Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
International guidelines have been developed that define death as “the permanent loss of capacity for consciousness and all brainstem functions, as a consequence of permanent cessation of circulation or catastrophic brain injury,” with permanent meant to represent a loss of function that will not return spontaneously and/or with intervention ( ). Brain death is a clinical diagnosis that is based on the absence of neurologic function with a known irreversible cause of coma ( ; ). Brain death is the cause of 16% of all pediatric intensive care unit (PICU) deaths ( ). There were 875 pediatric organ donors (<18 years old) in 2018, of which 742 (85%) were donations after neurologic determination of death (DNDD) based on data from the Organ Procurement and Transplantation Network (OPTN) as of July 31, 2019 ( Table 38.1 ).
| Age | To Date * | 2019 | 2018 | 2017 | 2016 | 2015 | |
|---|---|---|---|---|---|---|---|
| <1 year | Total | 3,358 | 81 | 106 | 123 | 135 | 150 |
| DNDD | 2,523 (75%) | 67 (83%) | 93 (88%) | 108 (88%) | 107 (79%) | 118 (79%) | |
| DCDD | 226 (7%) | 14 (17%) | 13 (12%) | 15 (12%) | 28 (21%) | 32 (21%) | |
| 1–5 years | Total | 6,639 | 123 | 226 | 197 | 229 | 244 |
| DNDD | 4,920 (74%) | 96 (78%) | 191 (85%) | 167 (85%) | 194 (85%) | 217 (89%) | |
| DCDD | 368 (6%) | 27 (22%) | 35 (15%) | 30 (15%) | 35 (15%) | 27 (11%) | |
| 6–10 years | Total | 4,373 | 70 | 104 | 113 | 125 | 126 |
| DNDD | 3,106 (71%) | 61 (87%) | 89 (86%) | 94 (83%) | 112 (90%) | 107 (85%) | |
| DCDD | 227 (7%) | 9 (13%) | 15 (14%) | 19 (17%) | 13 (10%) | 19 (15%) | |
| 11–17 years | Total | 16,402 | 270 | 439 | 463 | 445 | 419 |
| DNDD | 11,824 (72%) | 223 (83%) | 369 (84%) | 389 (84%) | 386 (87%) | 343 (82%) | |
| DCDD | 958 (6%) | 47 (17%) | 70 (16%) | 74 (16%) | 59 (13%) | 76 (18%) | |
| >18 years | Total | 188,127 | 6,171 | 9,846 | 9,390 | 9,037 | 8,140 |
| DNDD | 149,340 (79%) | 4,803 (78%) | 7,847 (80%) | 7,645 (81%) | 7,488 (83%) | 6,800 (84%) | |
| DCDD | 17,858 (9%) | 1,365 (22%) | 1,999 (20%) | 1,745 (19%) | 1,549 (17%) | 1,340 (16%) | |
* Data reflect donors recovered from January 1, 1988, to July 31, 2019. Total column percentages do not add up to 100% because DCDD data was not available prior to 1993.
Guidelines for determining brain death in children were first derived by a multisociety task force in 1987 ( ). These guidelines were subsequently updated in 2011, again with endorsements from numerous medical societies, citing original limitations such as limited clinical information at the time the first guidelines were published, lack of information on ancillary testing, rationale for age-based criteria, and little discussion regarding brain death in neonates ( ). In order for brain death testing to occur, several physiologic perturbations must be absent, including hypotension, hypothermia, and metabolic disturbances, which could all affect the neurologic exam ( ). In addition, the patient should be free from all sedatives, analgesics, and neuromuscular blocking drugs. However, testing can occur if sedative, analgesic, and anticonvulsant drugs are present in low to mid-therapeutic levels. Any patient where these medications are felt to be at supratherapeutic or high levels should undergo ancillary testing ( ).
There must be examinations performed by two different attending physicians involved in the patient’s care. Experienced attending physicians, particularly those with training in neurocritical care, should perform pediatric brain death exams. These two examinations must be separated by a time period of 24 hours for 37-weeks’ gestation to term infants 30 days of age or 12 hours for infants and children (>30 days old to 18 years of age) ( ). The intention of the first examination is to confirm that the child has met the criteria for brain death. The second examination is to confirm that the child has fulfilled the criteria for brain death. Given that the neurologic exam may be unreliable immediately after cardiopulmonary resuscitation or other severe acute neurologic injury, the initial exam should be delayed until 24 to 48 hours after the event ( ). The components of the neurologic examination for determining brain death in neonates, infants, and children, including apnea testing, are presented in Table 38.2 .
| Coma |
|
| Loss of brainstem reflexes |
|
| Apnea |
|
| Flaccid tone |
|
In addition to the clinical examination components for determining brain death, ancillary studies, such as those presented in Table 38.3 , may also be utilized. While these are not required components, they can be particularly useful when other aspects of the neurologic examination cannot be safely completed, if there is uncertainty regarding an examination result, or where medications may affect the results of the exam. However, these studies are not meant to substitute for the neurologic examination.
| Test | Pro | Con |
|---|---|---|
| Electroencephalogram | Noninvasive, routinely performed, can be done at bedside | Cannot be performed when hypothermic or hypotensive, reflects cortical activity |
| Cerebral angiography | Gold standard, absence of flow within cerebral vessels confirms brain death | Invasive, image variability, requires physician skilled/credentialed to perform |
| Radionucleotide angiography | Highly available and affordable, bedside test | Poorly assesses posterior fossa, thus there may be no flow visible when in fact there is brainstem perfusion |
| Computed tomography angiography | Usually readily available | No consensus on diagnostic criteria, nephrotoxic contrast, interpretation may be variable/difficult |
| Magnetic resonance imaging/angiography/venography | Noninvasive, accurate | Not readily available, not a bedside test, might miss slow flow |
| Magnetic resonance perfusion/spectroscopy | May demonstrate decreases in apparent diffusion coefficient in brain parenchyma | Poor sensitivity and specificity |
| Brainstem auditory evoked potentials | Noninvasive, high availability, can be performed in the presence of hypothermia and barbiturates | Only measure discrete pathways, thus not reflecting global brain function |
| Xenon computed tomography | Avoids contrast, noninvasive, rapid | Poorly available, only a few academic institutions capable of performing |
| Single-photon emission computed tomography | Fairly reliable outside of the newborn period | Limited availability |
| Transcranial Doppler ultrasound | Noninvasive, bedside, inexpensive, widely available | Susceptible to false negative studies |
| Atropine test | Noninvasive, bedside, inexpensive, widely available | Susceptible to false negatives, can affect pupillary exam |
Electroencephalography (EEG) is a commonly performed bedside test that is a useful ancillary test for determining brain death. An analysis of 12 different studies comprising 485 children suspected to be brain-dead showed that EEG results were consistent with brain death in 89% of the cases ( ). Radionuclide angiography is also a readily available, highly studied bedside ancillary brain death test. A benefit of this modality is that it does not require the use of nephrotoxic contrast agents, such as those that are necessary for invasive cerebral angiography and computed tomography angiography. Again, an analysis of 12 studies comprising 681 children showed absent cerebral blood flow in 86% of clinically brain-dead patients (89% in those with more than one scan) ( ).
A child is considered brain-dead once a second clinical exam and apnea test have provided evidence to suggest the irreversible absence of brain activity. In some instances, it may not be safe to conduct an apnea test given that the patient may be at high risk for desaturation, and the test should be stopped if Sp O 2 decreases to <85% ( ). For children on extracorporeal membrane oxygenation, apnea tests can still be performed by decreasing the sweep and/or adding carbon dioxide to the circuit to achieve hypercapnia ( ). Every component of both clinical exams must be documented, even if the results are inconclusive. In the event that ancillary tests are performed, the results must be fully documented. Children meeting the clinical criteria for being brain-dead with or without support from ancillary testing can be considered for DNDD. Children who do not meet the criteria for clinical brain death but have devastating injuries that will result in imminent death may still be candidates for donation after circulatory determination of death (DCDD).
Though all mortalities are unfortunate outcomes, those who are eligible as organ donors present an opportunity for the prolongation of life in another individual with life-threatening, irreversible end-organ disease. The ideal organ donor candidate would be one with an isolated intracranial central nervous system event rendering them brain-dead with little to no involvement of any other organ system. However, many patients will not meet criteria for brain death (see Pediatric Brain Death) but will still have suffered nonsurvivable insults or events that render them in a persistent vegetative state with a poor prognosis for survival. Thus while these patients do not meet criteria for DNDD, they may be candidates for DCDD.
Donation after circulatory determination of death is currently less common than DNDD. There were 875 pediatric organ donors (<18 years old) in 2018, of whom 133 (15%) were DCDD based on data from the OPTN as of July 31, 2019 ( Table 38.1 ). However, this modality presents an opportunity for parents to consent to organ donation for children facing imminent death who may otherwise not qualify and serves to enlarge the overall donor pool ( ).
A major difference between DNDD and DCDD is that in brain death, although all of the vital organs are being adequately perfused (aside from perhaps the brain), the patient is dead by medical and legal standards ( ). Thus organ donation is ethical because the patient is already dead. In circumstances of DCDD, withdrawal of life-sustaining therapy (WLST) is required. Unlike DNDD, where several specific criteria must be met to be declared brain-dead (see Pediatric Brain Death), for DCDD the permanent arrest of circulation is the criteria used for the determination of death, with the understanding that this state inevitably leads to brain death ( ). Therefore WLST, such as extubation or cessation of vasopressors and/or inotropes, precedes the circulatory arrest required for DCDD. Guidelines have recently been drafted for pediatric DCDD. Sixty-three Good Practice Statements were generated for these guidelines; 48 are presented in the publication and the rest are available in the full-text report ( ). The following is a description of selected Good Practice Statements from these 2017 DCDD guidelines ( ).
When determining which patients may be appropriate for DCDD, members of organ procurement organizations and members of transplant teams should not be a part of the decision-making process for WLST, and the decision should not be based on donor potential. Once the decision for WLST has been made, discussion regarding DCDD can occur, and arrangements for organ allocation and limits of warm and cold ischemic times must be determined. The whole process of DCDD must be explained to the patient’s family/surrogate decision makers. This includes, but is not limited to, the process of WLST and that this withdrawal may be delayed until all potential organs have been allocated; the procedures and methods of determining death; that there is a possibility that organs may not be suitable for donation; and that consent may be withdrawn at any time, even after WLST and the determination of death. No antemortem care should be provided that incurs any higher risk than the usual standard of care and should in no way be intended to hasten death.
The process of WLST can occur in the intensive care unit, near the operating room, or in the operating room, depending on the surrogate decision maker’s wishes and logistics of the institution. Family/caretakers should be allowed to be with the patient until the determination of death has been made. Predetermined limits for the time to determination of death should be made, given that organs may be hypoperfused during the time between WLST and determination of death, which could render them not suitable for donation. While the use of predictive modeling for estimating the time to death from WLST remains controversial, the requirement for high inotrope and/or vasopressor infusions, positive end-expiratory pressure >10 mm Hg, and receiving extracorporeal membrane oxygenation have been shown to be associated with a shorter time to circulatory arrest ( ). In addition to the time between WLST and the cessation of circulation, it is suggested there be a mandatory 5 minutes of hands-off time. After the cessation of circulation and the 5 minutes of hands-off time, death must be determined by two separate physicians, one of whom should be a critical care attending physician from the intensive care unit where the patient was being treated. After WLST, cessation of antegrade circulation (suggested to be determined with the use of an invasive arterial blood pressure monitor), the 5-minute hands-off period, and the confirmation of death, it is then considered suitable to begin the organ harvest process.
All actions surrounding DCDD must comply with the “dead donor rule,” stating that organs may only be procured from a dead person and that organ procurement cannot result in death ( ). Several ante- and postmortem practices remain controversial without a clear consensus in place due to the potential violation of this rule, which some believe may actually impede the care that may be in the best interest of the patient and their surrogate decision maker. For instance, the practice of heparinization to minimize thrombosis formation during periods of low and no flow is a potential violation, because it provides no direct medical benefit to the donor—that is, unless you consider their ability to more effectively donate their organs in alignment with their wishes. Also, any intervention that could potentially provide oxygenated blood to the brain must be avoided. Therefore any sort of extracorporeal membrane oxygenation must be used in a fashion that provides organ-/region-specific blood flow, excluding any cerebral circulation.
The overarching goal of pediatric organ donor (defined by the United Network for Organ Sharing [UNOS] as <18 years old) management is to maintain organ system homeostasis as best as possible in a brain dead patient (or one with imminent death from circulatory failure). This requires highly orchestrated, timely, and meticulous care to manage not only the primary injury responsible for the brain death but also the organ system insults that occur as a result of brain death or circulatory failure ( ). This pedantic care is of critical importance to maximize the number of organs able to be recovered and, through optimization of care, increase the likelihood of successful posttransplant organ function. Optimization of pediatric donors is important given the high mortality of pediatric patients on the transplant waitlist, necessitating the need for adequate organs. It is also important given that more than two-thirds of recovered pediatric organs are transplanted into adults ( ). According to data from the OPTN, pediatric donors constituted 8% (875/10,721) of all organ donors in 2018 ( Table 38.4 ). However, the number of organs procured per pediatric patient (4.2) was higher than that for adults (3.5), and the proportion of organs actually transplanted into a recipient per organ procured was also higher (95% of donated pediatric organs vs. 86% of donated adult organs) ( Table 38.4 ). The 11- to 17-year-old age group had the highest procurement rate, with nearly five organs obtained per donor, and also the highest percentage of procured organs transplanted (2073/2133, 97%). This suggests that the organs procured from pediatric patients may be of better quality, which may be due to the fact that pediatric patients experience less chronic disease such as hypertension, atherosclerosis, and diabetes and effects from alcohol, tobacco, and/or other substance abuse. An overview of organ-specific management goals is presented in Table 38.5 .
| Age Group | Donors | Recovered | Organs/Donor | Transplanted | Transplanted/Procured |
|---|---|---|---|---|---|
| <1 year | 106 | 345 | 3.3 | 292 | 0.85 |
| 1–5 years | 226 | 792 | 3.5 | 742 | 0.94 |
| 6–10 years | 104 | 387 | 3.7 | 369 | 0.95 |
| 11–17 years | 439 | 2,133 | 4.9 | 2,073 | 0.97 |
| Pediatric | 875 | 3,657 | 4.2 | 3,476 | 0.95 |
| 18–34 years | 3,155 | 13,592 | 4.3 | 12,759 | 0.94 |
| 35–49 years | 2,950 | 10,203 | 3.5 | 9,071 | 0.89 |
| 50–64 years | 2,946 | 8,447 | 2.9 | 6,403 | 0.76 |
| 65+ years | 795 | 1,952 | 2.5 | 1,148 | 0.59 |
| Adult | 9,846 | 34,194 | 3.5 | 29,381 | 0.86 |
| Organ | Specific Management |
|---|---|
| Heart |
|
| Kidney |
|
| Liver |
|
| Lung |
|
| Pancreas |
|
| Small bowel |
|
The OPTN was created when the National Organ Transplant Act was signed into law in 1984. The OPTN managed how organs were donated and shared by creating Organ Procurement Organizations (OPOs) throughout the United States and its territories. The OPOs manage 58 donation service areas within 11 UNOS territories. In 2015 a consensus statement from the Society of Critical Care Medicine, American College of Chest Physicians, Association of Organ Procurement Organizations provided recommendations for donor management goals (DMGs) within the domains of (1) hemodynamics, (2) lung recruitment and mechanical ventilation, (3) fluids/electrolytes, (4) endocrine function, (5) blood product utilization, and (6) thermoregulation ( ). The pediatric DMGs are presented in Table 38.6 . As of 2016, 39 out of 58 (67%) OPOs followed some form of pediatric donor guidelines, and 27 out of 58 (47%) utilized DMGs for pediatric donor management ( ). Sixteen of the 39 OPOs that employed guidelines used the Updated Pediatric Donor Management and Dosing Guidelines established by the North American Transplant Coordinators Organization ( Table 38.7 ) ( ).
| Hemodynamics | ||
| Normal systolic blood pressure for age (80 mm Hg + 2 × age in years) | Systolic | Diastolic |
| Neonate | 60–90 | 35–60 |
| Infant (6 months) | 85–100 | 50–65 |
| Toddler (2 years) | 90–105 | 50–65 |
| School age (7 years) | 90–115 | 60–70 |
| Adolescent (15 years) | 110–130 | 65–80 |
| Central venous pressure <12 mm Hg | ||
| Lower vasopressor doses (e.g., dopamine infusion <10 mg/kg per minute) | ||
| Normal serum lactate | ||
| Lung and Mechanical Ventilation | ||
| Pao 2 >100 mm Hg | ||
| Fio 2 , 0.40 | ||
| Paco 2 , 35–45 mm Hg | ||
| Arterial pH, 7.30–7.45 | ||
| Tidal volume, 8–10 mL/kg | ||
| Positive end-expiratory pressure, 5 cm H 2 O | ||
| Fluid, Electrolytes and Nutrition | ||
| Normovolemia | ||
| Urine output, 1 mL/kg per hour | ||
| Serum sodium, 130–150 mEq/L | ||
| Serum potassium, 3–5 mEq/L | ||
| Serum glucose, 60–150 mg/dL | ||
| Ionized calcium, 0.8–1.2 mmol/L | ||
| Continue nutritional support for liver donors and consider enteral feedings for small bowel donors in the absence of contraindications | ||
| Hormonal Replacement Therapy | ||
| Control of diabetes insipidus (desmopressin, vasopressin) | ||
| Consideration of thyroid hormone replacement (levothyroxine, triiodothyronine) | ||
| Consideration of adrenal steroid replacement (methylprednisolone) | ||
| Blood Products | ||
| Hemoglobin >7 g/dL | ||
| Thermoregulation | ||
| Core body temperature, 36°C–38°C | ||
| Empiric Antibiotics | ||
| No specific recommendations | ||
| Medication | Dose | Comment |
|---|---|---|
| Code Medications | ||
| Epinephrine | 0.01 mg/kg | Concentration: 0.1 mg/mL |
| Atropine | 0.02 mg/kg | Concentration: 0.1 mg/mL |
| 8.4% Na bicarbonate | 1 mEq/kg | Concentration: 1 mEq/mL |
| 10% Ca chloride | 20 mg/kg | Concentration: 100 mg/mL |
| Hormonal Resuscitation * | ||
| Desmopressin † | 0.5 mcg/hour | Titrate to decrease urine output to 3–4 cc/kg per hour |
| Vasopressin † | 0.5 mU/kg per hour | Titrate to decrease urine output to 3–4 cc/kg hour |
| Levothyroxine | 0.8–1.4 mcg/kg per hour | Bolus 1–5 mcg/kg can be administered |
| Triiodothyronine | 0.05–0.2 mcg/kg per hour | |
| Methylprednisolone | 20–30 mg/kg | May be repeated 8–12 hours |
| Insulin | 0.05–0.1 U/kg per hour | Titrate for blood glucose 60–150 mg/dL |
| Antiarrhythmics | ||
| Adenosine | 100 mcg/kg | Rapid IV push, repeat dose 200 mcg/kg (max 12 mg) |
| Amiodarone | 5 mg/kg | Infuse bolus over 30 mins, infusion 5–10 mcg/kg per minute |
| Atropine | 0.02 mg/kg | Minimum dose 0.1 mg, maximum 0.5–1 mg |
| Lidocaine | 1–2 mg/kg | Infusion 10–50 mg/kg per minute |
| Magnesium sulfate | 30 mg/kg | Infuse bolus over 10 minutes, repeat dose 10 mg/kg, maximum dose 2.5 g |
| Correction of Metabolic Acidosis | ||
| Na bicarbonate | 1 mEq/kg | Monitor for hypernatremia |
| Tromethamine 0.3 M | mL bolus = BD × weight (kg) | Contraindicated in renal failure |
| Inotropic Infusions ‡ | ||
| Milrinone | 0.25–0.75 mcg/kg per minute | Loading dose 50 mcg/kg, caution in renal dysfunction |
| Dopamine | 2–20 mcg/kg per minute | Titrate to desired blood pressure |
| Dobutamine | 2–20 mcg/kg per minute | Titrate to desired blood pressure |
| Epinephrine | 0.1–1 mcg/kg per minute | Titrate to desired blood pressure |
| Norepinephrine | 0.05–2 mcg/kg per minute | Titrate to desired blood pressure |
| Phenylephrine | 0.1–0.5 mcg/kg per minute | Titrate to desired blood pressure |
| Vasopressin | 0.0003–0.002 U/kg per minute | Dose different for treatment of polyuria |
| Antihypertensives | ||
| Na nitroprusside | 0.5–10 mcg/kg per minute | Can cause thiocyanate and cyanide toxicity |
| Esmolol | 50–250 mcg/kg per minute | Loading dose 100–500 mcg/kg, can cause bronchospasm |
| Labetalol | 0.2–1 mg/kg | Infusion 0.4–3 mg/kg per hour |
| Nicardipine | 1–3 mcg/kg per minute | |
| Hydralazine | 0.1–0.5 mg/kg | Maximum 20 mg, may be repeated every 4–6 hours |
* Polyuria treatment should aim to decrease but not completely stop urine output. Urine output should be replaced with ¼ to ½ normal saline and serum sodium concentrations should be maintained with pharmacologic agents.
† Should be considered in donor management and may facilitate inotropic drug weaning.
‡ Used to improve end-organ perfusion but blood pressure alone does not indicate adequate perfusion and other serum biomarkers, such as lactate, should also be followed.
The goals for hemodynamic management do not differ significantly between the pediatric organ donor and the critically ill child, in that the maintenance of euvolemia, cardiac output, vascular tone, and oxygen-carrying capacity is necessary to ensure adequate organ perfusion, oxygenation and nutrient delivery, and carbon dioxide and waste removal.
Forty-three of the 58 OPOs report following guidelines for determining age-appropriate blood pressure in pediatric donors ( ). Nearly all OPOs report using an invasive arterial line for the monitoring of blood pressure, nearly 80% monitored central venous pressure (CVP), and slightly more than 30% followed serum lactate levels ( ). Hemodynamic goals for pediatric donors are presented in Table 38.6 .
Hypovolemia is common in DNDD patients due to cold diuresis from hypothermia, neuroendocrine failure and polyuria, tissue edema from inflammation, and third spacing of fluid ( ). Crystalloid solutions such as 0.9% normal saline or lactated Ringer’s solution may be used for initial volume replacement ( ). Care should be taken to avoid the hypernatremia and hyperchloremia that may result from 0.9% saline, whereas the hypoosmolar effects of lactated Ringer’s solution may not be tolerated by some donors ( ; ). Colloid solutions such as 5% albumin, or packed red blood cells in the case of unacceptable anemia, may also be used ( ; ). Hydroxyethyl starch should be avoided because it has been associated with acute kidney injury, coagulopathy, and trapping in the hepatic reticuloendothelial system ( ). In addition, hydroxyethyl starch use in donors has been associated with delayed graft function and graft failure ( ). Donor hypovolemia may be detrimental for renal graft function, whereas hypervolemia may impair pulmonary graft function. A CVP of 4 to 6 mm Hg was determined to be optimal for lung graft function, and this restrictive fluid management (CVP <6 mm Hg) has not been found to be detrimental for kidney graft survival or delayed renal graft function ( ).
Among surveyed OPOs, dopamine was the most commonly used initial vasoactive infusion (24/58, 41%), followed by phenylephrine (8/58, 14%), epinephrine (4/58, 7%), norepinephrine (2/58, 3%), vasopressin (1/58, 2%), and dobutamine (1/58, 2%) ( ). Although dopamine historically has been used as the first-line vasoactive agent for hypotension, there is insufficient evidence to preferentially recommend its use over other vasoactive agents ( ). Dopamine, epinephrine, and dobutamine may be used for cardiac dysfunction, whereas norepinephrine, phenylephrine, and vasopressin are recommended for the vasodilatory component of donor hypotension ( ; ). Recommended vasoactive medications and their doses can be found in Table 38.7 . Excessive beta-agonist use should be avoided for potential heart donors, given evidence that these may lead to myocardial cellular energy depletion and desensitization of beta receptors ( ). Furthermore, excessive vasoconstriction may have detrimental effects, especially for coronary and mesenteric vasculature (i.e., heart and intestine/multivisceral donors) ( ).
The overarching goals for mechanical ventilation in donors are achieving adequate oxygenation and ventilation while avoiding detrimental volutrauma, barotrauma, and atelectasis. The pediatric DMGs for mechanical ventilation are presented in Table 38.6 . Donor lungs are extremely vulnerable; any cardiopulmonary resuscitative efforts can lead to blunt force injury to the lungs during chest compressions. In addition, blood product administration—either during attempts at resuscitation or after declaration of brain death to maintain adequate hemoglobin levels and coagulation function—could result in transfusion-related acute lung injury. Furthermore, the event of brain injury and death can lead to pulmonary injury via neurogenic pulmonary edema (NPE).
The incidence of NPE can range from 2% to 42% after subarachnoid hemorrhage and 20% to 50% after traumatic brain injury ( ). The exact mechanisms underlying the development of NPE are still not clearly understood; however, the prevailing theory is that the profound catecholamine surge associated with the initial cerebral edema and neural compression, especially of the brainstem, is the inciting factor ( ; ). The sudden rise in vascular resistance forces more blood through the pulmonary vascular system and this, coupled with capillary permeability, results in pulmonary edema ( ). The ventilation management of patients with NPE is similar to those with acute respiratory distress syndrome (ARDS), which includes the use of positive end-expiratory pressure and lung-protective ventilation with lower tidal volumes (4 to 8 mL/kg) ( ).
Regardless of whether a pediatric organ donor is a candidate for lung donation, care must still be taken to manage their ventilation to preserve function of other organs to be donated. Systemic inflammation, aspiration, infection, and injury from mechanical ventilation can all result in poor ventilation and oxygenation. The routine use of beta-agonists in lung donors has been cautioned against given a lack of benefit and the side effect of tachycardia ( ).
The absence of a functioning hypothalamic–pituitary access in addition to other systemic derangements can lead to massive diuresis and electrolyte abnormalities, necessitating fluid management aimed at maintaining intravascular volume and electrolyte management to prevent arrhythmias and organ dysfunction. Central venous catheters and pulmonary artery catheters can provide volume status information, such as central venous pressure and pulmonary artery occlusion pressure, as well as other markers of organ perfusion, such as mixed venous oxygenation, lactic acid levels, base deficit, and acid–base status ( ; ). Additional volume status measures include transthoracic echocardiography, which can also provide an assessment of cardiac function, and pulse pressure variation and systolic pressure variation obtained via an invasive arterial line can further help guide fluid management ( ). Fluid therapy guidelines aim to maintain age-appropriate blood pressure, >1 mL/kg per hour urine output, and minimize pressure utilization (i.e., dopamine <10 mcg/kg per minute) ( ). Generally, lactated Ringer’s and 0.9% saline have been used for intravenous fluids; however, hyperchloremic acidosis may limit the use of 0.9% saline ( ; ). At times sodium bicarbonate may be added to fluids to maintain a donor pH between 7.3 and 7.45 ( ). Goal-directed transfusion of blood and blood products may be indicated; however, optimal thresholds have not been determined for donor management ( ). Thirty-one of 58 (53%) OPOs reported standardized guidelines for fluid and electrolyte management of pediatric organ donors ( ).
Electrolyte abnormalities are not uncommon in DCDD organ donors, whether it be due to fluid resuscitation, the hyperosmolar agents used to try to avoid cerebral edema, or the diabetes insipidus that can occur in brain-dead patients ( ). Whereas the first two etiologies can be managed by altering intravenous fluid administration and discontinuing the use of agents like hypertonic saline and/or mannitol, diabetes insipidus may require more active management given that this can result in hypovolemia, hypernatremia, hypokalemia, hypomagnesemia, and hypophosphatemia ( ). Pediatric DMGs for electrolyte goals are presented in Table 38.6 . In particular, hypernatremia has been associated with impaired liver graft function, although these observations have been seen more so with adults, and pediatric associations are lacking ( ). Nonetheless, donor serum sodium <155 mEq/L has been recommended ( Table 38.5 ) ( ; ). Cerebral edema associated with rapid correction of hypernatremia is less of a concern in brain-dead patients; however, the rapid administration of hypotonic fluids can lead to hemolysis and hemolysis-associated kidney injury ( ). Among the 52 out of 58 (90%) OPOs reporting using serum sodium thresholds, 30 used the guideline-recommended 155 mEq/L (18 used 160 mEq/L; 4 used 150 mEq/L) ( ).
The OPTN has not provided guidelines regarding nutrition management in organ donors. Due to organ donors’ low metabolic rate, insulin resistance, and steroid-induced hyperglycemia, many centers eliminate nutrition (enteral and parenteral) altogether ( ). Fifty-three of the 58 (93%) OPOs report stopping nutrition unless otherwise instructed ( ). There is thought that since the liver is the major glycogen storage unit for the body, liver grafts may benefit from ongoing donor nutrition; however, no data is available to definitively support this theory ( ). In addition, continued enteral nutrition may preserve small bowel villi and thus potential small bowel donors may continue to receive enteral nutrition ( ; ). With regards to written guidelines for glycemic control, only 29 out of 58 (50%) OPOs utilized glucose thresholds, which ranged from lower levels of 69 ± 12 mg/dL to hyperglycemia thresholds of 171 ± 37 mg/dL ( ).
Brain-dead donors are susceptible to several endocrine abnormalities; most notably there can be relevant deficiencies of arginine vasopressin, thyroid hormone, and cortisol. Ischemia and infarction of the hypothalamic–pituitary axis places the donor at risk for endocrine dysregulation. Pediatric dosing guidelines for hormone replacement therapy are presented in Table 38.7 .
Arginine vasopressin is produced in the hypothalamus and released from the posterior pituitary. It has been observed that 80% of DNDD donors manifest diabetes insipidus, which makes it not surprising that 21 out of 58 (36%) OPOs empirically administer vasopressin (or another arginine vasopressin analog) ( ). Arginine vasopressin acts on the vasculature via V1 receptors to elicit vasoconstriction, the kidney via V2 receptors as an antidiuretic causing free water resorption from the collecting system, and the anterior pituitary via V3 receptors to regulate adrenocorticotropic hormone release ( ). Diabetes insipidus is the most common manifestation of arginine vasopressin insufficiency and is typically treated with either vasopressin or desmopressin ( ). Treatment should be initiated for urine output in excess of 2.5 to 3 mL/kg per hour, increased serum osmolality, inappropriately dilute urine (specific gravity <1.005 and/or urine osmolality <200 mOsm/kg H 2 O), or serum sodium >145 mMol/L in the absence of other abnormalities ( ; ). In general, diabetes insipidus with concurrent hypotension should be treated with vasopressin, whereas diabetes insipidus in the normotensive brain-dead donor can be treated with desmopressin to avoid hypertension, given that it is more selective for the renal V2 receptor ( ). Vasopressin use in pediatric donors has led to higher organ yields, especially cardiac donors, and therefore its use is recommended ( ; ). Of note, the high concentration of V1 receptors on splanchnic vasculature results in visceral hypersensitivity to arginine vasopressin. This is particularly troubling for intestine recovery, and we suggest optimization of fluid status and utilization of only polyuria doses of arginine vasopressin to avoid splanchnic vasoconstriction and end organ ischemia.
Empiric administration of systemic steroids is even more prevalent, with all but one OPO administering corticosteroids (51 out of 58 use methylprednisolone, 5 use hydrocortisone, and 1 uses both) ( ). Despite a lack of strong evidence to support hypocortisolism, the antiinflammatory and immunomodulatory benefits outweigh the few potential risks that exist ( ). While no significant effect on hemodynamics has been observed, some benefits for liver, kidney, heart, and lung donation from pediatric donors have been shown when those donors received corticosteroids ( ; ). Hyperglycemia is common after brain death and is likely multifactorial due to massive catecholamine release or exogenous catecholamine administration, infusions of dextrose-containing fluids, corticosteroid administration, peripheral insulin resistance from catechol exposure, and altered intracellular metabolism ( ). Insulin administration to organ donors has not shown an independent benefit; however, hyperglycemia can affect pancreatic islet cells and affect renal graft function ( ; ). Pediatric DMGs recommend a serum glucose target range of 60 to 150 mg/dL ( Table 38.6 ) ( ).
It has been suggested that cellular metabolism switches from aerobic to anaerobic metabolism leading to increased lactate in DNDD donors with thyroid hormone insufficiency, and this has been implicated in graft dysfunction and failure ( ). Thyroid hormone replacement has shown benefit when used in hypotensive donors not responding to vasopressors and thus has shown a vasopressor-sparing effect ( ; ). They have also shown benefit in cardiac donors with depressed ejection fraction ( ; ; ). Empiric thyroid hormone was administered to pediatric organ donors by 37 of 58 (64%) OPOs, with 19 of 58 (33%) reserving this form of hormone replacement therapy for low cardiac output and/or potential heart donors and 2 of 58 (3%) not administering thyroid hormone at all during pediatric organ donor management ( ). The majority of OPOs (51/56, 91%) that administered thyroid hormone, either empirically or situationally, used thyroxine (T4) alone ( ). Data has not shown one (T3 or T4) to be superior to the other ( ; ).
In brain-dead patients, perfusion to the hypothalamus and pituitary ceases, resulting in thermodysregulation through the loss of shivering and vasoconstriction ( ). Adverse effects of hypothermia include arrhythmias, diuresis, coagulopathy, and insulin resistance ( ). Forty-three of 58 (74%) OPOs utilized standardized guidelines for temperature regulation in pediatric organ donors ( ). Donor management goals recommend that donors be managed to maintain a temperature of 36°C to 38°C ( Table 38.6 ). There is some evidence that mild donor hypothermia (34°C to 35°C) leads to reduced delayed graft function after kidney transplantation; however, the long-term graft outcomes are pending at this time ( ). The use of mild hypothermia must take into consideration the criteria for determining brain death, which at least for determining brain death requires normothermia (see Pediatric Brain Death).
Positive blood cultures have been observed in nearly 20% of brain dead donors, and older age and being in the intensive care unit for three or more days increases this risk ( ). Although some infections such as viral meningitis, fungal infections, and active hepatitis B preclude brain-dead patients from being organ donors, potential donors with most other bacterial infections can still be candidates and should receive a minimum of 48 hours of antibiotic therapy specific to the cultured organism(s) ( ). Bacteremia and/or sepsis and even bacterial meningitis are not absolute contraindications for organ donation as long as culture-directed antibiotics have been administered for 48 hours and the same culture-directed antibiotics are continued in the recipient for 7 to 14 days ( ). While patients with hepatitis B are typically not considered for organ donation, patients who are seropositive for hepatitis C may be candidates for donation to hepatitis C–positive recipients ( ). Although there is no specific recommendation for donor antibiotic prophylaxis, 56 out of 58 (97%) OPOs include antibiotic prophylaxis in their procurement guidelines ( ). Cefazolin was most commonly used (35/58, 60%), with piperacillin–tazobactam (8/58, 14%) being the second most commonly employed ( ).
The objectives for anesthesia management during pediatric organ procurement are similar to the preoperative management goals. The anesthetic goal is not amnesia and analgesia but rather hemodynamic and ventilatory stability. The anesthesiologist must take care to perform a meticulous history and physical exam of the patient in addition to reviewing all imaging and laboratory results. Hemodynamic status and current vasopressor and inotrope requirements, mechanical ventilation settings and current pulmonary condition, volume status, and urine output must all be reviewed, and plans to maintain these conditions in the operating room must be made. Coordination of the OPO management goals in the intensive care unit must carry over into the operating room with collaboration between the anesthesia care team, the intensive care team, surgical teams, and OPO personnel. The OPO representative and anesthesiologist should review the DMGs specific to that OPO. While anesthesiologists are required for DNDD donors to continue to manage vasopressors, inotropes, fluids, and ventilation, the anesthesiology team may not be required for DCDD donors. In the instances they are required, it is often for the withdrawal of the life-sustaining therapies for the purposes of comfort measure continuation until the patient dies ( ; ).
Care begins with patient transport, which often involves transitioning the patient from mechanical ventilation to hand ventilation. Small disturbances like this, in addition to moving the patient and/or inadvertent changing or discontinuation of medications, can result in cardiac arrest ( ). If an arrest occurs, it is unlikely that the patient will have return of spontaneous circulation, and procurement should begin as soon as possible ( ). Advanced life support techniques should be provided with the goal of perfusing and oxygenating organs, and heparin should be readily available for vascular clamping. Once in the room, the patient is positioned supine on the bed, and standard American Society of Anesthesiologists (ASA) monitors are applied including electrocardiogram, blood pressure, body temperature, SpO 2 , and end-tidal CO 2 ( ). A warming strategy should be employed to maintain the patient in the normothermic range until cross-clamping and cooling. This could include any of forced-air warming devices, water thermoregulation blankets, and intravenous fluid warmers.
Spinal reflexes below the brainstem are intact in DNDD donors, and therefore these patients can move, especially with surgical stimulation ( ). It is recommended that skeletal muscle paralysis be provided to optimize surgical conditions ( ; ). Not only can spinal reflexes result in patient movement but they can also elicit neurogenic vasoconstriction and stimulation of the adrenal medulla, resulting in tachycardia, hypertension, and perspiration ( ). Therefore in addition to neuromuscular-blocking drugs, the anesthesiologist should be prepared to administer volatile or intravenous anesthetics to mitigate the effects of the spinal reflexes ( ; ). There is evidence that volatile anesthetics may reduce ischemia reperfusion injury (anesthetic preconditioning) and improve organ function in the recipient; however, long-term studies are lacking ( ). A recent single-center retrospective review of 138 donors exposed to volatile anesthetics and 75 unexposed did not reveal any significant difference in either early (30-day) or late (5-year) graft survival rates for kidney, liver, lung, or heart transplants ( ).
Many OPOs have adopted a standard practice of including a moment of silence to serve as a time to pause and reflect on the gift that the donor is giving and to appreciate the event that is occurring. Often families will prepare brief statements for the OPO coordinators to read to the team. This pause is important to all involved in the organ recovery and, to no surprise to anyone involved in this process, has been universally praised by organ recovery teams. Next, after a time-out confirming the UNOS patient identification and the organs to be procured, the procedure will begin with a midline laparotomy incision with or without a median sternotomy ( ; ). Vascular cannulation will occur depending on which organs are to be procured. Just prior to aortic cross-clamping, heparin (usually 300 to 400 U/kg) must be administered and allowed to circulate for 3 minutes ( ). After aortic cross-clamping, the preservation solution is infused, blood is drained, and the cavity is packed in ice ( ; ). In general, organs are procured in order of ischemic tolerance. Because hollow viscera rapidly warm with elevated ambient temperatures, the heart, lungs, and intestine are recovered first, followed by the liver and kidneys ( ; ).
In addition to deceased donors, living donors, both related and nonrelated, can provide organs to those in need. Most commonly, kidney and split liver grafts are donated from adults to children with end-organ disease. The benefit of living organ donation is the luxury of timing an elective procedure with a healthy donor as opposed to a deceased organ donor. Because of this, living-donor organ transplantation is associated with better outcomes compared with deceased donor transplantation ( ). Unlike deceased donors, exsanguination and diffuse cold preservation solution infusion cannot occur. Thus the selected organ/organ fragment must be harvested, typically after administration of intravenous heparin, with immediate infusion of a cold preservation solution during back table processing.
For living kidney donors, the left kidney is often desired due to its longer vasculature, and the procurement of this organ is often performed via laparoscopy ( ). Living kidney donors can often be managed with standard noninvasive monitors and one to two large-bore intravenous lines ( ). Intraoperative management consists of liberal fluid administration, surgeon-/center-dependent use of furosemide and/or mannitol, heparin prior to vessel cross-clamping, and avoidance of nonsteroidal antiinflammatory drugs ( ). Standard organ preservation solutions such as University of Wisconsin (UW) and histidine-tryptophan-ketoglutarate (HTK) solutions are used for living-donor renal preservation, with some suggestion that HTK may reduce delayed renal graft function compared with using UW solution in living donors ( ). While there may be some evidence that UW solution is superior for longer cold ischemic times, living-donor organ cold ischemic times are very short, thus HTK is preferable because it is much less viscous, allowing for easier flushing of the organ, and it has a lower potassium content (personal communication with Drs. Kyle Soltys, Armando Ganoza, and George Mazariegos, UPMC Children’s Hospital Transplant Program). Furthermore, although the functional outcomes may be comparable, HTK solution has been shown to cost less (33% to 50% less) than UW solution ( ; ).
The left lateral segment or a total left hepatic lobectomy is generally enough to provide sufficient liver mass for pediatric recipients ( ). In addition to large-bore intravenous access, invasive arterial monitoring and central venous access are also often acquired ( ). Keeping the central venous pressure <5 mm Hg has been suggested to reduce liver edge bleeding during splitting ( ). Postoperative analgesia may include epidural analgesia; however, this remains controversial given that one of the main concerns is postoperative coagulopathy from hepatic injury ( ). Living-donor livers are preserved in much the same fashion as livers from deceased donors, with preservation solutions such as HTK and storage on ice (see Organ Preservation) ( ; ). As mentioned in the living kidney donor section, while graft outcomes are comparable, HTK solution may provide some cost benefit ( ; ). Also, similar to living-donor kidney preservation, the lower viscosity of HTK compared to UW allows for easier flushing of the donor liver or liver segment, and the lower potassium content may make HTK a favorable solution.
Preoperative and intraoperative donor management is aimed at maintaining adequate oxygen delivery to and oxygenation of the vital organs to be transplanted. As long as any vital organ is metabolically active and consuming adenosine triphosphate, it will require sufficient oxygen and nutrient delivery as well as carbon dioxide and waste removal. Thus once circulation to these vital organs has ceased, all efforts must be aimed at organ preservation until perfusion is reestablished with the organ recipient. These preservation strategies attempt to reduce cellular metabolism via hypothermia, mitigate ischemia/reperfusion injury with the used of buffered preservation solutions, and, more recently, utilize machine perfusing systems to continue to provide ex vivo organ oxygenation ( ).
Initial preclinical organ preservation methods of the 1930s to 1950s consisted of normothermic ex vivo perfusion ( ). However, these initial modalities lacked sterility, which resulted in graft contamination until an aseptic system was developed. It was not until 1960 that hypothermic blood was used to perfuse the organs ex vivo, and clinicians realized organs could last much longer outside the body than previously appreciated ( ). In 1969 Collins preserved a canine kidney for 12 hours by placing it in iced saline and was able to subsequently prolong the time even further, to 30 hours, by infusing his preservation solution ( ). This ushered in the era of static cold storage, which remains the gold standard for organ preservation to this day.
Hypothermia serves as a first-line defense against hypoxic cellular injury, and its implementation in the early stages of organ transplant allowed for longer organ ischemic times. Cooling organs to 4°C to 6°C greatly reduces but does not completely stop cellular metabolism ( ). Static cold storage of organs at this temperature range causes significant changes in cellular, energy, electrolyte, transcriptional, and gene expression homeostasis ( ). Estimating from Van ‘t Hoff’s principle (expressed as Q 10 = (k 2 /k 1 ) 10/(t2−t1) ), varying the temperature of preservation is expected to reduce tissue metabolism to 10% to 18% in hypothermic (0°C to 12°C), 19% to 35% in midthermic (13°C to 24°C), 36% to 85% in subnormothermic (25°C to 34°C), and more than 86% in normothermic conditions (35°C to 38°C) ( ). In addition to maintaining organs in hypothermic conditions, recent evidence has shown that maintaining the donor at 34°C to 35°C is not only safe but also reduces delayed graft function and improves 1-year graft survival ( ; ).
Collins initial preservation fluid was intended to mimic intracellular fluid with high potassium and lower sodium and chloride in an effort to reduce cellular edema by maintaining ion concentrations ( ; ). Phosphate and bicarbonate served as buffers in this initial preservation solution, and glucose was the energy source; however, glucose may have contributed to increased lactate levels via anaerobic metabolism ( ).
Since 1989, UW solution has served as a gold standard preservation solution ( ). Like Collins solution, UW solution is considered an intracellular fluid–like solution with a high potassium content. The UW solution added the adenosine triphosphate precursor, adenosine, and also removed bicarbonate and glucose ( ). Adenine breakdown products such as hypoxanthine contribute to the accumulation of reactive oxygen species ( ). The UW solution helps to combat this by containing allopurinol. There is additional antioxidant support from reduced glutathione, and the addition of raffinose and lactobionate helps minimize cellular edema ( ). Stated drawbacks to UW solution include its high potassium content, which may contribute to hyperkalemia in the recipient upon reperfusion, and its high viscosity ( ; ). Combined UW solution with blood at 4°C results in fluid 1.3 times more viscous than blood at normothermic temperatures, which may hinder organ washout upon reperfusion ( ).
Newer alternative solutions to UW include HTK solution, Celsior solution, and Institut Georges Lopez-1 (IGL-1) solution ( ). Each of these presents a lower-potassium, lower-viscosity alternative to UW solution. The HTK solution was named for its buffering constituents, membrane stabilizer, and energy substrate, respectively ( ). Celsior solution incorporates characteristics of both UW and HTK solutions including a lactobionate osmotic carrier and histidine buffer, respectively ( ; ). One main difference is Celsior’s higher sodium content compared with UW and HTK solutions. The IGL-1 solution resembles UW solution except that it replaces the hydroxyethyl starch colloid with a less viscous colloid, polyethylene glycol, and it, much like Celsior solution, has sodium and potassium concentrations that resemble that of the extracellular space as opposed to the intracellular milieu ( ; ; ). Large retrospective studies from the UNOS have failed to definitively demonstrate superiority of any of these newer preservation solutions over UW solution, aside from evidence that Celsior may provide greater benefit as the preservation solution for lung preservation ( ).
Novel additives to preservation solutions have received increasing attention over the past decade. These include small-molecule bioregulators and mitochondria-targeted antioxidants. Of the small-molecule bioregulators, the gaseous additives for preservation solutions include carbon monoxide (CO), hydrogen sulfide (H 2 S), and nitric oxide (NO). Each of these molecules interacts with circulating metals, enhancing cellular signaling ( ). These have also been shown to be protective for the endothelium during organ preservation ( ). In addition, microcirculatory vasodilation improves tissue perfusion. Organs preserved with CO showed reduced apoptosis, whereas the molecules H 2 S and NO are potent vasodilators, which act to enhance tissue microperfusion ( ; ). Investigation of the use of mesenchymal stem cells has also been a recent area of interest. These multipotent stem cells can be obtained from both adult and fetal stromal tissue and act to facilitate damaged tissue repair. These stem cells are capable of secreting numerous factors that can affect the local immune system, enhance angiogenesis, prevent reactive oxygen species production and cellular apoptosis, and stimulate survival, proliferation, and differentiation of resident tissue cells ( ).
Reactive oxygen species are a major contributor to the ischemia–reperfusion injury observed in organ transplantation, with the graft mitochondria being the largest source of reactive oxygen species during static cold storage and reperfusion. Thus various additives have been studied to reduce mitochondrially derived reactive oxygen species. As previously mentioned, allopurinol present in UW solution inhibits xanthine oxidase–derived reactive oxygen species, glutathione is present in both UW and Celsior solutions to serve as a cellular redox buffer, and in HTK solution tryptophan acts as a reactive oxygen species scavenger ( ). A mild uncoupling of oxidative phosphorylation, as is observed with 2,4-dinitrophenol, has been shown to result in a lower mitochondrial respiratory rate, preservation of adenosine triphosphate, and less reactive oxygen species generation ( ). In addition, novel antioxidants combine the cation triphenylphosphonium (TPP+) with derivatives of ubiquinol (MitoQ) or plastoquinone (SkQ) to result in some of the most advanced mitochondria-targeted antioxidants ( ). These additives have been shown to increase intramitochondrial antioxidants more than 1000-fold compared with the extracellular compartment ( ).
While the earliest transplants were facilitated by organ preservation via perfusion techniques, these were widely replaced by static cold storage after the success of the preservation solutions ( ). However, there has been an ever-increasing interest in machine perfusion techniques, as there is evidence that there may be less graft failure for organs from marginal donors, thus enlarging the potential donor pool to help meet transplant needs ( ). Several different temperature ranges have been studied given the balance that must be achieved between oxygen delivery and cellular metabolism that is experienced within each temperature range. The machine perfusion techniques mitigate the negative effects of cold ischemic time as well as ischemia-reperfusion injury ( ). However, the use of machine perfusion techniques can be expensive and technically more difficult. For example, the dual blood supply of the liver necessitates cannulation of both the hepatic artery and portal vein.
Hypothermic machine perfusion occurs at temperature of 0°C to 12°C, since above 12°C energy-dependent enzymatic activity increases ( ). Perfusion within this temperature range does not require significant oxygen-carrying capacity (i.e., erythrocytes and/or hemoglobin-based oxygen carriers), and preservation solutions are able to be used as the perfusate because they provide adequate substrates for continued adenosine triphosphate synthesis ( ). Midthermic machine perfusion occurs within a temperature range of 13°C to 24°C. The goal of perfusing in this range was aimed at balancing the negative effects of cold ischemia with the increased oxygen utilization at normothermic temperatures. One of the main benefits is the decreased perfusate viscosity, which allows for improved microcirculatory perfusion ( ). Subnormothermic machine perfusion utilizes temperatures between 25°C and 34°C. One of the main applications of subnormothermic perfusion is for controlled oxygenated rewarming after static cold storage of organs. This allows for gradual warming of the organ until within the subnormothermic range. Normothermic machine perfusion occurs between 35°C and 38°C, and at these temperatures perfusates based on concentrates of red blood cells, plasma, nutrients, cofactors and insulin, antibiotics, electrolytes, and buffers are required ( ). The benefits of perfusing within the normothermic range include the avoidance of hypothermia altogether and monitoring of synthetic organ function such as bile secretion by the liver, since bile synthesis is arrested during hypothermia ( ).
Much has changed in pediatric transplantation since the first pediatric kidney transplant was performed in 1959. Advances in surgical technique coupled with improved immunosuppressive therapies have increased life expectancy and the quality of life for children with end-stage renal failure. A more detailed historical perspective on pediatric kidney transplant has been reviewed by . Advances and development of immunosuppression therapy, the pretreatment use of monoclonal antibodies and tacrolimus monotherapy to reduce and even eliminate the need for corticosteroids in some patients ( ), coupled with multidrug regimens involving corticosteroids, calcineurin inhibitors (cyclosporine or tacrolimus), monoclonal antibodies, antimetabolites such as azathioprine, and the purine synthetase inhibitor mycophenolate mofetil (MMF) to successfully prevent rejection, have all helped improved survival and quality of life ( ; ; ; ). In addition, the use of deceased adult and living-related adult kidneys has changed the incidence of graft thrombosis and delayed graft function that occurred when infant and small child donors were used ( ; ; ; ). With these advances, pediatric renal transplant patients can be transplanted at an early age and thus have a better quality of life than pediatric patients requiring dialysis ( ; ; ; ; ; ; ; ; ; ; ; ; ).
Though much has changed in the surgical and medical management of pediatric renal transplantation, the epidemiology and underlying renal pathology of pediatric patients with end-stage kidney disease has remained relatively constant over the past 40 years. The overall incidence in children 0 to 19 years is approximately 14 per million. Pediatric recipients of renal transplants differ from their adult counterparts in several ways. With the exception of focal segmented glomerulosclerosis, immunoglobin A, and hyperoxalosis, most causes of pediatric end-stage renal disease are congenital anomalies of the urinary tract, such as obstructive nephropathy or hypoplastic kidneys, while glomerulonephritis and diabetes are leading causes in adults ( ). Approximately 30% of children who receive a transplant have never had prior dialysis. Of the children who are on dialysis, approximately half are receiving peritoneal dialysis and the other half are receiving hemodialysis ( ; ; ; ).
Approximately 750 pediatric patients undergo kidney transplantation each year in the United States. Approximately 450 patients receive deceased donor transplants and 300 receive living-related transplants. The overall number of transplants has remained constant over the past 10 years, but fewer pediatric patients are receiving living-related transplants ( Fig. 38.1 ). However, the majority of transplants in children less than 2 years of age are from living-related donors ( ) ( ).
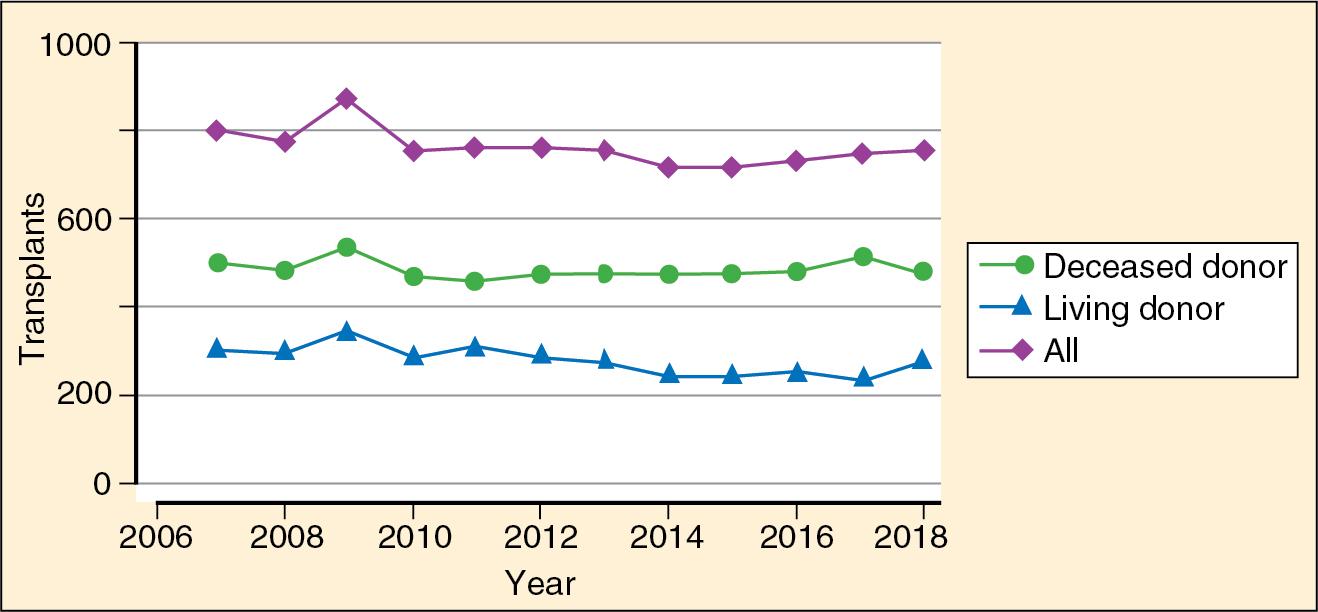
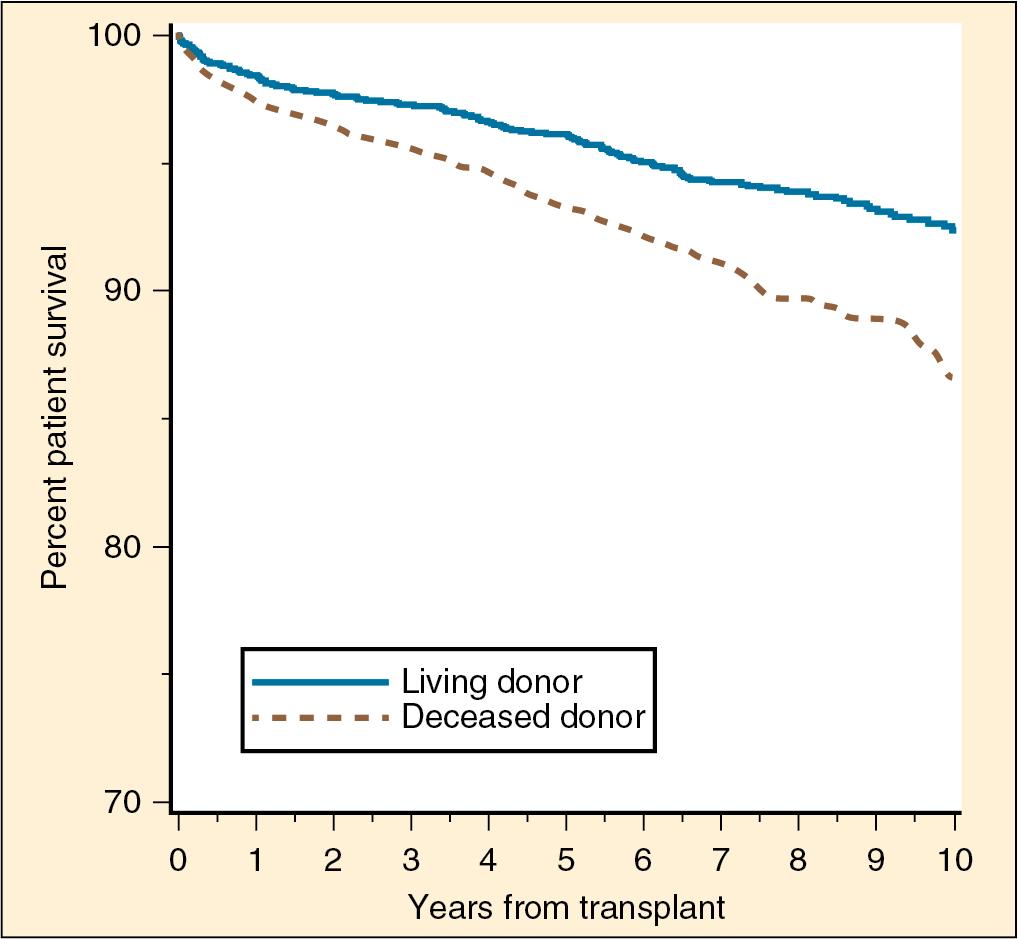
The age distribution of recipients has changed little over the years. Children aged 11 to 17 years are the most common age group (58.1%), followed by ages 6 to 12 years (18.7%), 2 to 5 years (23%), and under 1 year of age (0.1%). Pediatric living-donor kidney transplantations have been shown to have increased half-lives and graft survival in patients 2 to 5 years of age compared with transplants from deceased donors ( Fig. 38.2 ) ( ; ; ; ). Factors affecting graft survival in both living and deceased donors that are associated with worse outcomes include African American race of the recipient, no antibody induction, more than five transfusions, and retransplantation and pretransplantation dialysis ( ; ; ).
End-stage renal disease (ESRD) in the pediatric patient has significant effects on growth, metabolic homeostasis, cardiovascular function, and neurologic development. Chronic acidosis, hyperparathyroidism, and electrolyte imbalance (sodium, potassium, calcium, phosphorus, and magnesium) occur secondary to the kidney’s inability to excrete metabolic waste products. Anemia is common because as renal function fails, erythropoietin production decreases. The renin angiotensin system increases secondary to a decrease in renal perfusion. Hypertension, left ventricular hypertrophy, diastolic dysfunction, and hyperlipidemia are known cardiovascular consequences of ESRD. With a decreased ability to clear free water and sodium, volume overload is common. As glomerular filtration rate decreases, phosphorus levels increase. The elevated phosphorus binds to the serum calcium and magnesium, resulting in hypocalcemia, hypomagnesemia, and an increase in parathyroid hormone (PTH). Fractures in children can occur as calcium is leached from the bones secondary to the effect of PTH. Increased levels of PTH coupled with chronic metabolic acidosis and vitamin D deficiency can result in renal osteodystrophy. Developmental delay and seizures can also occur. Growth is impaired secondary to protein and calorie malnutrition, anemia, growth hormone resistance, vitamin D deficiency, chronic acidosis, and renal bone disease. Linear growth impairment may be further affected by the chronic use of corticosteroid therapy to treat an underlying disease state causing the ESRD.
In children, the most common primary diseases causing renal failure are congenital or inherited in nature (renal dysplasia, obstructive uropathy, reflux nephropathy) and acquired glomerular diseases (focal segmental glomerulosclerosis). Educational guidelines were published in 1998 by the Pediatric Committee of the American Society of Transplantation on the indications for pediatric transplantation. It suggested that the following pediatric patients with renal disease receive a kidney transplant: patients with ESRD unresponsive to medical management, patients with growth failure in spite of optimal nutritional management, patients with developmental delay, children with progressive renal osteodystrophy in spite of optimal medical management, and patients who fail to thrive ( ). Age is no longer a contraindication in transplanting children under 2 years of age. Infants who receive kidney transplants have better growth and development than those managed on dialysis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here