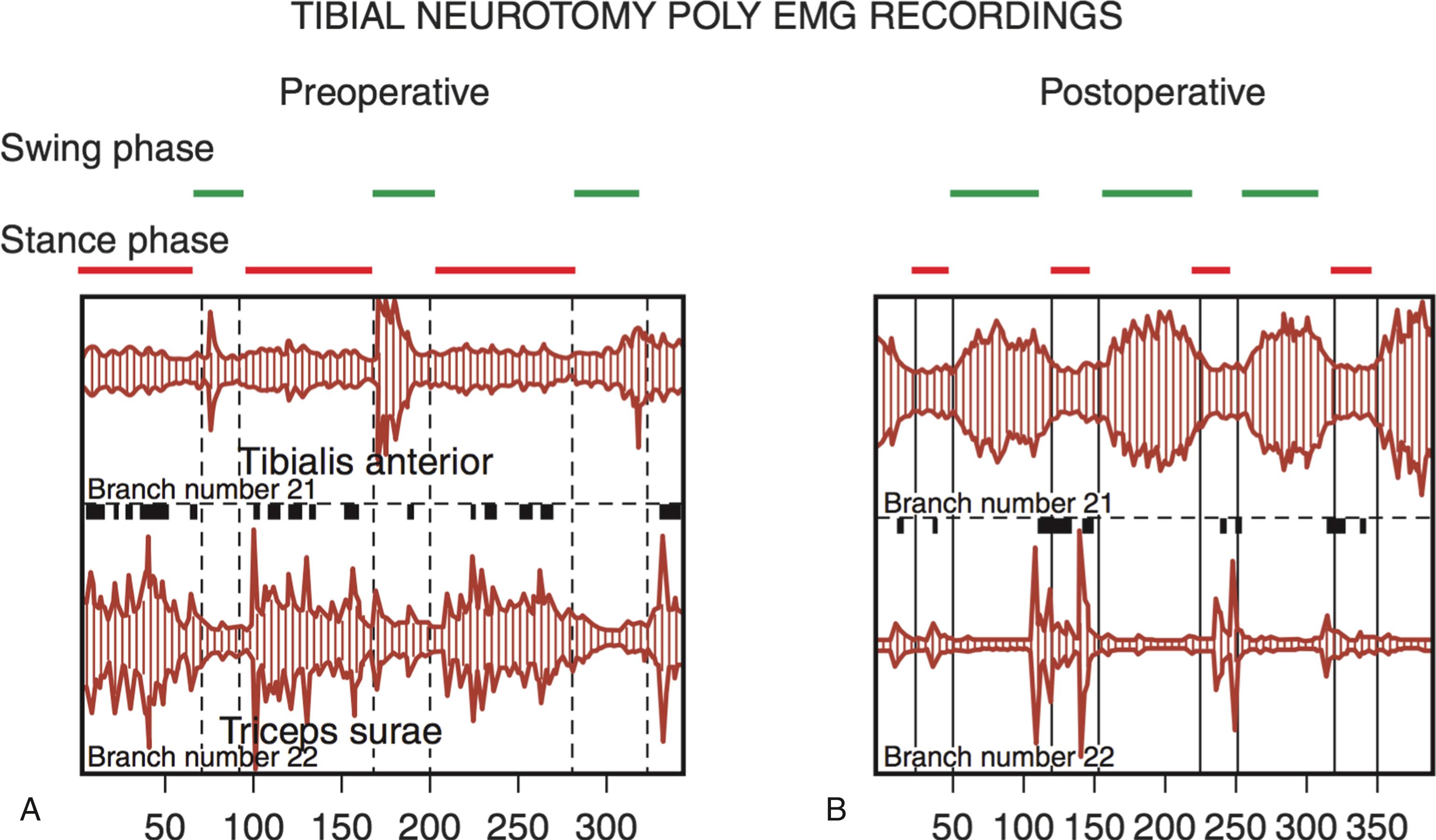
General principles as follows ( Fig. 113.5 ):
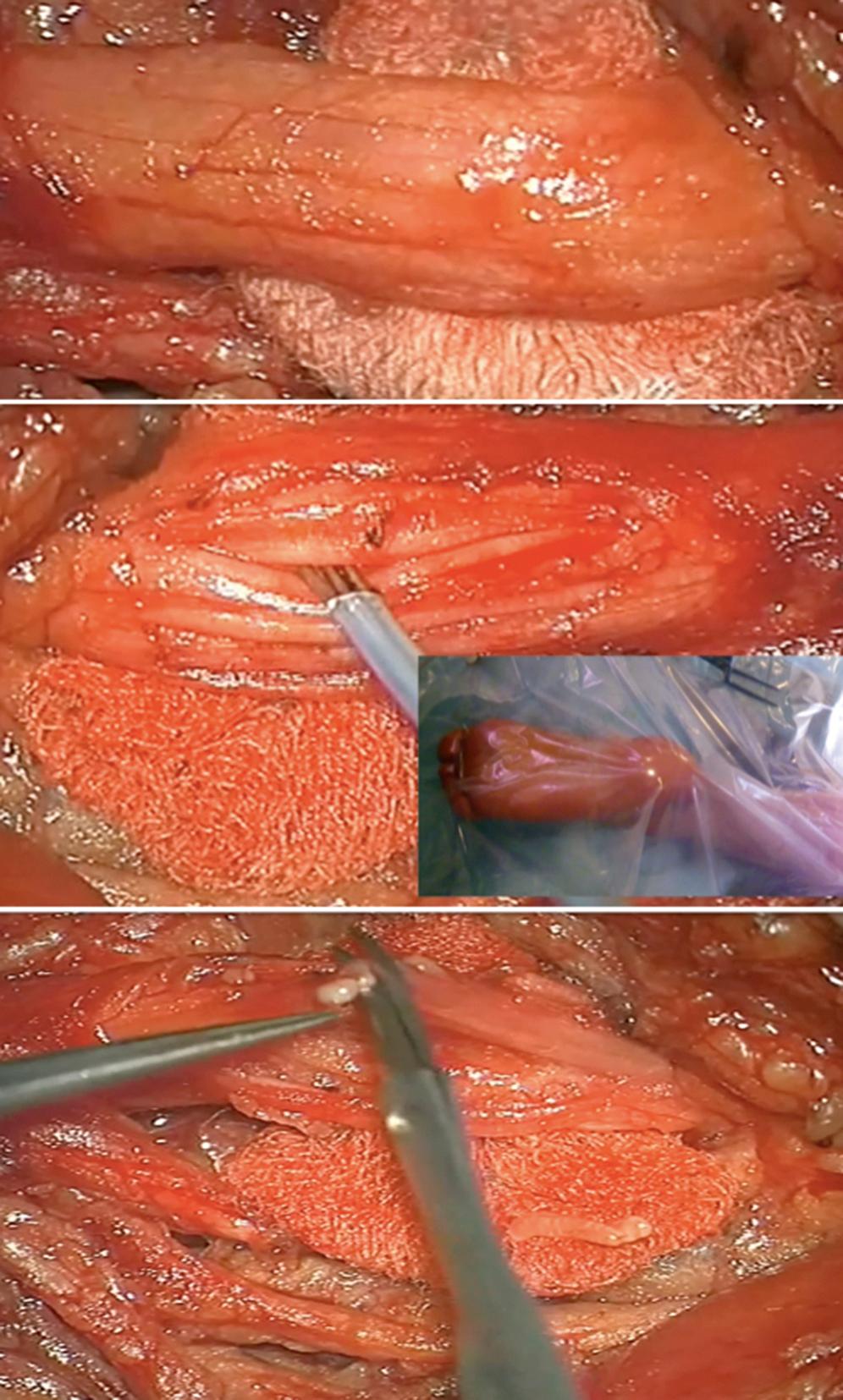
Before recommending PN, local nerve blocks with a long-lasting anesthetic (2 to 3 hours with bupivacaine) can be performed to evaluate the possible hidden motor power of the antagonist muscles and to determine whether the eventual articular limitations result from spasticity or musculotendinous contractures/articular ankyloses. Also botulinum toxin injections can be used as a “prolonged” test for several months, as they mimic the effect of corresponding neurotomy(ies). This strategy of using preoperative tests allows both the patients and their medical team to estimate the benefit that would follow the neurotomies.
General anesthesia must also be induced without long-lasting myorelaxants so that the muscular responses to electric stimulation can be detected. Muscle relaxants must be avoided; nitrous oxide and propofol are contraindicated because they modify reflex excitability.
Use of the operating microscope and individual mapping of branches/fascicles is required because of the frequent variations in the emergence and distribution of the nerve branches. Bipolar stimulation is preferred to monopolar stimulation to avoid spread of the electric current. Stimulation, such as using the Innopsys Nimbus I-Care multifunctional neurostimulator (manufactured by Innopsys Parc d’Activités Activestre, Carbonne, France, www.innopsys.com ), is performed using a frequency at 2 Hz at low intensity (currently 200 μA) to avoid electric diffusion and therefore incorrect interpretation. When fascicles are tightly apposed, a triple stimulation probe composed of an anode between two cathodes is the more accurate way of stimulating because of less current diffusion. The response to stimulation is visualized in the form of clinically observable muscular responses of the corresponding muscles by the surgeon. If considered useful, the surgeon may have recourse to electromyogram (EMG) recordings. Extreme care should be taken to recognize false-positive responses by diffusion of current to neighboring fascicles and false-negative due to short circuits of current caused by serosities between contacts of stimulation probe.
Once all motor branches/fascicles have been identified, those considered to be targeted are marked separately with different-colored threads. According to preoperative program variable proportions (50% to 80% depending on the degree of preoperative spasticity) of the selected motor branches/fascicles are cut. The effect of each interruption is then evaluated by comparing the intensity of the muscular responses to electric stimulation, proximally and then distally to the section. If the response after proximal stimulation is still intense, further sectioning is performed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here