Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spinal muscular atrophies (SMAs) are genetic disorders that are clinically characterized by progressive muscle weakness and atrophy, associated with degeneration of spinal and, in the most severely affected patients, lower bulbar motor neurons. Classic proximal SMA is the most common form of SMA and the leading cause of infant mortality; it seems to be found in practically all populations but is diagnosed more frequently in infants and children, rather than adults. In the early 1890s, the Austrian clinician Guido Werdnig and the German physician Johann Hoffman were the first to describe the severe form of SMA, at the University of Graz, Austria, and in Heidelberg, Germany, respectively. Their reports described the neuromuscular phenotype of the disease and the associated loss of anterior horn cells in the spinal cord.
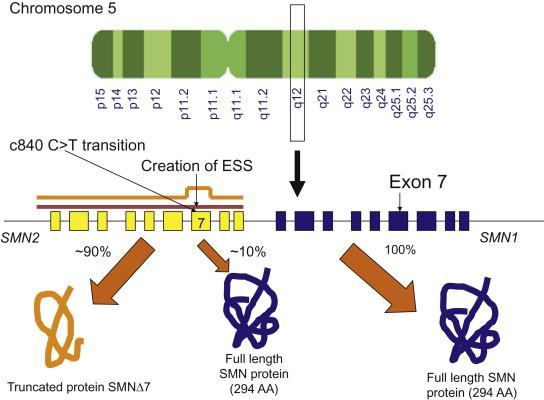
The disease results from homozygous deletions or mutations involving the “survival of motor neuron” ( SMN ) gene at locus 5q13. The SMN gene is present in 2 copies on each chromosome 5, designated SMN1 and SMN2 , forming an inverted duplication ( Figure 8.1 ). Five nucleotide changes which do not change amino acids differentiate SMN2 from SMN1. A single nucleotide change that creates an exonic splicing suppressor in exon 7 of SMN2 is the critical one and leads to exclusion of exon 7 in most transcripts ; thus the duplicated ( SMN2 ) gene produces less functional SMN protein. Most patients with 5q proximal recessive SMA harbor homozygous deletions involving exon 7 of the SMN1 gene, but maintain at least 1 copy of SMN2 . There is a rough correlation between SMN2 gene copy number, which varies normally in the population, the level of SMN protein, and severity of disease. The role of the SMN protein is still under active investigation. Modifying genes appear to be present as well, serving other roles in motor neuron function. SMA may involve dysfunction of more than lower motor neurons, with abnormalities of the neuromuscular junction noted in animal models, and abnormal muscle development in the most severely affected patients. There are also many other types of SMA, known as non-5q SMAs, related to mutations in various genes that are expressed in a wide range of tissues, including the nervous system.
The incidence of SMA has been estimated at 1 in 6000–11,000 live births or about 7.8–10 per 100,000 live births and at 4.1 per 100,000 live births for SMA type I. A 2005 study in Cuba found a reduced incidence of type I SMA among the Cuban population overall (3.53 per 100,000 live births) and especially among those of African ancestry (0.89 to 0.93 per 100,000 live births). The estimated pan-ethnic disease frequency is approximately 1 in 11,000. The carrier frequency for mutations in the SMN1 gene has been estimated at from 1:38 to 1:70. A 2009 epidemiologic study sought to determine the carrier frequency in different ethnic groups in North America. The carrier frequency was highest in Caucasians (1 in 37, or 2.7%) and was lowest in Hispanics (1 in 125, 0.8%). Ashkenazi Jews (1 in 46, 2.2%) and African Americans (1 in 56, 1.8%) were of intermediate frequency. It should be noted that, despite the high carrier frequency, the incidence of SMA is lower than expected. It has been postulated that this may reflect that some fetuses have a 0/0 SMN1 / SMN2 genotype (i.e. no SMN protein is present at all), which is known in other species to be embryonic lethal.
Though most patients with spinal muscular atrophy have deletions or mutations involving the SMN1 gene, a range of phenotypic severity permits division into four broad clinical subtypes. It is recognized that the subtypes represent a phenotypic continuum extending from the very severe, with onset in utero , to the very mild, with onset during adulthood; there is also a spectrum of severity within each of these groups ( Table 8.1 ). For the purposes of clinical classification, or of guidelines developed for standards of care, the “maximal functional status achieved” approach, which classifies type I patients as “non-sitters,” type II patients as “sitters,” and type III patients as “walkers,” has been used. Patients with a mild phenotype and onset during middle or late age are classified as type IV. The age at onset is also considered in the classification but because of potential overlap between subtypes and the difficulty in accurately determining the onset of symptoms, it has not been considered as the sole determinant of disease subtype.
| SMA Type | Other Names | Age at Onset | Life Span | Highest Motor Milestone Achieved | Other Features | Proportion of Total SMA (%) |
|---|---|---|---|---|---|---|
| Type IA | Prenatal, Congenital SMA, Werdnig-Hoffman disease | Prenatal | <6 months | Mostly unable to achieve motor milestones |
|
60 a |
| Type IB, Type IC | Werdnig-Hoffman disease, Severe SMA (“non-sitters”) | Type IB (0–3 months) Type IC (3–6 months) | <2 years without respiratory support | Never sits supported |
|
60 a |
| Type II | Intermediate SMA (“sitters”), Dubowitz disease | 6–18 months | >2 years ~70% alive at 25 years of age | Sits independently. Never stands or walks |
|
27 |
| Type III | Kugelberg-Welander disease, Mild SMA (“walkers”) | >18 months Type IIIA (prior to 3 years) Type IIIB (after 3 years) | Almost normal | Stands and walks |
|
12 |
| Type IV | Adult SMA | >21 years | Normal | Normal | 1 |
a SMA types I, IA, IB, and IC all have a 60% proportion of total SMA.
Following the initial description of infantile SMA by Werdnig and Hoffman in the early 1890s and further descriptions made by Sylvestre in 1899 and Beevor in 1903, infantile or type I SMA was described again in detail, both clinically and pathologically, by Randolph Byers and Betty Banker at Boston Children’s Hospital in 1961. Patients with type I SMA, also known as Werdnig-Hoffman disease, present between birth and 6 months of age. SMA type I has been further subdivided into three groups: type IA (or type 0 in certain reports ) with onset in utero and presentation at birth, type IB with onset of symptoms prior to 3 months of age, and type IC with onset between 3 and 6 months of age. Infants with SMA type I have progressive proximal weakness that affects the legs more than the arms. They have poor head control, hypotonia that causes them to assume a “frog-leg” posture when lying and to “slip through” on vertical suspension, and areflexia. They are never able to sit (“non-sitters”) ( Table 8.1 ). There is also weakness of the intercostal muscles with relative sparing of the diaphragm, producing a bell-shaped chest and a pattern of paradoxical or “belly”-breathing. Infants with type I SMA also classically exhibit tongue fasciculations and eventually have difficulty swallowing, with risk for aspiration and failure to thrive. Other cranial nerves are not as affected, although facial weakness does occur at later stages of the disease. Cognition is normal, and they are often noted at diagnosis to have a bright, alert expression that contrasts with their generalized weakness. Infants with type I SMA usually develop respiratory failure by age 2 years, or much earlier, and in the past most did not survive past 2 years; however, there has been some increase in survival in recent years with the use of assisted ventilation (to be described in more detail in later sections) and other interventions. Prior natural history studies have reported shortened life span, with 68% mortality within 2 years and 82% by 4 years of age. The application of nutritional and respiratory interventions has reduced the mortality to approximately 30% at 2 years, but about half of the survivors are fully dependent on noninvasive ventilation.
It was previously believed that SMA is a purely motor neuron disorder. However, recent studies have shown that severe type I SMA can result in various organ manifestations, apart from spinal cord motor neurons such as brain, cardiac, vascular, and even sensory nerve involvement. Recent autopsy studies have shown increasing evidence of congenital heart disorders in severe SMA, with the most common association being hypoplastic left heart syndrome. In a study, 3 out of 4 (75%) patients with 1 SMN2 copy had congenital SMA (SMA type 0) associated with hemodynamically significant atrial or ventricular septal defects, which underscores the relevance of the SMN protein for normal cardiac development ; despite the reported cases, however, a chance association has not been firmly excluded. Severe SMA mouse models have shown autonomic disturbances including bradyarrhythmia and progressive heart block, as well as dilated cardiomyopathy and decreased contractility ; in humans with molecularly proven SMA there has been no evidence of dilative or congestive cardiomyopathy, nor have there been reports of AV conduction anomalies or arrhythmias besides bradyarrhythmias. Studies done on various mouse models have also found that severe SMN protein deficiency might present as vasculopathy. This was also noted in the case reports of two unrelated patients with severe SMA type I. Both of these infants developed ulcerations and necroses of the fingers and toes. Sural nerve biopsy studies on them showed thrombotic occlusion of small vessels without inflammation resulting in perfusion abnormality and subsequent tissue necrosis in the absence of a significant peripheral neuropathy. Autonomic dysfunction is thought to be the primary cause of this vasculopathy. While some of the reported patients had only one SMN2 copy, two cases of infants with SMA type I in whom a distal necrosis developed had two SMN2 copies. In a different biopsy study done by Rudnik-Schöneborn et al. on 19 patients with infantile SMA, significant sensory nerve pathology was found in severe SMA type I patients, whereas no sensory involvement was found in SMA type II and type III patients. Out of these, axonal degeneration was noted in seven and abnormal sensory conduction in five SMA type I patients. The abnormal nerve conduction study patients were followed with biopsy, which showed marked fiber density reduction in four patients, thus indicating that severe SMA type I patients can show both clinical and morphological sensory nerve involvement.
This intermediate form of SMA was first reported in 1893 at the University of Edinburgh, United Kingdom, and described again by Byers and Banker in 1961 and in detail by Dubowitz in 1964. Patients with type II SMA, also known as intermediate SMA or Dubowitz disease, are able to sit unsupported at some point (“sitters”), but are never able to stand alone or walk ( Table 8.1 ). The onset of symptoms is between 6 and 18 months of age. They have progressive proximal weakness affecting legs more than arms, hypotonia, and areflexia. They also develop progressive scoliosis which, in combination with intercostal muscle weakness, results in significant restrictive lung disease as they grow older. They develop joint contractures and can have ankylosis of the mandible. They exhibit tremor, or polyminimyoclonus, of the hands. Although their body mass index may be low (at the 3 rd percentile or less when compared with normal children), the high-functioning, nonambulatory patients have a higher relative fat mass index and are at risk of becoming overweight. Cognition is normal and verbal intelligence may be above average. In a study done on 240 type II patients, survival rates were found to be 98.5% at 5 years and 68.5% at 25 years. Patients may live into the third decade, but life expectancy is shortened due to the risk of respiratory compromise.
In 1956, Kugelberg and Welander described a much milder form of SMA characterized by prolonged ambulation. Patients with type III SMA, also known as Kugelberg-Welander disease, are able to stand alone and walk at some point (“walkers”). The onset of symptoms occurs after the age of 18 months; it has further been subdivided into type IIIA (onset between 18 months and 3 years) and type IIIB (onset after 3 years). They have progressive proximal weakness affecting legs more than arms and may ultimately need to use a wheelchair, but they generally develop little to no respiratory muscle weakness or severe scoliosis. Loss of ambulation increases the risk of these complications. They may have tremor or polyminimyoclonus of the hands. Sometimes, the calves of these patients can be prominent and hence type III SMA can be confused with Becker muscular atrophy. Life expectancy is not significantly different compared with the normal population ( Table 8.1 ). Case Example 8.1 describes the disease progression of a particular type III patient.
An 11-year-old girl was evaluated at age 19 months for delayed motor development. At age 4 months, hypotonia was noted, but her early gross motor development had been normal. She sat at 8 months, crawled at 9 months, and pulled up to stand and cruised at 12 months. By age 25 months, she was taking a few steps without assistance and initiating walking. At 19 months, after the clinical diagnosis of SMA was confirmed by molecular testing, she was enrolled in early intervention and aqua therapy programs, and physical therapy was continued through preschool. An intensive PT and aqua therapy program was initiated after she entered elementary school.
At age 19 months, she was evaluated by pediatric neurology because of delayed motor milestones, hypotonia, and weakness. The bilateral weakness involved the axial and proximal limb muscles to a greater degree than the distal muscles, and the weakness was asymmetric with greater involvement of the right arm and left leg. The muscle bulk was reduced. Eye, facial and tongue muscles were normal. Tendon reflexes and Babinski signs were absent. The spinal contour was normal but the lumbar lordosis was accentuated. She was remarkably intelligent. Electromyography showed ongoing denervation with evidence of reinnervation in multiple muscles of legs and arms; her sensory nerve conduction studies were normal whereas the motor responses were of low amplitude but with normal conduction velocities; these findings suggested motor neuron disease. The diagnosis of spinal muscular atrophy was confirmed by DNA testing which showed a homozygous deletion of SMN1 and three copies of SMN2 gene.
At the age of 5 years, she was enrolled in a prospective multicenter natural history study at the Columbia University Spinal Muscular Atrophy Clinical Research Center; she was evaluated at baseline (age 6 years) and subsequently at months 2, 4, 6, 9, and 12. She continued to be followed every 6 months for a total of 84 months (age 13 years). The outcome measures included motor function, pulmonary function, muscle strength, electrophysiological measures (CMAP and MUNE), and patient-reported outcomes. From 6 to 7.5 years, her motor function improved but from 7.5 to 11 years, her motor function declined with continued body growth. Manual muscle testing showed only minimal decrease in strength. This patient walked with assistance until age 9 years, longer than expected, probably due to the intensive rehabilitation she continued to receive. In fact, her motor function improved during the first 18 months of observation, but it was then followed by a slow functional decline. These observations are consistent with the results of published natural history studies of spinal muscular atrophy types II and III, which show stability over the initial 12-month period (with younger patients often showing improvement), followed by gradual functional decline over years. After infancy and with continued weight bearing, the patient developed a progressive thoracolumbar scoliosis, pelvic obliquity, and recurrent painful hip subluxation ultimately requiring multiple orthopedic procedures to correct the advancing musculoskeletal deformities and persistent joint pain. The orthopedic deformities accelerated after ambulation was lost and the patient spent her waking hours in a motorized wheelchair. Respiratory complaints also supervened with intercurrent pulmonary infections demanding aggressive proactive management to protect the airway and to ensure adequate ventilation.
This case illustrates several points. Dating the onset of symptoms is often in the “eye of the beholder.” This patient has SMA type IIIA with onset before age 3 years. These children often regress functionally and lose the ability to stand and walk after years. In fact, this patient had neuromuscular symptoms dating back to early infancy with hypotonia. She was cruising by 12 months, but she did not take steps until age 19 months, suggesting underlying weakness, sensory disturbance, or incoordination. There were no cerebellar signs and the sensory nerve studies were normal. Her remarkable motor accomplishments, despite the early signs of hypotonia and weakness, underscore possible mitigating factors that can alter the natural history of SMA. Early intensive rehabilitation clearly benefited this patient’s natural course. It also highlights the fact that motor function can worsen over long periods of time despite relatively preserved strength and optimal rehabilitative care. Growth during development also adds additional physical burden to a compromised neuromuscular system, and contributes to the subtle functional decline during childhood and adolescence. Whether the disease process worsens in parallel with the functional decline remains a subject of debate, and the answer is important particularly as it relates to the anticipated response to therapeutic intervention. Respiratory insufficiency and musculoskeletal deformities, often with considerable associated pain, are inevitable complications of SMA and should be addressed proactively to forestall and minimize the resulting acute and chronic morbidity that otherwise erodes the patient’s quality of life.
There has been much debate about the appropriate classification of patients into these three types of SMA because, as mentioned, there are patients within these categories who exhibit phenotypes of differing severities. A classification system based on a continuous rather than discrete variable (e.g. SMA “type 1.8” in the case of a less severely affected type I patient) has been proposed to better capture the clinical spectrum of these patients.
There are also patients who are outliers on either end of the phenotypic spectrum. As discussed above, an SMA “type IA” (formerly known as type 0) has been used to describe neonates who present with severe weakness and profound hypotonia, probably of prenatal onset, as well as with a history of decreased fetal movements. The majority do not attain any motor milestones. Other findings include areflexia, facial diplegia, atrial septal defects, and joint contractures. In SMA type IA, respiratory failure forms an important cause of morbidity and mortality, requiring noninvasive ventilation and endotracheal intubation at birth. Life expectancy is reduced and most of them are unable to survive beyond 6 months of age ( Table 8.1 ). Furthermore, arthrogryposis multiplex congenita (congenital joint contractures involving at least two regions of the body) has been noted in SMA patients with SMN1 gene deletions, and congenital axonal neuropathy involving motor and sensory nerves in conjunction with facial weakness, joint contractures, ophthalmoplegia, and respiratory failure at birth has been reported in three newborn siblings with deletions in the SMA chromosomal region.
A milder adult-onset SMA, or type IV SMA, has also been described with onset of symptoms after age 21 years and essentially normal life span. Most patients with the SMA type IA and IV phenotypes have homozygous deletions of exon 7 in SMN1 , but as it will be discussed in the Genetics section, the SMN2 copy number is usually only 1 in SMA type IA and 4 to 5 in SMA type IV.
The non-5q13 associated spinal muscular atrophies are a heterogeneous group of motor neuron diseases associated with mutations in a variety of different genes (e.g. X-linked and autosomal dominant or recessive spinal muscular atrophies), distal or segmental spinal muscular atrophies, or distal hereditary motor neuropathies. Patients with these disorders generally have some clinical characteristics that can help differentiate them from those with 5q13-associated or classic SMA. The differential diagnosis of SMA is detailed in the respective section.
Linkage analysis studies showed that all three forms of SMA map to chromosome 5q11.1-13.3. In 1995, Lefebvre et al. identified the SMN gene within this region, which was absent or interrupted in 98.6% percent of the patients in their group. The structure of this region is complex, with a large inverted duplication of a 500 kb element. This contains the SMN1 gene, which is deleted or interrupted in patients with SMA and is evolutionarily older, in the telomeric portion of the region; and the SMN2 gene, a duplication of SMN1 that differs from it by only 5 nucleotides, in the centromeric portion ( Figure 8.1 ). The critical difference between SMN1 and SMN2 is a C to T transition that creates an exon splicing suppressor in exon 7 of SMN2 . While this splice modulator change is translationally silent (i.e. it does not change the amino acid sequence), it affects the alternative splicing of the gene, so that exon 7 is spliced out of or excluded from most SMN2 mRNA transcripts. This altered mRNA results in production of a truncated version of the SMN protein, which does not oligomerize efficiently and is degraded. Since exon 7 is not always spliced out of all SMN2 mRNA, a small amount of full-length transcript and hence functional protein is produced by SMN2 , but it yields only on average about 10% as much as that produced by SMN1 . In patients with SMA both copies of the SMN1 gene are deleted or disrupted, so the individual is left with only the small amount of SMN protein produced by the remaining copies of SMN2 . The amount of SMN protein is inversely correlated with the severity of disease.
About 95% to 98% of patients with SMA harbor deletions of the telomeric SMN1 gene ( Table 8.2 ). The remainder have small intragenic mutations or have undergone gene conversions from SMN1 to SMN2 . De novo mutations occur at a rate of about 2%, which is relatively high, and explained by the fact that this region of chromosome 5 is unstable, containing not only the inverted repeat of SMN1 and SMN2 , but other surrounding low copy number repeats. This leads to a high rate of unequal crossover and de novo mutations (especially paternally derived), which could explain the relatively high carrier frequency despite the mortality rate for the most severe forms of the disease. More mildly affected patients seem more likely to undergo gene conversions of SMN1 to SMN2 rather than deletion of SMN1 .
| Type of Mutation | Test applied | Mutation Detection Rate |
|---|---|---|
| Homozygous deletion of exon 7 a | SMN1 Targeted mutation analysis PCR/restriction enzyme analysis or multiplex ligation probe amplification (MLPA) methodologies |
~95–98% |
| Compound heterozygosity (Deletion of SMN1 exon 7 [allele 1] and an intragenic mutation of SMN1 b [allele 2]) |
Targeted mutation analysis combined with SMN1 gene sequence analysis c | 2–5% |
| SMN2 copy number d | Quantitative PCR analysis and other methodologies e | N/A |
a Testing for exon 8 deletion is not necessary.
b Small intragenic deletions/insertions and nonsense, missense, and splice site mutations.
c Whole-gene deletions/duplications are not detected.
d SMN2 copy number ranges from 0 to 5.
e MLPA, long-range PCR, chromosomal microarray (CMA) that includes the SMN1 , SMN2 chromosomal segment.
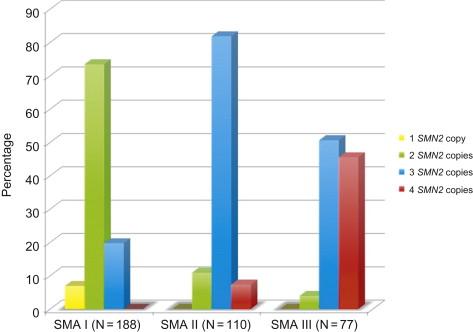
The number of copies of SMN2 per chromosome 5 varies among normal individuals, and 10–15% of the population possess no copies of SMN2 . Among patients with SMA, a clear correlation has been established between SMN2 copy number and phenotypic severity. Feldkotter et al. in 2002 found that in their series 80% of patients with type I SMA had one or two copies of SMN2 , 82% of patients with type II had three copies of SMN2 , and 96% of patients with type III had three or four SMN2 copies ( Figure 8.2 ). Studies by Mailman et al. in 2002 and Arkblad et al. in 2009 found similar results (95–100% of type I patients had one or two copies of SMN2 , and all type III patients had at least three copies of SMN2 ). However, this correlation is not so perfect as to permit the absolute prediction of clinical severity based on SMN2 copy number, especially in the intermediate forms of the disease where there is some overlap (patients with three copies of SMN2 have been described with all three phenotypes). One reason for the overlap is that not all copies of the SMN2 gene are equal; in terms of full-length SMN protein production, some copies probably produce less and some more than 10% functional protein. In general, though, it can be said that a patient with one or two copies of SMN2 is highly likely to present with SMA type IA, IB, or IC. Interestingly, unaffected family members with homozygous SMN1 deletions and five copies of SMN2 have been described, which suggests that SMN2 copy number alone cannot be the sole modifying factor in disease severity, since there are patients with type III SMA who also have five copies of SMN2 . In Smn (−/−) knockout mice, the introduction of eight copies of human SMN2 can rescue the phenotype and result in essentially normal mice. The presence of only two copies of SMN2 in mice results in a severe SMA-like phenotype and death at 5 days. The addition of an SMN transgene lacking the exon 7 sequence ( SMNΔ7 ) along with two copies of SMN2 prolongs the lifespan of the transgenic mouse to about 14 days.
A positive modifier has been identified in the SMN2 gene. A single base substitution in exon 7 in three unrelated patients’ DNA (c.859G>C) creates a new exonic splicing enhancer that increases exon 7 inclusion and thus the amount of full-length protein by about 20%. The phenotype in these patients was less severe and did not correlate with their SMN2 copy numbers, supporting the positive modifying effect of this sequence change and the notion that not all SMN2 copies are equal. Of note, this variant has been identified in two copies in type IIIB patients with a mild phenotype, in one copy in type II patients, and not at all in severe type I patients. It seems that a 20% increase in full-length protein in patients with two copies of SMN2 will probably result in mild type IIIB SMA; therefore, it is fair to speculate that a 25% increase in full-length SMN protein in the same patients may result in total rescue of the phenotype. There are also modifiers that do not involve the SMN1 or SMN2 genes. It is well known that haploidentical siblings with the same SMN2 copy number can have SMA of different severities. Higher expression of plastin 3 ( PLS3 ) was also identified as a gender-specific protective modifier of SMA in asymptomatic SMN1 -deleted females, carrying the same number of SMN2 copies as their affected sibling. A subsequent study, however, showed that PLS3 may be an age- and/or puberty-specific and sex-specific modifier as the expression of the gene was greatest in postpubertal female patients with SMA type III, intermediate in SMA type II, and lowest in SMA type I patients, underscoring an association with disease severity as well. Further, high plastin levels have also been detected in female siblings with more severe SMA presentation. It is possible that the plastin 3 modifier is not an important modifier of SMA phenotype or it may be female-specific and incompletely penetrant. No changes in the plastin 3 gene itself have been reported; thus, the modifier role of plastin 3 in SMA remains uncertain. Sequence variants within splicing regulators of the SMN1 and SMN2 genes, either polymorphisms or mutations, may explain divergent phenotypes in haploidentical siblings.
Regarding the level of SMN protein itself, although there is in general an inverse correlation between the level of SMN protein and severity of disease, this correlation does not seem to be quite as close as that between SMN2 copy number and phenotype.
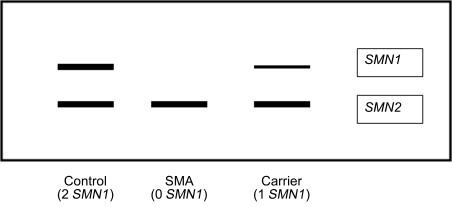
Genetic testing for SMA can be performed with PCR-based targeted mutation analysis using a restriction enzyme that digests exon 7 ( Table 8.2 ). If SMN1 exon 7 is homozygously deleted (as it is in about 95–98% of patients) then the restriction fragments will be absent, the DNA will not be amplified, and a band will not be seen on the gel. If an individual is a carrier (heterozygous deletion) then a lighter band will be present ( Figure 8.2 ). However, multiplex ligation probe amplification (MLPA) methodology is currently applied in most DNA diagnostic laboratories for deletion analysis of exon 7 of the SMN1 gene in potential probands and carriers. This type of targeted mutation testing in conjunction with sequence analysis can also detect individuals who are compound heterozygotes with a deletion of exon 7 in one SMN1 allele and an intragenic point mutation in the other allele. In such a case (approximately 2–5% of individuals with clinical diagnosis of SMA), sequence analysis of the SMN gene will detect the mutation; this sequence testing, however, will not detect exonic deletions or duplications and will not determine whether the point mutation is in the SMN1 gene or SMN2 gene (if one of these genes is not deleted). Fortunately, certain point mutations have been described in more than one SMA patient already and thus the detection of a previously reported mutation supports its pathogenicity and its location in the SMN1 gene. The use of long-range PCR or subcloning can also allow specific analysis of SMN1 and confirm that the mutation is present in SMN1 . See Figure 8.3 .
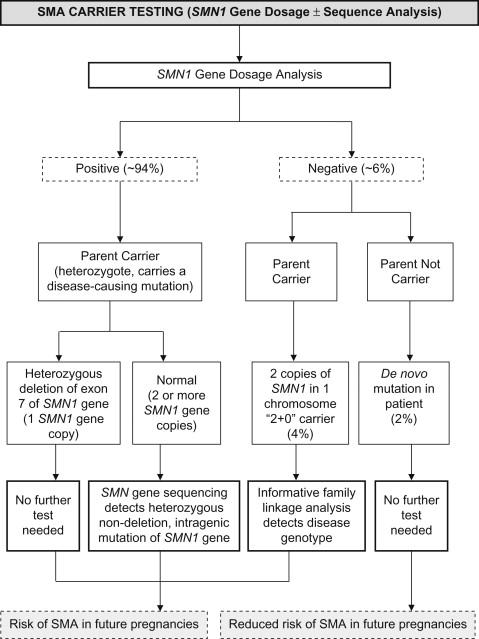
Carrier testing is feasible and accurate in the parents of patients with homozygous deletions of exon 7 or compound heterozygosity using a PCR-based dosage assay, known as “SMN gene dosage analysis” ( Figure 8.4 ). It can also be performed using other techniques such as long-range PCR, MLPA, and chromosomal microarray (CMA) that includes this gene segment. Sequencing of the SMN gene will detect point mutations in nondeletion carriers. Rarely, carriers may have two copies of SMN1 on one chromosome (a so-called “2+0 carrier”); the incidence of this genotype is about 4% of the general population. In the “2+0 carriers”, the SMA dosage carrier test will be falsely normal and thus one may need to pursue other methods such as family linkage analysis to identify the disease-associated genotype in families in which a deletion mutation has been transmitted more than once from a parent with two copies of the SMN1 gene on gene dosage testing. Due to the occurrence of de novo mutations in 2% of patients with SMA, one of the parents may not be a carrier. So, in approximately 6% of parents of a child with SMA secondary to a homozygous SMN1 deletion, SMN1 gene dosage testing will be normal.
As mentioned, a general correlation has been found between SMN2 copy number and disease severity, and it is relatively straightforward to determine SMN2 copy number in individual patients using various methodologies. However, this correlation is not so strict that the severity or type of disease can be reliably predicted based on copy number, and it is hence not advisable to offer families prognostic information based on SMN2 copy number assays.
The potential for newborn screening for SMA has been of great interest because the ideal time to initiate therapy would be prior to the initial degeneration of motor neurons, and newborn screening may help to identify presymptomatic individuals. Swoboda et al. performed a prospective study in prenatally diagnosed infants with type I SMA and found that, associated with the initial onset of symptoms or decline in function in these young infants, there was electrophysiologic evidence of precipitous denervation (assessed with serial measurements of compound motor action potential amplitude and motor unit number estimation), suggesting that motor neuron loss occurs very early. This provides further support for the potential usefulness of newborn screening to identify patients prior to the period of greatest motor neuron loss, should a therapeutic intervention become available.
Regarding the issue of routine preconception and prenatal screening for SMA, the American College of Obstetricians and Gynecologists Committee on Genetics published an opinion recommending against preconception and prenatal screening for SMA in the general population, citing insufficient evidence as to its cost-effectiveness and concerns regarding the education of the public and physicians about the complex issues raised by prenatal diagnosis of this disorder. However, various other professional medical societies have stressed the importance of screening. To address this issue and create a consensus, a meeting was organized by National Institutes of Health in late 2009. The conclusion of the meeting was that a carrier screening is technically feasible but its implementation will require addressing broader issues of screening in general.
In patients with spinal muscular atrophy the serum creatine kinase may be 2- to 4-fold elevated, but not more than 10 times normal. Nerve conduction studies demonstrate normal sensory potentials, but may show diminished compound motor action potential amplitudes. Needle EMG in type II/III patients demonstrates a neurogenic pattern with high amplitude, long duration motor unit potentials with reduced recruitment pattern. Needle EMG in type I patients shows denervation changes but may not show evidence of reinnervation, as there may not have been enough SMN protein and/or time for this to occur yet. As will be discussed in more detail in the Pathology section, muscle biopsy in all types of SMA demonstrates a neurogenic pattern, with grouped atrophy; the small atrophic fibers are of both types (type I and type II) whereas the large ones are always type I fibers. Although a neurogenic pattern with large group atrophy is common, notably, a fetal appearance has been noted in muscle biopsy specimens from patients with type I SMA, leading some to question whether type I SMA may actually result from arrested development of the motor unit rather than degeneration of the motor neuron. Indeed, a morphometric analysis of fetuses with type I SMA at 12–15 weeks showed delayed maturation of myotubes when compared with controls. While EMG continues to be used in the diagnosis of SMA in selected atypical cases, the use of muscle biopsy has become essentially obsolete.
SMN is a 38 kDa protein found in all cells, located in both the cytoplasm and in the nucleus, where it localizes to structures known as Gemini of Cajal bodies, or simply “gems” ; therefore, its expression is ubiquitous. In patient fibroblasts, the number of gems correlates with SMA severity. SMN is also localized in motor neuron axons. The SMN protein in conjunction with several Gemin proteins forms an SMN complex whose chaperone function facilitates the assembly of spliceosomal small nuclear ribonucleoprotein particles (snRNPs), essential components of the spliceosome complex, and hence plays a critical role in pre-mRNA splicing. The SMN protein may also be essential in assisting arginine methylation of some splicing-related proteins, transporting axonal mRNAs in motor neurons and perhaps in other processes in muscle and neuromuscular junction. The role of SMN protein in axonal mRNA trafficking and mRNA splicing may explain the selective vulnerability of spinal cord motor neurons to decreased SMN protein; albeit not the only ones, these neurons have long axons and many targets, particularly in large muscles, and thus may be very dependent on axonal mRNA transport. Further, they may be extremely vulnerable to the mRNA splicing defect. Nevertheless, it continues to be unclear whether a splicing defect due to SMN protein deficiency, disruption of an additional axonal SMN function, an unknown function, or a combination of these is responsible for the pathogenesis of SMA.
The complete absence of the SMN protein in cells is embryonic lethal in mice and other organisms. The reason that motor neurons are specifically vulnerable to partial SMN deficiency is unclear. Mouse and zebrafish models have been used to explore this question, but because the SMN2 gene is present only in humans, animal models have been created via knockout of SMN with insertion of a human SMN2 transgene. In addition to its role in spliceosome assembly, which is essential in all cells, SMN seems to associate with actin, which is involved in motor axon pathfinding and outgrowth during development. Two groups have demonstrated aberrant motor axon outgrowth and pathfinding in zebrafish models of SMA. It has been shown using laser capture microdissection that the amount of full-length SMN from SMN2 is lower in motor neurons of normal transgenic mice than in other neuronal cell types; this may explain the selective vulnerability of motor neurons. In both Drosophila and mice, SMN reduction leads to disrupted splicing of a minor intron in the Stasimon gene and hence reduction of a spliced isoform in motor neurons and proprioceptive neurons. Expression of Stasimon in both sensory and cholinergic neurons of the SMN-deficient fly corrects some of the larval neuromuscular junction and muscle defects and suggests that Stasimon has a role similar to that of SMN in the motor circuit function. Further, SMN seems to be required for the correct splicing of Stasimon and probably of other genes. Furthermore, SMN is required for normal sensory-motor circuit function in Drosophila ; this circuit includes not only proprioceptive sensory neurons but also interneurons. However, whether this circuit is operative in the pathogenesis of SMA in humans remains unknown.
Patients with SMA also appear to have structural and physiological abnormalities of the neuromuscular junction. Kong et al. showed that in severely affected SMA mice, prior to anterior horn cell death and/or axonal degeneration there is synaptic dysfunction at the neuromuscular junction in the form of decreased synaptic vesicle density at motor terminals, reduced quantal content, and slowed maturation of the acetylcholine receptor with prolonged retention of fetal characteristics. Kariya et al. also demonstrated structural and functional abnormalities at the level of the neuromuscular junction that precede overt symptoms in mice, as well as structural abnormalities in the neuromuscular junctions of humans with SMA, and hence proposed that SMA may be a “synaptopathy.” Based on this concept and the electrophysiologic data suggesting dysfunction of the neuromuscular junction in patients with SMA types II and III, treatment approaches directed towards enhancing neuromuscular transmission may also be beneficial to patients with SMA.
The differential diagnosis of 5q SMA is listed in Box 8.1 and is described in detail in this section.
Neoplasms (SMA types I, II, III)
Other myelopathies (SMA types I, II, III)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here