Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term radio-surgery was possibly first used in a medical context more than 90 years ago, when Dr. Francis Hernaman-Johnson described in a lecture to the Royal Society of Medicine an assortment of indications where x-ray therapy might be combined with surgery for benign and malignant indications. From the modern perspective, Dr. Hernaman-Johnson's oration is quaint but wonderfully lyrical, at times invoking Biblical metaphors and Greek fables. Prophetically, though, he concluded with the message that “no human mind can compass the whole field of medicine. Hence the hope of the future lies in specialism [ sic ] tempered by co-operation.”
Fast-forward to the mid-20th century, and with the loss of a hyphen along the way, the term radiosurgery was repurposed to describe the procedure now widely used as treatment for a variety of benign and malignant intracranial neoplasms as well as a few selected functional disorders. Applying principles of stereotactic surgery and harnessing the tissue- and tumor-ablative potential of ionizing radiation, the Swedish neurosurgeon Lars Leksell designed the first prototype unit for stereotactic radiosurgery (SRS), opening up new clinical opportunities and launching a Hernaman-Johnsonian interspecialty cooperation between neurosurgeons and radiation oncologists that continues to provide valuable clinical service to patients and new insights into tumor and normal tissue biology.
In this chapter we review the distinct technical aspects of SRS, the current understanding of SRS radiobiology, and clinical outcomes after SRS for common indications.
In the half-century following Leksell's pioneering work in SRS, numerous other investigators around the world made important contributions to the technical development of SRS. Whereas Leksell eventually settled on a design involving a hemispherical pattern of multiple cobalt-60 sources shielded and arranged so that their output gamma rays would converge on the target, in the 1980s and 1990s various linear accelerator-based SRS platforms were also developed. Many of these newer commercially available systems are also capable of delivering other forms of radiotherapy, and there was a period when the commonly used terminology describing SRS and non-SRS forms of radiotherapy were somewhat loosely interchanged. Ultimately, the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, and the American Society for Radiation Oncology (ASTRO) agreed that a uniform description of SRS was required to avoid confusion, and the consensus definition is as follows.
SRS is a distinct discipline that uses externally generated ionizing radiation in certain cases to inactivate or eradicate a defined target or targets in the head or spine without the need to make an incision. The target is defined by high-resolution stereotactic imaging. To assure quality of patient care, the procedure involves a multidisciplinary team consisting of a neurosurgeon, radiation oncologist, and medical physicist.
SRS typically is performed in a single session, using a rigidly attached stereotactic guiding device, other immobilization technology, and/or a stereotactic image-guidance system, but can be performed in a limited number of sessions, up to a maximum of five.
Technologies that are used to perform SRS include linear accelerators, particle beam accelerators and multisource cobalt-60 units. To enhance precision, various devices may incorporate robotics and real time imaging.
Contained within this definition are references to several essential ingredients of SRS. The treatment is noninvasive and involves external radiation sources or beams. As it is also elaborated in other ASTRO statements concerning SRS, the adjective stereotactic implies that the target lesion is localized relative to a fixed three-dimensional spatial coordinate system, using either a rigid head frame or reliable internal fiducial markers (bony landmarks or implanted markers). And, most important, SRS is a multidisciplinary endeavor in which the quality of patient care is of paramount importance. Appropriate patient selection, treatment delivery, and follow-up care including complication management is best accomplished by a multidisciplinary team.
Regarding the issue of patient safety, in 2011, ASTRO issued a white paper concerning quality and safety considerations for SRS and the extracranial application of high dose-per-fraction irradiation, stereotactic body radiation therapy (SBRT). This report highlights structure and process elements that are essential for establishing and operating a clinical program with the proper recognition of the risks involved and attention to detail that maximizes the chance for successful treatment while minimizing the chance of error.
As for any clinical activity involving radiation therapy, developing a culture of safety is crucial. Collegiality and a nonjudgmental atmosphere can contribute to an environment that fosters proactive recognition of ways to avoid systematic problems that might produce potentially harmful errors. In the context of SRS, mistakes can be magnified beyond what might be seen in conventionally fractionated radiotherapy treatments. That an entire treatment course is delivered in only a single or perhaps a few fractions means that a targeting inaccuracy greatly increases the odds of tumor progression via geographic miss. Furthermore, that each treatment involves a large amount of ionizing radiation dose deposition means that the risk of injury to normal tissue may be escalated beyond an acceptable range if the delivered dose hotspots drift into adjacent normal brain parenchyma rather than remain in the target volume. A thorough discussion of quality assurance methods in SRS is outside the scope of the present chapter. Among the high-priority tasks are accurate initial commissioning and periodic calibration of the treatment delivery technology, proper personnel training, and various patient-specific preparation routines.
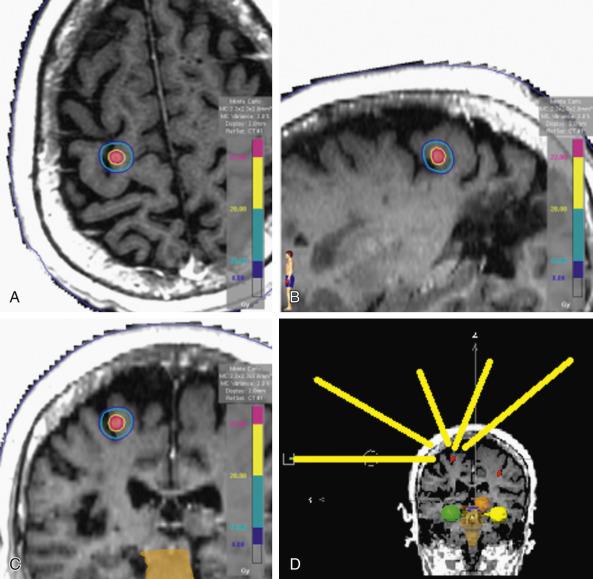
Common to all delivery technology for SRS is the use of multiple nonopposed radiation beams that converge on a target in the brain, thus avoiding a high dose to normal tissues in the entrance and exit paths of the beams while depositing an ablative dose within the target volume. The ideal treatment delivery plan achieves a high level of conformality, implying that the volume receiving the prescription dose closely approximates the target volume, thus minimizing the volume of normal tissue exposed to a high dose ( Fig. 27.1 ). Unlike the case in some applications of conventionally fractionated radiation therapy, wherein dose homogeneity within the target volume is clinically valuable for cosmetic or other endpoints, for SRS it is generally advantageous to allow for a dose hotspot to be present within the target volume, both for the purpose of increasing the intensity of therapeutic effect and steepening the gradient of dose falloff into surrounding normal tissues in the brain. This latter goal in particular is often best achieved under the conditions whereby the beam's-eye view of the tumor nearly surrounds the contour of the target itself, perhaps with even a “negative margin” where the beam is slightly smaller than the target in cross section—effectively exploiting that the maximum slope of dose falloff outside the beam is generally at the midpoint of the lateral penumbra, a consideration also applicable in SBRT.
Upon recall of the classic “4 Rs” of radiobiology, it is readily apparent that SRS involves considerations that depart from the traditional views of conventionally fractionated radiotherapy. For a single treatment course, the interfraction processes of repair, repopulation, redistribution, and reoxygenation are not relevant realities. Furthermore, for single or extremely hypofractionated regimens, the utility of the popular linear-quadratic model of radiobiological potency has been challenged given the lack of agreement with some preclinical observations and emerging awareness of dose threshold effects that affect tumor and normal tissue responses via vasculature-related events.
By the 1980s, SRS had become appreciated as a safe and effective therapy for arteriovenous malformations (AVMs), and efforts were initiated to understand the nature of the therapeutic histologic effect on blood vessels in particular. Interestingly, a wide variety of animal models (e.g., goat, baboon, cat) of SRS have been used to study normal tissue effects. Some of these early reports included experimental single fraction doses on the order of 150 to 200 Gy, and thus their relevance to modern clinical practice, which frequently uses doses an order of magnitude smaller, is uncertain. However, using a dose more closely in line with current human clinical practice, Acker et al. at Duke University studied the pial vasculature in a rat using a window chamber model that allowed for repeated direct visual inspection of the in vivo microcirculation.
The experimental setup at Duke included a 4-MV LINAC fitted with a 2.2-mm diameter collimator. Doses in the range of 15 to 30 Gy were administered in a single exposure, and serial observations were made to characterize the effect on blood flow, vessel density, and leukocyte-endothelial cell interactions. Acute reductions in vessel length density and blood flow were observed at 24 hours postirradiation and continued to become more pronounced 30 days after exposure, without a suggestion of dose dependence above the level of a 15-Gy dose. Notably foreshadowing a future area of intense focus by others, morphological changes that included extensive sections of endothelial cell loss were also observed within weeks after irradiation. The authors found this apparently apoptotic effect to be somewhat curious, speculating on its relationship to changes observed in white blood cell interactions with the vessel walls and hypothesizing that platelet-activating factor might play an important role.
This possibility that the tumor vasculature is an important target of radiotherapy is not an especially modern concept and was suggested at least as long ago as 1930, when James Ewing commented that with regard to certain tumors whose cells were thought to be relatively radioresistant, “it seems to me highly probable that the influence is mainly upon the blood vessels, which eventually shrink and cut off the blood supply.” There was rather limited investigation into this topic specifically between that statement and the SRS-inspired work of the late 20th and early 21st centuries. Many of these studies are cataloged in a review by Park et al., who concluded that there appears to be a suggestion of a threshold effect occurring at a fraction size on the order of 10 Gy, above which there appears to be substantial vascular damage that contributes indirectly to a tumoricidal effect.
Perhaps best exemplifying these studies, while also adding fundamental mechanistic insights, is the work of Garcia-Barros et al. from Memorial Sloan-Kettering Cancer Center. In the experiments reported in 2003, these investigators implanted MCA/129 fibrosarcomas and B16F1 melanomas into mice that were either genetically wild type or deficient in acid sphingomyelinase (ASMase), an enzyme needed for endothelial cell apoptosis. For both tumor cell types, host ASMase deficiency was associated with radioresistance as evidenced by significantly enhanced tumor growth delay after a single dose of 15 Gy. Confirmatory assays of endothelial apoptosis reported in the same paper demonstrated that the effect occurs acutely, peaking within 3 to 6 hours after exposure. Additionally, there is an apparent threshold dose for inducing endothelial apoptosis between 7 Gy, where essentially zero apoptosis was seen, and 11 Gy, where the percentage of apoptotic cells in ASMase wild type jumped up to approximately 20%. There was a continued increase in percentage of apoptotic endothelial cells up to approximately 60% with a dose of 25 Gy, the upper limit of dose evaluated in this study. As will be discussed, the profound vascular effects of SRS are also well-illustrated clinically by the frequently successful obliteration of complex intracranial AVMs when using single-fraction doses in the upper range of what was tested here preclinically.
For fractionated radiotherapy, the most frequently applied mathematical model to relate radiation dose to expected tumor cell kill is the linear-quadratic (LQ) model, based on a formula that first appeared in Lea and Catcheside's description of the relationship between radiation dose and incidence of chromosomal translocations, using a plant model. Although this model has been the most popular in recent decades and is discussed in Chapter 1 , sometimes overlooked in the original publication by Lea and Catcheside is recognition that for high-dose exposure, there would in principle need to be a correction applied to the LQ model that accounted for a decaying effect when the dose is delivered over a prolonged interval, presumably related to what amounts to intrafractional repair as some radiation-induced single-stranded breaks recombine without translocation. Experimental data supporting this possibility in mammalian cells may be found in the work of Eley et al., who used a glioma cell line and modeled SRS-type dose effects, comparing radiosensitivity when the same 12-Gy dose was given either in a single brief exposure or else in a series of smaller exposures spread out over 1 hour. The intent was to simulate SRS treatments that might be delivered clinically using multiple beam angle and table position changes, effectively prolonging the time of dose delivery. The results revealed that the cells underwent detectable cell-cycle arrest at the G2/M after the first subfraction in intermittent exposure conditions and that this effect was associated with relative radioresistance, consistent with the Lea-Catcheside predictions.
In view of the vascular threshold dose effects and possible intrafraction repair effects, among other differences from fractionated radiotherapy, an argument can be made that alternatives to the LQ model are needed. Indeed, numerous groups have proposed alternative models to characterize the relationship between radiation dose and tumor cell kill when doses in the realm used for SRS or SBRT are used. For example, Guerrero and Li proposed a modified LQ model based largely on the lethal-potentially lethal model of Curtis, except they proposed a new term, δ, to account for repair kinetics related to dose rate. In a model that combines elements of LQ formalism and a multitarget model, Park et al. at the University of Texas–Southwestern Medical Center have described a “universal survival curve” that involves a step function: LQ estimates apply below a certain dose per fraction, but above a transition dose, D T , there is a correction that effectively straightens the curve to maintain a linear relationship between dose and log cell kill above D T . The purpose of this construct is to match the true relationship between high-dose exposure and log cell kill, which tends to be overestimated by the LQ model. The universal survival curve model may be used to derive a convenient index of radiobiological potency, the single fraction equivalent dose (SFED), by which different SRS or SBRT regimens might be compared. When the dose per fraction, d , exceeds D T , SFED is calculated as follows:
where D is the total dose, D 0 is the dose required to reduce the surviving fraction of cells to 37%, n is the total number of fractions, and D q is the quasi-threshold dose of the multitarget model. The SFED metric has been demonstrated to describe a dose-control relationship for a variety of tumor types treated with SBRT.
To gauge the true dose-related normal tissue toxicity risk in clinical settings, even the best predictive mathematical models are not a substitute for careful analyses of actual clinical data. The Quantitative estimates of Normal Tissue Effects in the Clinic (QUANTEC) project was sponsored jointly by the American Association of Physicists in Medicine and ASTRO. Medical physicists, radiobiologists, and radiation oncologists from across North America, Europe, and Asia participated. The group's charge was to catalog, review, analyze, and summarize all published literature concerning the quantitative relationship between dose of ionizing radiation and injury to normal tissues, with the intent of identifying practical guidelines for normal tissue dose constraints based not on models but on actual patient observations. Of direct relevance to SRS are the QUANTEC reports on radiation effects in the brain, brainstem, and optic nerves and chiasm.
In each case, there are acknowledged limitations concerning the quantitative data available for analysis; nevertheless, the QUANTEC papers do offer dose-volume parameters that are clinically useful in the design and evaluation of SRS treatment plans for individual patients. Largely influenced by the synthesis of numerous reports involving the treatment of AVMs and subsequent risk of radionecrosis, the authors of the QUANTEC brain paper concluded that toxicity increases rapidly once the volume of the brain exposed to >12 Gy is >5 cm 3 to 10 cm 3 . An added caveat is that eloquent areas of the brain such as the brainstem or corpus callosum require extra caution.
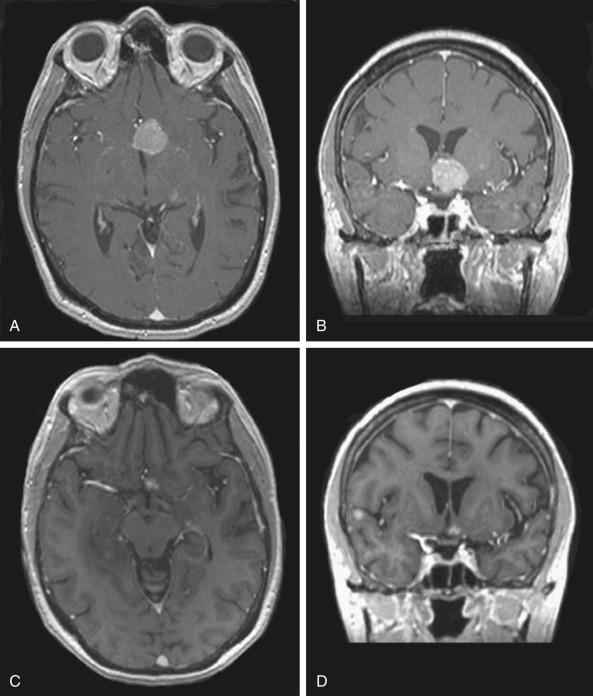
The QUANTEC brainstem summary is mainly concerned with tolerance to fractionated radiotherapy, given the larger number of studies available for that setting relative to the setting of brainstem SRS and, in particular, the paucity of long-term reports after SRS to brainstem metastases. One clinical indication for SRS in which long-term follow-up is typically available is for the treatment of acoustic schwannomas, a benign condition with negligible risk of mortality to the patient. Here, the dose to the brainstem is primarily just a glancing focus of scattered dose away from the actual target; a maximum point dose of less than 12.5 Gy is associated with a low risk of new cranial neuropathy in that setting. Regarding injury to the optic nerves or chiasm, the QUANTEC reviewers found that the risk of radiation-induced optic neuropathy in the more modern context of magnetic resonance imaging–aided planning is low, with a maximum point dose of ≤12 Gy to the optic apparatus. Fig. 27.2 provides an illustration of a patient treated with SRS where the tumor approached the chiasm, necessitating caution with the optic chiasm dose.
In Leksell's report describing the first 762 cases treated with SRS at the Karolinska Institute, the three most common diagnoses were AVMs, Cushing disease, and acoustic neuromas. No brain metastases were treated, and a large percentage of the cases were functional disorders such as trigeminal neuralgia, intractable pain elsewhere in the body, or pituitary tumors. Nowadays, the most common indication for SRS is brain metastases. Although many of the diagnoses treated in the early years by Leksell are still managed by SRS today, others such as Parkinson disease, anxiety, or obsessive-compulsive disorder are more frequently managed with other interventions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here