Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Evidence supporting the efficacy of implantable cardioverter-defibrillators (ICDs) in their ability to correct ventricular fibrillation (VF) is extensive and secure. , The conventional transvenous ICD (TV-ICD) has evolved considerably after being initially somewhat cumbersome and requiring surgical placement of epicardial patches. Abdominally placed generators with long, subcutaneously tracked but nonetheless transvenous leads followed. The major strategic technical advance was the simplified unipolar approach allowing devices to be placed in deep and then prepectoral locations. Subsequent increasing use in core task defibrillation set the stage for complex pacing applications and, later, cardiac resynchronization with high energy capability. Each of these later advances depended on the evolution of intravascular leads ( Fig. 123.1 ).
Landmark studies supporting primary prevention , , led to predictions for several hundred thousand additional ICD implants each year. Evidence of potential efficacy was not, however, followed by the anticipated expansion in demand but rather a grumbling, persistent disinclination from referrers, especially for primary prevention indications. , Patients had also become aware of adverse events mostly related to the leads, which blunted their enthusiasm despite the proven lifesaving potential. The usual well-recognized problems with transvenous pacemaker leads such as vascular obstruction, thrombosis, and systemic infection were joined by a veritable flood of other concerns, with many related to design and manufacture. , Specifically, it also became apparent that performance deteriorated over time with some leads, especially those with a deliberately low profile, showing accelerated aging and potentially severe consequences, including structural breakdown, the possibility of inappropriate shocks (IASs), and a need for premature replacement. Transvenous leads became viewed as the most vulnerable component and, indeed, the weakest link in the effective delivery of ICD therapy. The fact that, in response to advisories leads often needed extrication from the heart and vasculature, further blunted a community view of singular benefit. ,
It was against this background that the concept of the subcutaneous ICD (S-ICD) developed with a key objective of returning to pure goal-directed defibrillation not confounded by the safety issues necessarily attached to intravascular leads. During the long S-ICD development program, TV-ICDs were also transformed technically with more reliable leads , ; generators with improved sensing algorithms and longer battery life; and clinically, mainly through more rational programming. , The S-ICD has gained considerably through these advances to become an established option in the therapeutic toolbox of the electrophysiologist. Although the S-ICD should, according to guidelines, , be considered in patients who do not carry a pacing indication (bradycardia, conduction system disease, requirement for cardiac resynchronization therapy [CRT]), the community’s familiarity with and the ease of deployment of TV-ICDs underpins a continuing preference by many for these long-established approaches, although S-ICD implant rates do continue to grow.
The first question to be addressed in system development was what energy was needed for defibrillation with an electrode placed subcutaneously? Exploratory studies demonstrated defibrillation was possible in patient volunteers employing subcutaneous electrodes and an active can emulator; furthermore, generators placed in a lateral location were reasonably well tolerated. , , Subsequently, in dogs paced into VF, electrode configurations for defibrillation were identified that would also be open to implementation in humans. Next-stage clinical research established optimal system location for defibrillation in humans , ; these protocols required that patients undergoing TV-ICD implantation were paced into VF with defibrillation thresholds (DFTs) then obtained for a range of subcutaneous device/lead configurations. It was thereby established that a laterally placed emulator with an 8 cm coil running parallel to and approximately 2 cm away from the left sternal border (parasternal) resulted in the lowest DFTs. The headline was that the observed DFT (35 J average with reasonably tight confidence intervals) supported the idea that an 80 J device would provide a clinically acceptable margin of safety. , In a later study required for European CE approval during implant testing, all 137 episodes of induced VF were cardioverted reliably at 65 J using the now established optimal generator/electrode relationship. This observation of high efficacy was then reproduced in the pivotal IDE trial.
The first generation S-ICD (SQ-RX 1010, Cameron Health/Boston Scientific Inc.) had a significantly larger volume (70 cm 3 ) and was heavier (165 g) than contemporaneous conventional devices; these dimensions were required to accommodate the capacitors that provided higher charge outputs. One design advantage of the S-ICD was that the lead was unconstrained by the requirements for intravascular placement. For example, it needed neither inner coils nor a hollow core and could therefore be configured primarily to have long-term durability based on multistrand core cables and polyurethane insulation. Because of this it was predicted to be robust. The lead is not without risks of failure, although at an annual rate of less than 0.07%; a 2020 Safety Advisory highlighted 27 reports of body fractures occurring just distal to the proximal sensing ring out of 47,000 implants. Although the mechanism remains unresolved, we should keep in mind careful attention to handling during implant and telling patients that subcutaneous leads have such rare vulnerabilities. The system characteristics have changed little from the initial vision ( Figs. 123.2 and 123.3 ) with downsizing of the generator as the key step change in design. It was clear that even the first iteration was well tolerated in the lateral location, , and in one comparative study S-ICD and TV-ICD implantation were associated with similar indices of quality of life over time but depression and anxiety were lower with the S-ICD.
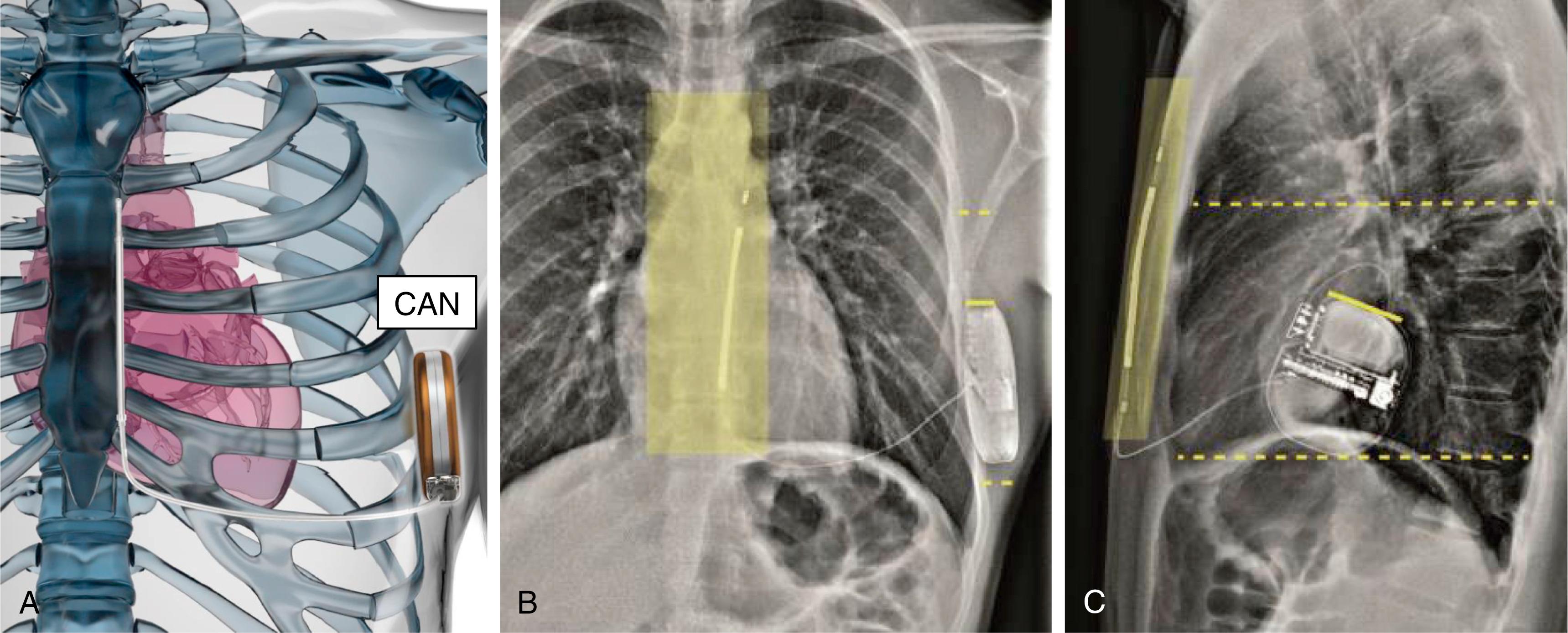
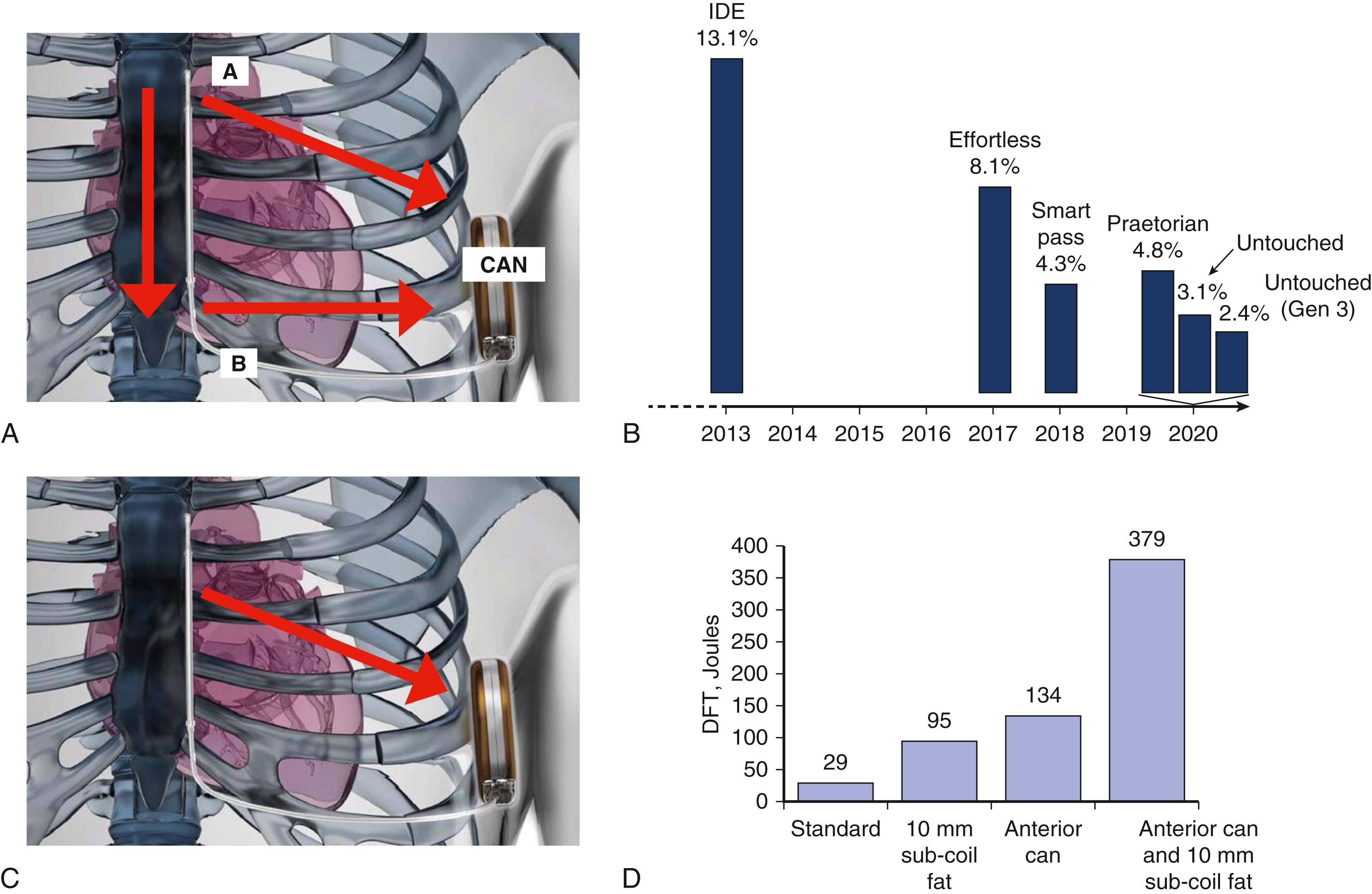
The second-generation EMBLEM S-ICD (A209) was launched in March 2015, and the third magnetic resonance (MRI)-compatible EMBLEM S-ICD (A219) of similar dimensions was released in 2018. These devices are smaller (20% reduction in thickness, 15% reduction in volume, 10% reduction in weight) than the first generation and have a modestly revised shape. They also have increased battery longevity (predicted out to 7.3 years) with engineering based on principles common to the same manufacturer’s transvenous devices that are also enabled for use on its Latitude remote monitoring platform. , , The shock remains a nonprogrammable biphasic 80 J and is delivered after a longer charge time than for TV-ICDs. These charge times were seen initially by some to be a hazard, but a move to programming delayed therapy delivery for TV-ICDs , quelled much of that concern and is consistent with benefits in reducing unnecessary shocks. The time to therapy, mainly caused by capacitor charging, typically takes 15 to 20 seconds. If an initial shock is unsuccessful then the S-ICD shock polarity is automatically inverted for the next shock. The device is also capable of postshock pacing for 30 seconds although, of course, not for sustained bradycardia support. The engineering needed to provide a device for effective defibrillation and postshock pacing was provided relatively early, and the main technical hurdle was development of software algorithms and associated hardware for effective sensing and discrimination of the cardiac rhythm.
The first implanted system was placed in Auckland, New Zealand, on July 28, 2008. Studies required European CE marking (approval) then followed involving 55 patients with the primary trial end points being acute efficacy in VF correction at the time of implant and short-term safety, although longer-term follow-up data were subsequently available. The S-ICD IDE Clinical Investigation required approval in the United States, then began recruitment in January 2010. This was a prospective, single-arm comparison to an objective performance criterion (OPC) with a design based on those used in prior TV-ICD trials. The efficacy end point was again that of acute VF conversion with the safety endpoint being the 180-day system complication-free rate. The study enrolled 330 patients, with a distribution of primary and secondary prevention indications, 70% having an ejection fraction (EF) less than 35% similar to those recorded in the National Cardiovascular Data Registry (NCDR) registry. , The primary effectiveness end point was met, and in 304 patients conversion was achieved in 100% of cases with all analyses exceeding the OPC. Furthermore, clinical efficacy was also observed with 22 episodes of monomorphic ventricular tachycardia (VT) and 16 episodes of VF correction with high first (92%) and second (97%) shock success being observed during follow-up. The primary safety end point was also met with 180-day type 1 complication-free rate of 99%. The overall conclusions of this pivotal study were that both the efficacy and safety end points had been met leading to almost unanimous US Food and Drug Administration (FDA) advisory panel approval when presented in Washington, DC, on April 26, 2012. The thoughtful, reasonable points of concern offered by the dissenting panel member were subsequently presented in detail.
The techniques for S-ICD implantation resembled in many aspects those developed for abdominal transvenous systems with tunneled leads and were established in the first few experimental cases. Accordingly, movies made several years ago still provide the template for didactic animations and confirm a striking consistency of approach over time. It was clear from the outset that following a simple set of rules resulted in good outcomes, and the learning curve for implantation is therefore steep. , Anatomic deterrents are few and implantation is consistently predictable in terms of duration at approximately 65 to 75 minutes, with limited unforeseen consequences. It does seem though that based on sheer practicalities the rapid average implantation times seen for single-lead transvenous systems are unlikely to be beaten.
In many labs the procedures are still conducted under general anesthesia, although several operators have now moved to conscious sedation for most patients, which in some geographies exceeds 70%. Conscious sedation needs to be robust and a relatively long-acting local anesthetic (e.g., bupivacaine) is beneficial in ensuring early recovery is comfortable. Effective local anesthetic use requires infiltration not only around the pocket but into the deep fascia and along the route taken by the lead. Surgical preparation should be meticulous with diligent skin preparation, removal of local hair, exclusion of the axilla, and antibiotic prophylaxis according to local guidance with physician involvement at all stages. The incision needed to accommodate the main body of the device is made around the left inframammary crease at the level of the fifth intercostal space centered in the mid thoracic line. In initial iterations (the three-incision technique) two other small incisions were made at the lower sternal border (just to the left of and above the sternal notch) and then at the upper left sternal border at a measured caudocranial distance (determined by the 8 cm length of the electrode), taking into account the characteristics of the individual patient to accommodate the electrode. The lead in all cases is then placed absolutely parallel to the sternum using proprietary tunneling tools.
In the three-incision technique the tip at the superior (distal) end is fastened securely to the fascia to avoid lead drift. This recommendation was supported by the need to avoid the downward movement observed in some cases included in the initial CE study and may protect against lead retraction leading to twiddler syndrome and reduce the likelihood of air entrapment. The alternate two-incision technique, pioneered by Knops and associates, requires upward tunneling with bespoke tools and is now the most widely used technique. Long-term observational follow-up from Amsterdam (268 patients total: 237 two-incision; 31 three-incision) showed similar complication rates for the two approaches. Two-incision procedures tend to be shorter and give the lowest Praetorian Score, but they may be associated with higher IAS rates (hazard ratio [HR], >3.5) and although the mechanisms are uncertain need further evaluation. Under all circumstances in patients with prior sternotomy, proximity to sternal wires/clips should be avoided. ,
The system is placed without any mandated radiographic requirement, although in early post approval cases some still advocated imaging to verify position. Accordingly, the implanter does not usually require radiation protection, so in early presentations we suggested there would be reduced risks for occupational injury. Location within an intermuscular pocket is now preferred to minimize both DFTs and IAS, to maximize comfort, and to protect against erosion. , , In this variation the generator is placed in a muscular pocket between the anterior surface of serratus anterior and the posterior aspect of latissimus dorsi. Care should be taken with hemostasis, but under direct vision blood vessels can be avoided such that hematoma is infrequent and erosion is strikingly rare.
The leads were designed to favor adhesion formation, although lead extraction has both a high procedural success and low-risk tools may be required. Simple traction was sufficient for removal in 19 of 32 patients (59.4%) in whom the lead had been implanted a median of 9.3 months. Additional incisions were required in 3 patients and mechanical sheaths in 9 (28.1%) total to free up the lead from adhesions. The obvious limitation is the absence of a central lumen so that locking stylets cannot be used. If same site parasternal reimplantation is envisaged avoidance of fibrosed tunnels is advisable as higher impedances and DFTs are likely. , Experience with generator replacement remains limited, although it has been reported to be safe. In the largest published experience of 72 cases in whom battery depletion was observed, at approximately 6 years device position was reassessed and if Praetorian score was greater than 90 then the generator was relocated. At 1.9 years after replacement in this series there have been two complications.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here