Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter summarizes how the hepatobiliary system develops, from a sheet of undifferentiated cells at 3 weeks’ gestation to an organ capable of performing a myriad of metabolic, synthetic, and detoxifying functions weeks later. The chapter divides hepatobiliary development into two related processes: structural development and functional development. From a clinical standpoint, this chapter can help practitioners better diagnose and treat congenital liver conditions by helping them understand the steps that occur before and after birth during normal development. In addition, the chapter will help providers appreciate new cell-based therapies, by discussing cell fate regulation mechanisms that underlie these innovative treatments.
Hepatobiliary development involves a sheet of undifferentiated cells undergoing a series of steps to become liver, gall bladder, and bile ducts. This dramatic transformation is guided by certain principles. First, the initial steps of hepatobiliary development are conserved across species. As a result, studies in mouse, chicken, frog, and other model organisms have contributed deeply to our understanding of hepatobiliary development. These studies have demonstrated that the initial sheet of undifferentiated cells comes from the endoderm, one of the three germ layers formed at gastrulation. The endoderm also gives rise to the tubes of the digestive and pulmonary systems, as well as the pancreas and thyroid. Endodermal cells transition from undifferentiated to differentiated cells though the developmental steps of competence, specification, morphogenesis, and ultimately differentiation (described below) ( Fig. 67.1 ). During this time, liver progenitor cells go through multiple cell-fate decisions, which ultimately affect organ size, organization, and the capacity to regenerate.
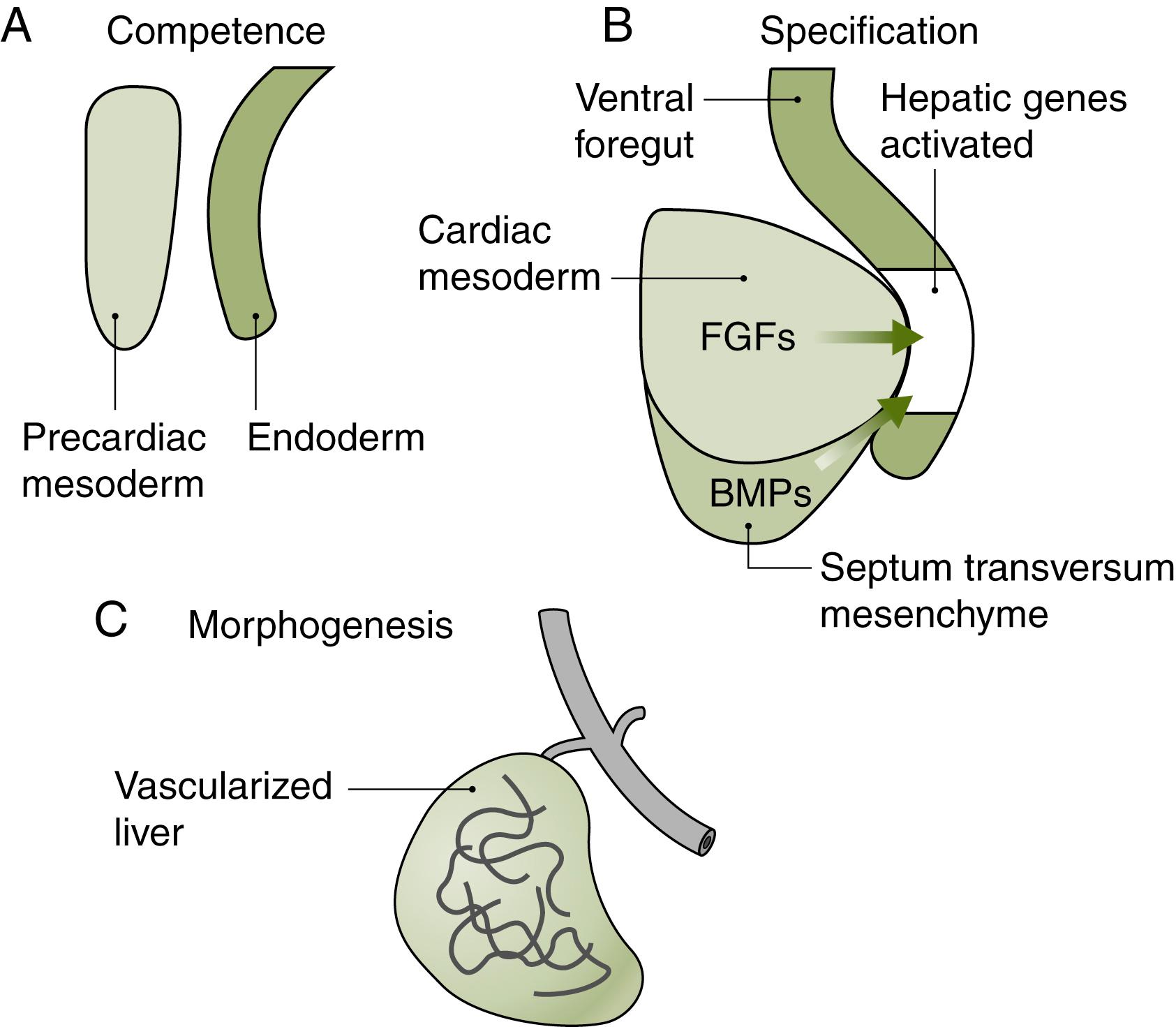
Second, cells respond to intrinsic and extrinsic cues as they transition through the different developmental states toward becoming mature hepatobiliary tissue. The extrinsic cues come from mesodermal tissues adjacent to the endoderm. Two mesodermal tissues are especially important. The first mesodermal tissue, the precardiac mesoderm, lies ventral and just anterior to the developing hepatobiliary system. The second mesodermal tissue, the septum transversum, is also ventral to the developing hepatobiliary system but posterior to the precardiac mesoderm, thus separating the pericardium from the peritoneum. Both the precardiac mesoderm and septum transversum secrete important signals that pattern the overlying endoderm. In addition, the septum transversum contributes cells that will migrate and contribute to parts of the liver.
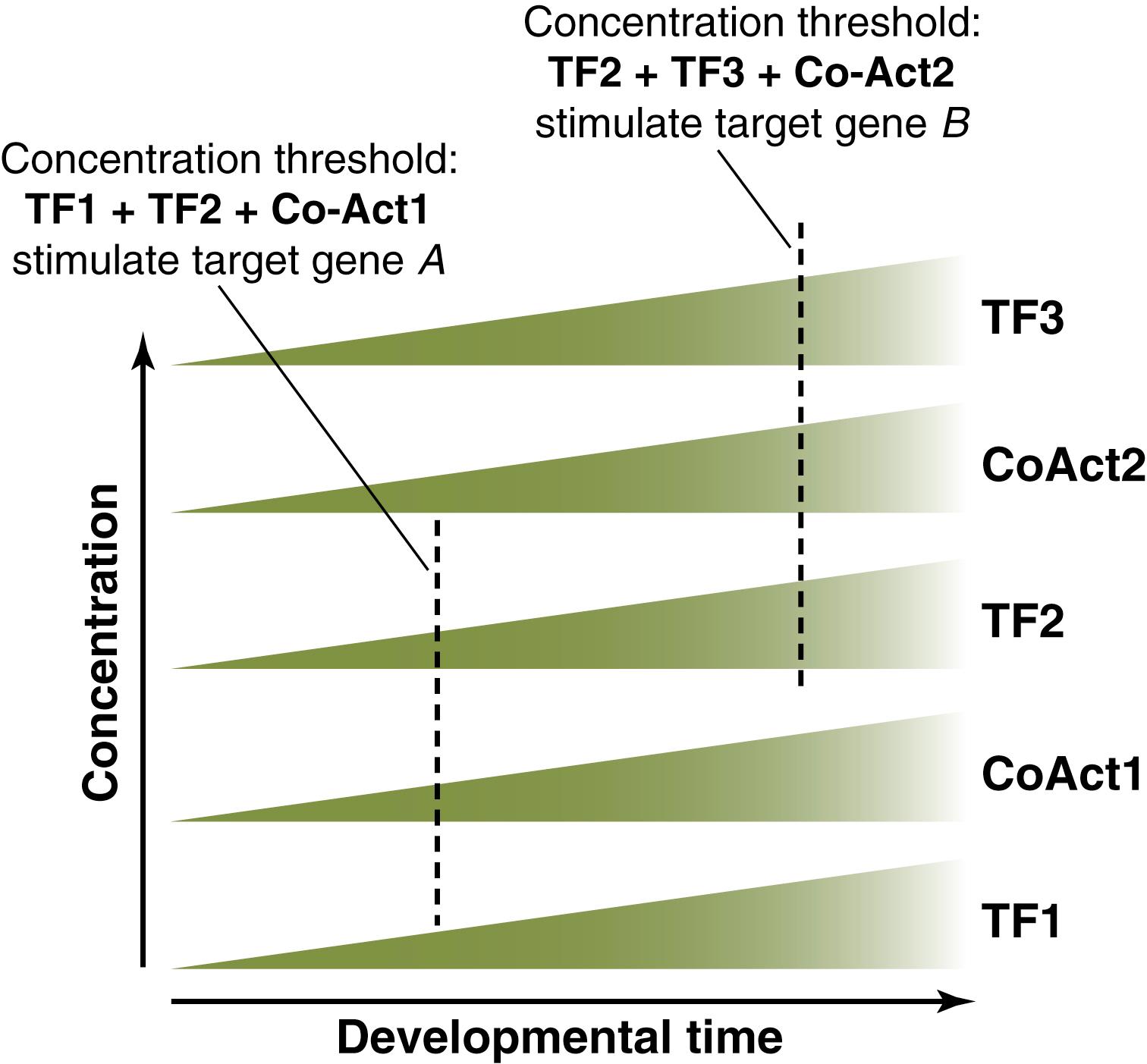
Third, cell differentiation is controlled by a “dynamic transcriptional network.” This network involves a set of liver-enriched factors that interact synergistically, creating increasing numbers of regulatory loops as development proceeds. According to the “dynamic transcriptional network” model, the complexity and number of interactions between the different factors and regulatory molecules are threshold-dependent and increase as development proceeds, thereby activating and inhibiting genes in a timely fashion ( Fig. 67.2 ). Table 67.1 summarizes the phenotypes of the knockouts of some of the members of the “transcriptional network.” Importantly, during liver development, there is a precise temporal regulation of genes, such that genes “used” for one purpose in very early liver development can be reactivated later in development for another mission. For example, Foxa3 participates in early cell-fate decisions at the beginning of development, and later controls glucose homeostasis in hepatocytes. Another example is HNF1α, which is involved in biliary development at early stages and glucose, amino acid, and fatty oxidation metabolism later.
| Gene | Phenotype |
|---|---|
| Hnf3β − / − (FoxA2) | Embryonic lethal |
| Hnf3αα − / − (FoxA1) | No liver phenotype |
| Hnf3γ − / − (FoxA3) | 50% reduction in expression of hepatocyte genes: tyrosine aminotransferase, PEPCK, transferrin. Compensatory increase in FoxA1 and FoxA2 |
| FoxA1 − / − FoxA2 − / − | No liver |
| Hnf6 − / − | Absence of gallbladder and abnormal differentiation of intrahepatic bile ducts, atretic hepatic vasculature |
| Hnf4α − / − | Diminished expression of pregnane X receptor, Hnf1 α, albumin, α-fetoprotein, apolipoproteins A1, A4, B, C3, and C2, PAH, erythropoietin, retinol binding protein |
| Hnf1 α − / − | Hepatic expression of PAH is totally absent in these mice. There is decreased expression of albumin, α 1 -antitrypsin, and β-fibrinogen |
| C/EBPα − / − | Increased hepatocyte proliferation and decreased postnatal expression of glycogen synthase, glucose-6-phosphatase, and PEPCK. In the adult mouse there is also diminished expression of bilirubin UDP-glucuronosyltransferase and factor IX |
As mentioned earlier, undifferentiated cells go through the developmental steps of competence, specification, morphogenesis, and differentiation to become mature hepatobiliary tissue. In the first step, undifferentiated endodermal cells become competent ; that is, they gain the ability to give rise to a restricted number of tissues in response to a given signal (see Fig. 67.1A ). , In this step, which begins at the third week of human gestation, endodermal cells located in the ventral foregut become “primed” by early signals that “activate” the cells’ potential to become liver, gallbladder, pancreas, thyroid, or lungs. , Understanding how endodermal cells become competent is critically important in cell-based liver therapies. For example, to generate hepatocytes from undifferentiated cells, cells must be in a “primed” developmental state to respond predictably to a soluble signal in vitro . Clinically, mutations in genes regulating competence may never become apparent, because mutations are often lethal in the mouse embryos and likely have the same effect in humans. However, it is unclear if partial loss of function is compatible with a term delivery and postnatal life.
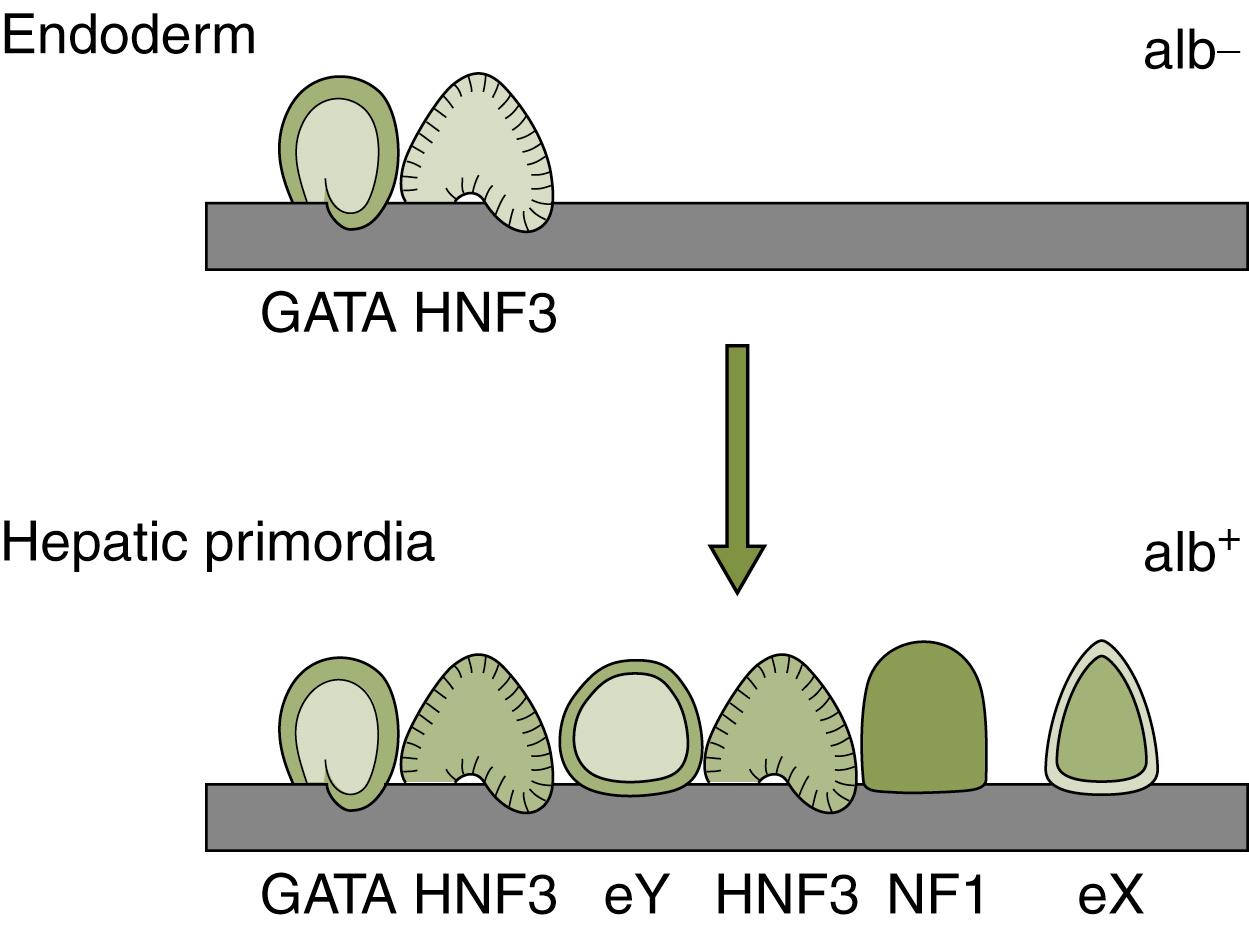
At a molecular level, competence involves a number of mechanisms. Endodermal cells become competent after responding to signals from adjacent tissues. For example, a subset of endodermal cells respond to Wnt inhibitors, allowing them to maintain a foregut identity and initiate the hepatic program. , Within the endodermal cell, competence also involves changing chromatin structure so that areas of the genome are open for genes to be expressed. For example, molecules such as Hdac1 remodel chromatin and thereby direct cells toward the hepatic (and pancreatic) lineage. The effects of this chromatin remodeling at the albumin locus have been well-studied. In early prehepatic endoderm, chromatin at the albumin locus is compact and closed. Transcription factors such as FoxA1, FoxA2, and GATA4 can bind this closed chromatin at specific sites at the albumin locus. This binding allows for the chromatin to open, allowing cells to become competent to respond to other signals from adjacent tissues that promote albumin expression and the liver fate ( Fig. 67.3 ). , Because FoxA1, FoxA2, and GATA4 bind compact chromatin at the albumin locus and open it for other factors to bind, FoxA1, FoxA2, and GATA4 are considered “pioneer factors” for hepatic competence. ,
The next important step in hepatobiliary development occurs when competent cells are specified to become liver; that is, they are committed to a hepatic fate (see Fig. 67.1B ). The specified cells are a subset of competent cells and form a structure comprised of endodermal cells that have transitioned from cuboidal to pseudo-stratified epithelium to form a distinct endodermal structure called the hepatic diverticulum. The hepatic diverticulum becomes specified by at least two external signals secreted by cells in the neighboring mesoderm: (1) fibroblast growth factor (FGF) signals, made by the adjacent precardiac mesoderm, and (2) bone morphogenetic protein (BMP) signals from the septum transversum mesenchyme. Studies in the mouse have shown that if the FGF signal is absent, the competent cells become pancreatic tissue. Subsequent investigations have revealed that the signal is more than just an on–off decision; in other words, it is not the presence or absence of the secreted FGF or BMP signal that regulates the fate of the endoderm but rather the amount of signal. Indeed, the relative amount of FGFs can direct competent cells in the anterior endoderm to become lung, liver, or pancreas. Likewise, by disrupting the location of the FGF source and thereby losing the inductive signal, the early hepatic diverticulum undergoes apoptosis. Taken together, these findings illustrate the temporal and spatial complexity of cell-fate decisions necessary to direct the early morphogenesis of different organs from the same tissue and highlight challenges in recreating these developmental paradigms when trying to “make liver in a dish.”
Following specification, the third step in hepatobiliary development is growth of the hepatic diverticulum ( Fig. 67.4 ). The hepatic diverticulum diverges into cranial (toward the head) and caudal (toward the tail) branches. The cranial branch has been well-studied and ultimately gives rise to the liver. For the caudal branch, there are at least three fates: gall bladder, extrahepatic bile duct tissue, or ventral pancreas. For the gall bladder/bile duct fate, two molecules, Sox17 and Hes1, are critical. When Sox17 or Hes1 is removed in mouse caudal branch cells, extrahepatic bile duct fails to form and is replaced by extra pancreatic tissue. , Similarly, when Sox17 is overexpressed, caudal branch cells overwhelmingly become bile duct cells at the expense of the ventral pancreas. Future studies are needed to investigate how Sox17 and Hes 1 interact, as well as the molecular signals that help further pattern biliary caudal branch cells into gall bladder versus cystic duct structures.
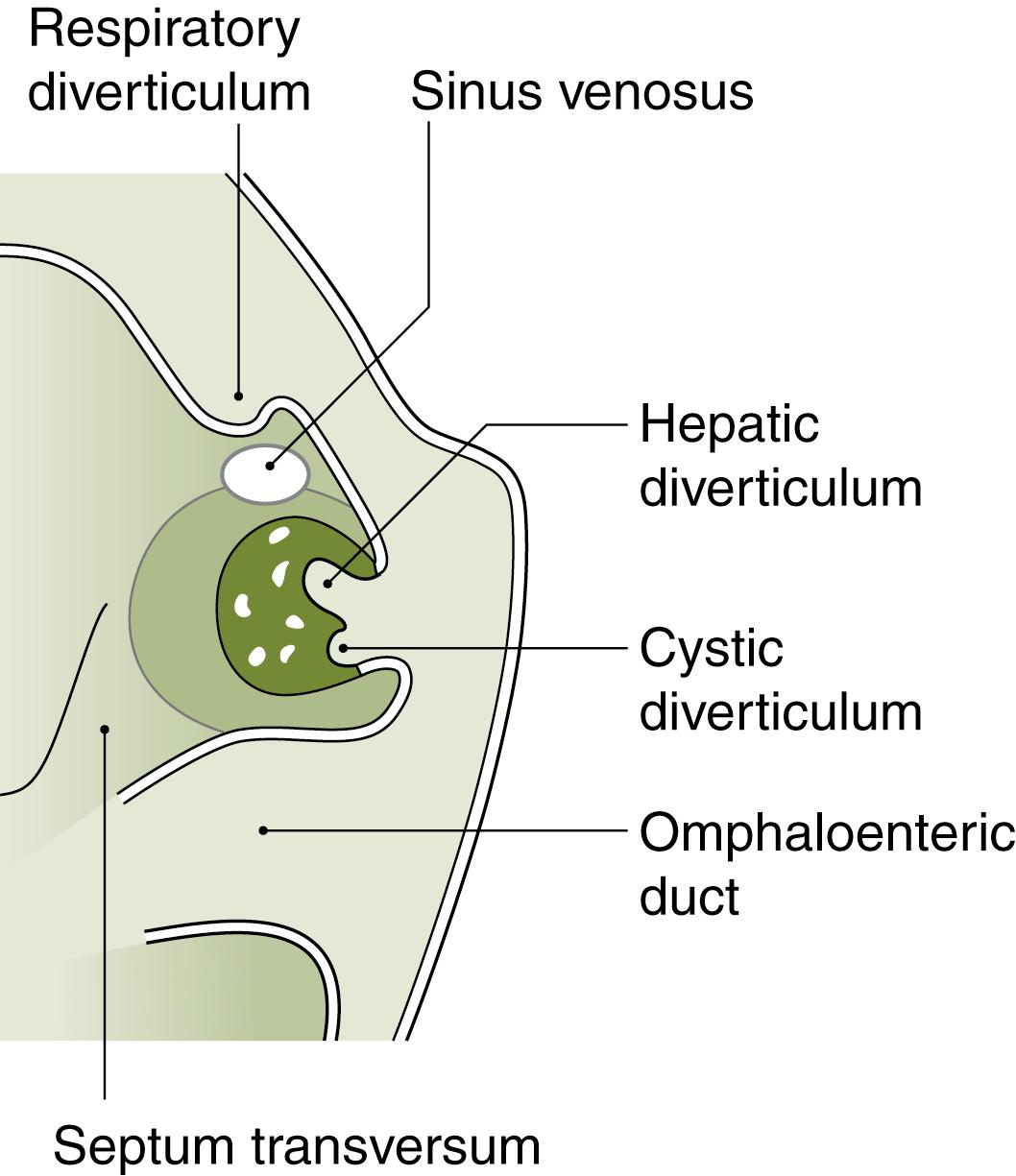
The cranial branch of the hepatic diverticulum gives rise to the liver bud, the precursor to the liver. The liver bud emerges at the beginning of the fourth week of human gestation. The process begins when endodermal cells, which were specified to become liver, differentiate into a new cell type called the hepatoblast. Hepatoblasts can respond to multiple signals and delaminate from the original sheet of endodermal cells. The delaminated hepatoblasts invade the adjacent septum transversum mesenchyme, joining hematopoietic and endothelial precursors, which are also invading the septum transversum. Together, these cells form the liver bud. Cross-talk between the different cell types is crucial for normal liver development: in the absence of endothelial cells, for example, the liver bud fails to develop.
At the molecular level, hepatoblast division, delamination, and then condensation to form the liver bud require a complex network of transcription factors, signaling, and extracellular matrix molecules. First, hepatoblast differentiation and proliferation is regulated by Prox1, Hex, and GATA6. In the absence of Prox1, endodermal cells do not express hepatoblast markers such as albumin and α fetoprotein. Likewise, in the absence of Hex and GATA6, endodermal cells express albumin and α fetoprotein initially but the expression is transient. Second, hepatoblasts delaminate and invade the septum transversum in a process controlled by an array of genes. Among these, Prox1 and Tbx3 contribute to the migration into the septum transversum. , It is probable that these transcription factors act upstream of extracellular matrix molecules and enzymes such as the metalloproteases as well as molecules affecting cell shape and movement such as the integrins and cadherins. ,
In the liver bud, the hepatoblasts and accessory cells undergo intense growth and architectural organization to form the hepatic anlage. By the end of the sixth week of human gestation, the hepatic anlage resembles the adult lobulated structure. Multiple cell-fate decisions involving both the epithelial and mesenchymal components contribute to the determination of organ size, hepatoblast differentiation into hepatocytes or cholangiocytes (see below), and spatial organization of cells into functional zones. These processes are not quite complete at birth and continue into the first 2 months of life. After this, cells in the liver replicate slowly but sufficiently to maintain an organ mass proportional to the size of the child. It is notable that a small proportion of hepatoblasts do not differentiate, but rather retain their bipotentiality of becoming either hepatocytes or cholangiocytes. These hepatocytes may participate in the ability of the adult liver to respond to injury (see section on liver regeneration).
Regulation of organ size depends on a tight balance between cellular proliferation and control of apoptosis, controlled both by cell-autonomous and paracrine signals. Multiple epithelial growth factors participate in hepatoblast proliferation and organ growth. These include hepatocyte growth factor (HGF), hepatic growth factor receptor (HGFR or c-Met), c-Jun N-terminal kinase (JNK), transforming growth factor β (TGF-β), and hepatoma-derived growth factor (HDGF). β-Catenin, best known for its role as the intracellular mediator of Wnt signaling, appears to be at the convergence of multiple signaling pathways (Wnts, FGFs, and HGF) that promote liver growth and cellular proliferation. Its role in liver growth is critical. In liver-specific knockouts of β-catenin, liver size is compromised consistent with its role in proliferation. Its contribution to organ size and shape is also readily illustrated by the grossly impaired liver morphology in chick models where β-catenin loss of function in peripheral growth zones leads to altered gross hepatic structure and organ size.
Liver size regulation is not only a cell-autonomous process. Rather, there are several examples of signals from the surrounding mesenchyme and mesothelium influencing the adjacent developing parenchyma. For example, Wnt signals from the hepatic endothelium help control hepatocyte number and patterning. Another example is retinoic acid, which is secreted from the mesothelium and thought to initiate signaling in parenchymal cells expressing the retinoic acid receptor. Finally, the mesenchymal homeobox transcription factor Hlx appears to be upstream of paracrine growth factors. In the regulation of organ size, the logical corollary to cellular proliferation is limiting apoptosis. To this end, Iĸβ kinase in the tumor necrosis factor (TNF) pathway and c-Raf-1 in the Fas-L pathway are essential factors to protect the hepatoblasts from excessive apoptosis. ,
One morphogenesis step that is still poorly understood is how a connection forms between liver bud cells (cranial branch cells that delaminate) and hepatic duct cells (cranial branch cells that do not delaminate). This connection is critical to support normal bile flow out of the liver, and defects in this connection may explain diseases such as biliary atresia. The connection is thought to occur by 12 weeks of human gestation. The connection may involve remodeling of the hepatic ducts outside the liver and their “docking” with ducts inside the liver, which have been newly formed from hepatoblasts (see below).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here