Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Crohn disease (CD) is a chronic inflammatory disease of the intestinal tract with an unknown etiology and an unknown cure. The characteristic transmural inflammation can progress to refractory inflammatory disease, stricturing disease, and fistulizing disease—all potential indications for surgery when medical management has been exhausted. An important tenet to remember is that surgery is not curative but is rather an adjunct to maximal medical therapy. Thus bowel preservation is imperative because up to two-thirds of patients will require subsequent operations in their lifetime.
Because there is no cure for CD, medical management is used with the intent of symptom control and, ideally, maintaining disease remission. With such vast number of medications now approved for the treatment of CD, management can be compartmentalized by phenotype and severity of disease. The three classic phenotypes include inflammatory, fibrotic, or fistulizing disease. And within each of these subtypes, disease may be mild to moderate, moderate to severe, severe, or refractory.
In the setting of mild to moderate disease, patients are often treated with 5-aminosalicylate products, such as sulfasalazine, oral mesalamine (Pentasa, Asacol), and rectal mesalamine (Rowasa). For ileal, ileocolonic, and colonic disease, sulfasalazine as a 3- to 6-g daily divided dose is effective treatment. Multiple trials across the United States and Europe have demonstrated its benefits over placebo. However, its ability to induce or maintain remission is significantly less than corticosteroids, and sulfasalazine and mesalamine are significantly less effective than corticosteroids in managing active CD. In contrast, budesonide is equally as effective as conventional oral corticosteroids in the treatment of mild to moderate ileal and right colonic disease. However, controlled-release formulations of budesonide (9 mg/day) are limited to treatment of disease in the ileum and right colon and are not effective for treating disease in the proximal small bowel or rectum.
For patients with moderate to severe disease, corticosteroids are the cornerstone of medical management until symptoms resolve. Patients may require up to 40 to 60 mg daily until clinical improvement is seen. Alternatives to steroid therapy effective for inducing remission and closing fistulas include azathioprine at 2 to 3 mg/kg per day and 6-mercaptopurine at 1.5 mg/kg per day. In steroid-dependent or refractory CD, methotrexate at 25 mg/week may be effective.
Since the US Food and Drug Administration (FDA) approval of infliximab in 1998 for the treatment of CD, we have entered a new era of biologic medical therapy, which has largely supplanted the aforementioned classes of medications or certainly added to the armamentarium. Anti–tumor necrosis factor (TNF) monoclonal antibodies (infliximab, adalimumab, certolizumab) were the first class to be introduced and are the most commonly prescribed. Additional biologic therapies have since been introduced for patients with contraindications, complications, or loss of response to the anti-TNF-α class. These include antibodies that target integrins (natalizumab, vedolizumab) and interleukin (IL)-12/23 (ustekinumab). Initially, biologic agents were only given to patients with severe disease, half of whom had already required surgery for complications, and a third of whom had failed to respond to thiopurines. However, post hoc analysis of the large randomized controlled trials found that overall remission rates with biologics were greater when administered to patients within 2 years of diagnosis. Thus the preferred patient for introducing biologics is one with active disease, prior to the development of strictures, who is at higher risk of complications. Unfortunately, up to 60% of patients experience recurrence of symptoms after induced remission with anti-TNF-α agents. With more and more biologic classes being FDA approved, these patients, rather than going to surgery, are now often given an alternative class of biologic therapy. Thus, patients are generally arriving to the operating room with more advanced disease activity and a longer duration of immunosuppression. However, it is worth noting that in the prebiologic era, up to 30% of CD patients who underwent surgery would require another operation within 5 to 10 years, and now in the era of biologic therapy, only 10% of CD patients will require another operation within 8 years of the index case. Furthermore, meta-analyses have shown that biologic therapy, although not without complication, has a significant impact on preventing disease recurrence.
Although biologics have significantly enhanced medical management, they are not without potential morbidity and significant cost. Patients are at increased risk of opportunistic infection or reactivation of latent infections, malignancy (especially lymphoma), worsening of congestive heart failure, and eczematous skin lesions. Thus treatment must be individualized, and careful consideration given to patients with significant comorbidities or of advanced age.
Despite making significant advances in medical therapy, up to 70% of patients eventually require an operation. The leading indication for surgery is disease that is refractory to medical management. The end result presents as obstruction, fistulas, abscesses, gastrointestinal bleeding, or perforation. In addition, less common indications for surgery include growth retardation in children, toxic megacolon, and fulminant colitis.
It is important to remember that a multidisciplinary approach is central to the management of this patient population. The patient, gastroenterologist, and surgeon should be in close communication as the patient's disease severity increases and surgery becomes increasingly likely. Before exhausting all medical options, a patient should at least have had a surgical consultation to understand the risks and benefits of an operation versus ongoing medical management. Ideally, a consensus will be reached by all parties involved before the patient is taken to the operating room. At that time, careful preoperative planning in a joint fashion should ensue.
The decision to operate is made in the context of the patient's preoperative nutritional status, immunosuppressive regimen, and any undrained sources of infection. The patient's nutritional status is often compromised by severe disease and long-standing poor nutrition. Total parenteral nutrition (TPN) may be indicated if the patient is severely malnourished (defined as loss of more than 5% of body weight in 1 month or 10% in 6 months, a body mass index less than 19 kg/m 2 , or an albumin level <3 g/dL) to achieve improvement in wound healing and prevention of anastomotic leaks. This finding was demonstrated in a study of 395 malnourished patients who received 1 week of TPN before surgery and had significantly fewer noninfectious complications as compared with the controls (5% vs. 43%).
The impact of immunosuppressive medications on postoperative complications remains controversial. Recently, there has been a significant focus on whether biologic therapy increases postoperative complications. A previous series from our institution concluded that anti-TNF-α therapy did not increase the rate of postoperative complications after surgery for CD. However, recent meta-analyses including up to 18 studies concluded infliximab does increase the rate of postoperative complications, especially postoperative infectious complications, which are reported to occur at a rate of 15% to 17%. Thus the timing of surgery as it relates to the most recent dose of biologic therapy becomes important to consider.
Patients with abscesses should be drained nonoperatively prior to going to the operating room, unless contraindicated due to need for emergent surgery. Interventional radiology can be used to drain intraabdominal abscesses, and examination under anesthesia can be used to drain perirectal abscesses. For intraabdominal abscesses, adequate drainage may obviate the need for surgery altogether; if not, it at least minimizes the degree of intraabdominal inflammation, allowing for a more limited bowel resection. If infection or abscesses are present at the time of the operation, the surgeon should consider postoperative antibiotic therapy and delayed closure of operative incisions.
After it is decided that the patient will proceed with surgery, the surgical plan should incorporate detailed information from imaging, endoscopy, and prior operative reports. Cross-sectional imaging with computed tomography (CT) or magnetic resonance (MR) enterography provides important information about the distribution and extent of disease, any undrained fluid collections, anatomic location of fistulizing disease, alteration in anatomy due to prior operations, and an estimate of remaining small bowel length. MR enterography has the added advantage of no radiation, with improved anatomic detail, especially important in this young population who will likely require several repeat abdominal images over the course of their lifetime. Thus it is has largely supplanted fluoroscopic imaging, which is now primarily used for complex fistulizing disease requiring careful preoperative planning, and assessment of distal patency prior to stoma reversal.
A final important step prior to taking a patient to the operating room is a discussion regarding the potential need of a permanent or temporary stoma. Because anxiety around stoma formation is common among CD patients, early comprehensive education and support from an enterostomal therapist is important. In addition, it is important to consider preoperative stoma marking if the patient has any suspicion of requiring a stoma, especially in complex reoperative CD in which unexpected intraoperative findings or technical difficulties may mandate stoma construction.
After patient optimization and preoperative planning are completed, the initial operation for CD should focus on bowel preservation, careful measurement and description of remaining bowel, and how to maximize a minimally invasive approach. Because surgery is not curative and the dreaded fear of short bowel is real, more conservative operative approaches have been adopted over time. Resections are performed to macrospically uninvolved rather than microscopically uninvolved bowel, and more aggressive use of long stricturoplasty to preserve bowel may be used. When confronted with challenging cases in the operating room, it is important to keep in mind that when more than 70% of the small bowel is resected, supplemental parenteral nutrition is nearly always required. If less than 50% of the small bowel is resected, patients can still experience malabsorption of fat-soluble vitamins and lactose.
There are at least three aspects of the index operation that can aid any subsequent operations. The first is the measurement and dictation of the length of remaining small bowel. To do this, the bowel is kept in a relaxed state and a suture of known length, usually a 60-cm silk suture, is manually used to measure the small bowel from the ligament of Treitz to the ileocolonic anastomosis or ileostomy. Second, a laparoscopic approach should be used whenever safe and feasible to prevent adhesive disease formation. Studies have found laparoscopy to be safe in both index and reoperative CD cases, with added benefits including reduction of adhesive disease, earlier return of bowel function, decreased length of hospital stay, improved cosmetic satisfaction, and decreased rates of small bowel obstruction. One of the main indications to convert a laparoscopic approach to an open one in CD patients is the thickened, vascularized mesentery. If this is encountered, a laparoscopic approach will likely need to be abandoned. Third, the use of antiadhesion barriers, such as Seprafilm, reduces pelvic and abdominal adhesive disease and may make the next operation easier and safer when entering the abdomen or reversing a stoma.
Indications for an operation in the setting of stricturing CD include persistent obstructive symptoms that do not resolve with maximal escalation of medical therapy, chronic requirement for steroids, weight loss, or the need for chronic narcotic pain medication. Repeated episodes of inflammation, remodeling, and scarring of the bowel wall occur more often in small bowel than the colon. Regardless of the location, the normal pliable tissue is replaced with thickened nondistensible segments that narrow the lumen.
The length of the stricture, number of strictures, number of prior operations, and remaining small bowel length all contribute to the intraoperative decision making of bowel resection versus stricturoplasty. At an initial operation, a long-segment stricture may be resected if the remaining small bowel looks healthy. However, if there are multiple segments or the remaining small bowel has areas of narrowing, strictureplasty should be considered due to the concern for bowel shortening.
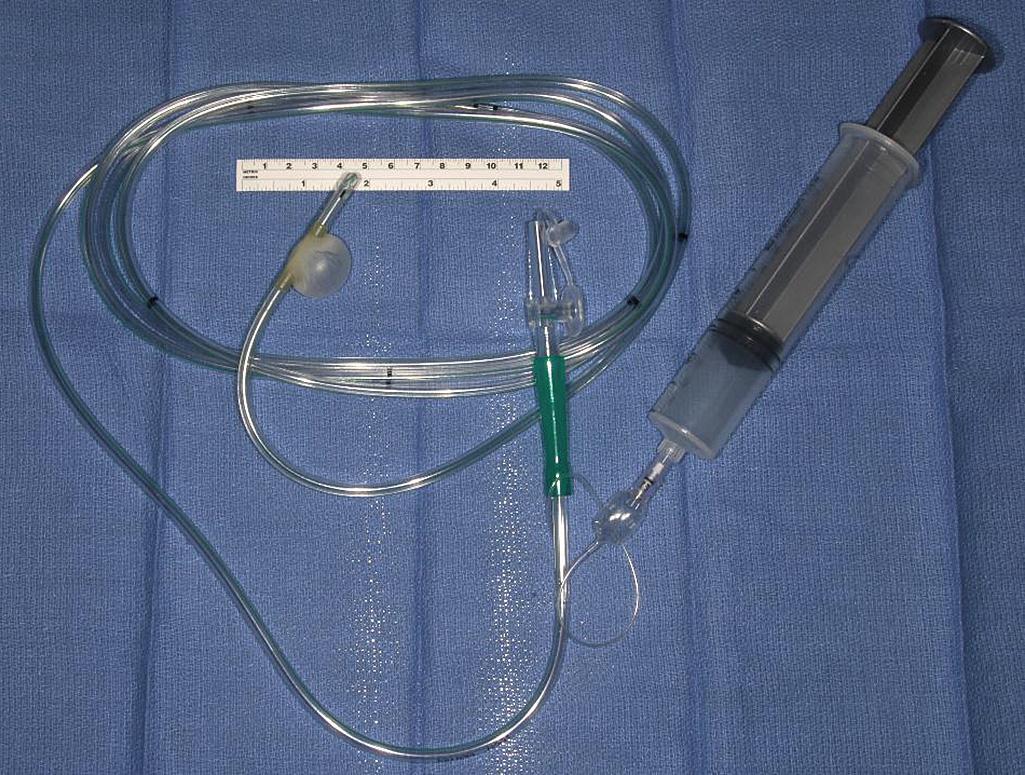
On initial exploration in the setting of known obstructive disease, the entire bowel should be examined for any evidence of disease. Obvious strictures should be marked with sutures. If there are any questionable areas of narrowing during visual and tactile examination of the bowel, a sizing device can be run through the small bowel to assess diameter and distensibility. A balloon-tipped catheter may be passed through an enterotomy made at an obvious stricture site and advanced through the bowel looking for additional strictures ( Fig. 163.1 ). Our preference is the use of surgical steel balls of varying diameter that pass through the bowel much easier and with less manipulation. As the balls are passed through the bowel, each additional area of narrowing is marked with a suture, and assessment of bowel length and the number and spacing of required strictureplasty is assessed to determine which surgical approach will be used. Fortunately strictureplasty is appropriate to perform at any place along the length of bowel, including the duodenum, jejunum, ileum, and even colon. However, it should not be performed if there is an associated phlegmon or perforation.
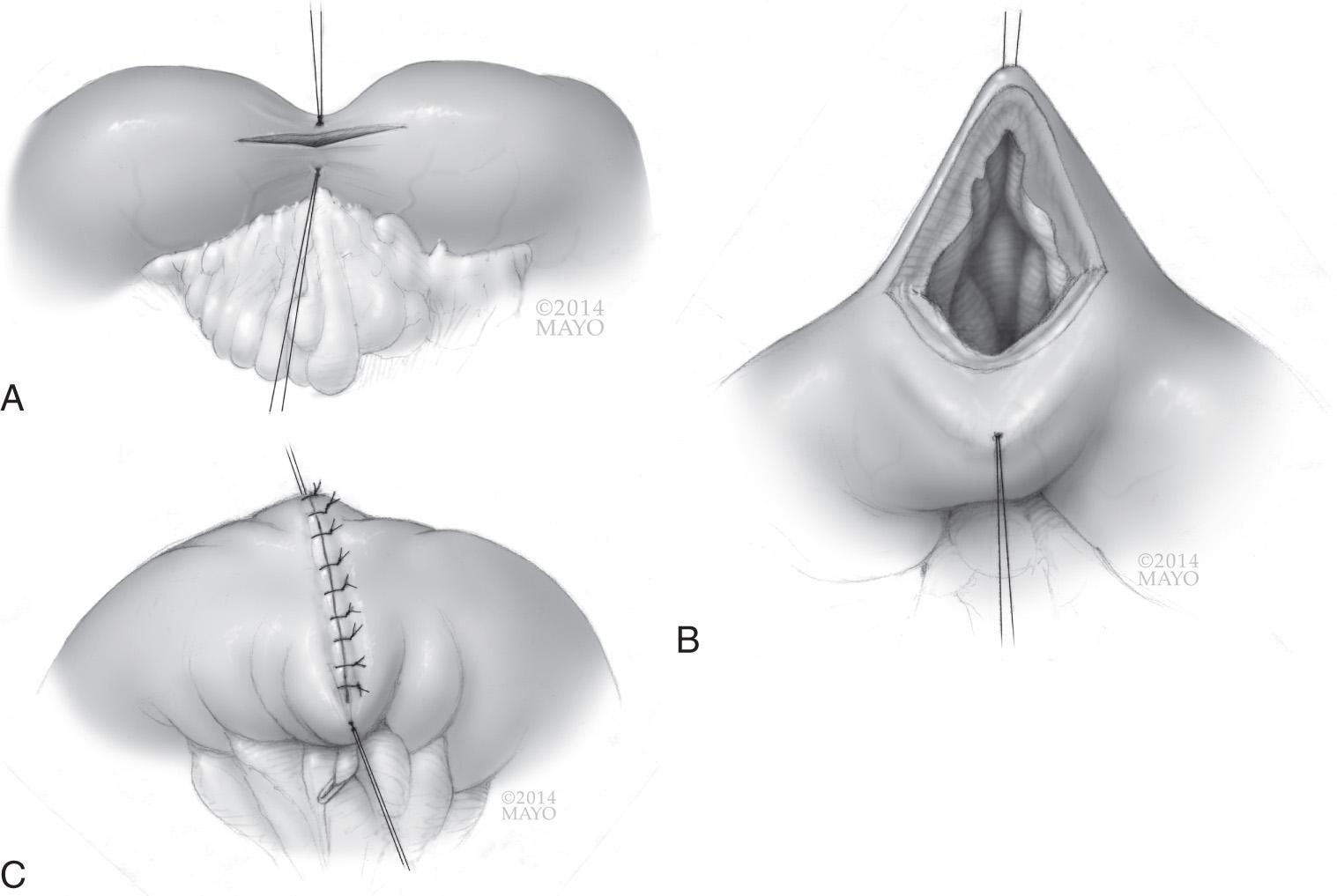
The first reported strictureplasty for the treatment of CD was by Lee and Papaioannou in 1982. Since then, many techniques for strictureplasty have been described, and more surgeons are advocating the use of strictureplasty in place of resection for first-time ileocolic disease. For strictures less than 4 to 5 cm in length, a Heineke-Mikulicz technique can be used, opening the stricture longitudinally and closing the bowel wall transversely ( Fig. 163.2 ). Two sutures are placed at the midpoint of the stricture, to be used as stay sutures to open the strictureplasty. An antimesenteric longitudinal incision is made over the stricture using cautery and extended onto normal bowel of equal distance in both directions. If the patient has had long-standing inflammation or the stricture has been present more than 5 years, biopsy of the mucosa may be prudent to rule out dysplasia or malignancy intraoperatively because this would change the operation performed. The enterotomy is then closed in a transverse, two-layered hand sewn fashion. For strictures longer than 4 to 5 cm in length, a Finney strictureplasty can be used to prevent narrowing at the inlet or tension on the transverse closure. A Finney strictureplasty is similar to a side-to-side anastomosis and is useful in the setting of a single long stricture or multiple short-segment strictures in close proximity. Two options exist. First, if the strictured area is mildly stenotic and the bowel has remaining pliability, the strictured area can be opened and sutured to normal bowel in a hand sewn side-to-side fashion. The second option is to exclude the strictured segment. If the segment is severely narrowed and nonpliable, normal bowel proximal and distal to the strictured segment can be overlapped and sewn together in a side-to-side fashion, effectively bypassing the area of strictured bowel. The concerns with this technique are the possible complications, including bacterial overgrowth and malignant degeneration of the excluded segment of bowel.
Significant dilation proximal to a strictured segment that results in significant size discrepancy between the normal proximal and distal bowel discourages a Heineke-Mikulicz strictureplasty. In this much less common scenario, a Moskel-Walske-Neumayer strictureplasty can be performed. The stricture is opened along the antimesenteric border as a Y -shaped enterotomy with the Y portion in the dilated bowel just proximal to the stricture. The strictured segment is then pulled apart and the antimesenteric segment of the proximal bowel is advanced into the strictured area and closed in a transverse fashion with one side of the closure being normal bowel along the entire length and the other being the two strictured bowel edges.
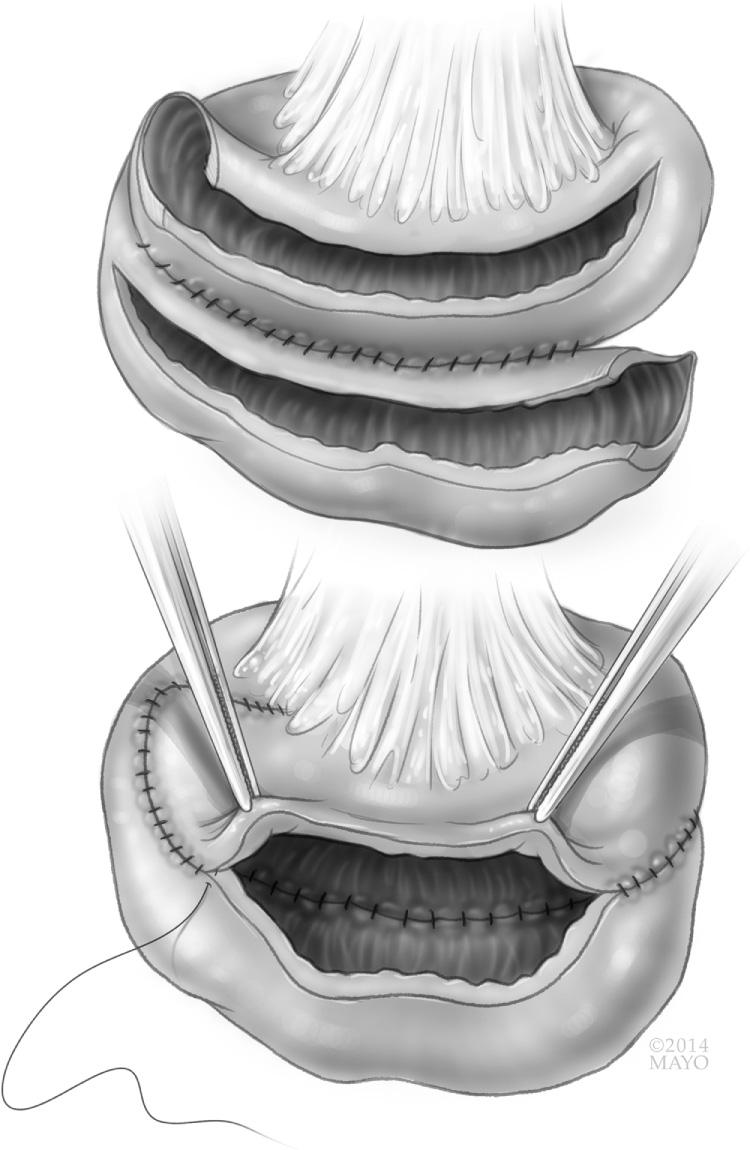
The most difficult type of stricture to address is the very long, greater than 20 cm, stricture or series of strictures in close proximity. Due to the length of compromised bowel, resection is not recommended. Fortunately, Michelassi developed and published a unique technique to address this anatomic challenge. A side-to-side isoperistaltic strictureplasty is made by completely dividing the bowel transversely in the middle of the strictured segment. The mesentery is then divided perpendicular to the long axis of the bowel to permit the now two segments of strictured bowel to lay side by side along the entire length of strictured bowel ( Fig. 163.3 ). Both strictured segments are then opened along the antimesenteric border and sewn to one another in an isoperistaltic fashion. This technique does not exclude any bowel but rather incorporates all strictured areas. Shatari et al. published an initial series of 21 patients describing this technique as safe for long-segment strictures. A meta-analysis of 148 patients followed; this technique did not increase postoperative morbidity. The largest series of 184 patients across six international centers found the technique safe, with 11% morbidity and 0% mortality, and effective, with only 23% of patients requiring reoperation at 5 years.
As strictureplasty has become more widely adopted, several analyses regarding safety and efficacy have been published. Dietz et al. reported an overall morbidity of 18% and a septic complication rate of 5% among 314 patients with 1124 strictureplasties. Tichansky et al. found that 90% of strictures in their series of 506 patients across 15 articles were less than 10 cm in length, of which 85% were successfully treated with a Heineke-Mikulicz technique. Interestingly, the authors found a trend toward lower recurrence with the Finney technique. Yamamoto et al. found a 4% rate of septic complications among 1112 patients who underwent 3259 strictureplasties and a site-specific recurrence rate of only 3%. Campbell et al. compared the type of strictureplasty performed and found no difference in immediate- and long-term complication rates between the conventional Heineke-Mikulicz and nonconventional Finney and Michelassi technique among 1516 patients with 4538 strictureplasties. Putting these results together, regardless of the operative technique used, bowel can be safely preserved with a low recurrence rate at the site of strictureplasty.
Ongoing transmural inflammation can result in fistula formation between loops of bowel, bowel to bladder, bowel to abdominal wall, or bowel to any other structure or organ in the abdomen and pelvis. Historically, intraabdominal CD fistulas were treated with bowel rest, intravenous nutrition, proximal diversion, and occasional resection. The introduction of infliximab in 1998 changed the paradigm of operative indication given its efficacy in closing abdominal wall and perianal fistulas. In the initial multicenter, double blind, randomized trial assessing the efficacy of infliximab for fistulizing CD, 55% of all patients had closure of their fistulas and 70% had a reduction in the number of actively draining fistulas. In the follow-up trial of long-term outcomes, 36% of patients had complete absence of fistulas at 1 year compared with 19% in the placebo. Another more recent study found a third of patients with enterocutaneous fistulas achieved closure within 3 months of infliximab initiation. Following these studies, patients now undergo a 3-month trial of infliximab prior to operating for fistulizing disease, in hopes of healing the fistula without an operation.
After it has been decided an operation is needed to address the fistulizing disease, the type of operation performed depends on the anatomic location of the fistula and degree of associated sepsis. For enterocutaneous fistula, the goal is to resect the bowel and skin communicating via the fistula. This should be attempted only after the patient has exhausted maximal medical therapy, the skin surrounding the fistula is soft and pliable, sepsis related to the fistula has been adequately drained, and the patient demonstrates adequate nutritional parameters. At this stage, interventional radiology may be required to drain any intraabdominal sources of infection, TPN may be necessary to improve a patient's nutrition prior to a major operation, and preoperative planning for potential mesh placement with loss of abdominal domain is imperative. The operation is performed in an open manner, taking care to lyse adhesions without inadvertent enterotomies. When resecting the small bowel, the consistent principles of bowel conservation should be kept in mind. The bowel is resection often with a primary anastomosis, and the skin and abdominal wall are excised back to healthy tissue. If the abdominal wall cannot be closed, mesh can be inserted. Prosthetic material, such as Prolene or Gore-Tex, is contraindicated due to infection risk, but biologic meshes, now abundantly available, can be used. Biologic mesh has the advantage of slow tissue ingrowth, decreased adhesion formation, and the ability to not becoming chronically infected in the setting of local contamination. The mesh should be placed as an underlay with normal surrounding tissue and drains placed under any flaps to prevent seroma formation. If the mesh is left exposed, a wound vacuum closure device can be used with continuous drainage to facilitate tissue ingrowth, or transition to placement of a skin graft.
Entero-enteral fistulas may not be clinically significant unless a large segment of bowel is bypassed. If found preoperatively, preoperative planning should include imaging to best delineate the anatomy before an operation. When an operation is undertaken, often only one segment of bowel is actively involved with CD, whereas the other is a bystander and is not actively inflamed. When this is seen, the actively inflamed segment with fistula should be resected while the other can be repaired primarily following fistula takedown. This allows for increased bowel preservation with minimal morbidity and mortality.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here