Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lipoma represents the most common mesenchymal soft tissue tumor of adults. Most commonly, lipomas are localized to the subcutaneous adipose tissue, presenting as a painless mass/swelling, although examples may arise in intramuscular locations and intrathoracic/intra-abdominal/retroperitoneal regions. If associated with peripheral nerves, there may be tenderness. Unlike ordinary lipomas, angiolipomas present as small and often painful subcutaneous masses, frequently in adolescence and young adults. Myolipomas (extrauterine lipoleiomyomas) are typically an incidental finding, originating in the abdominal cavity (usually retroperitoneum), but may also be found in the soft tissues of the trunk and extremities. Chondroid lipomas are generally deep-seated, painless lesions, most commonly occurring in the proximal extremities and limb girdles.
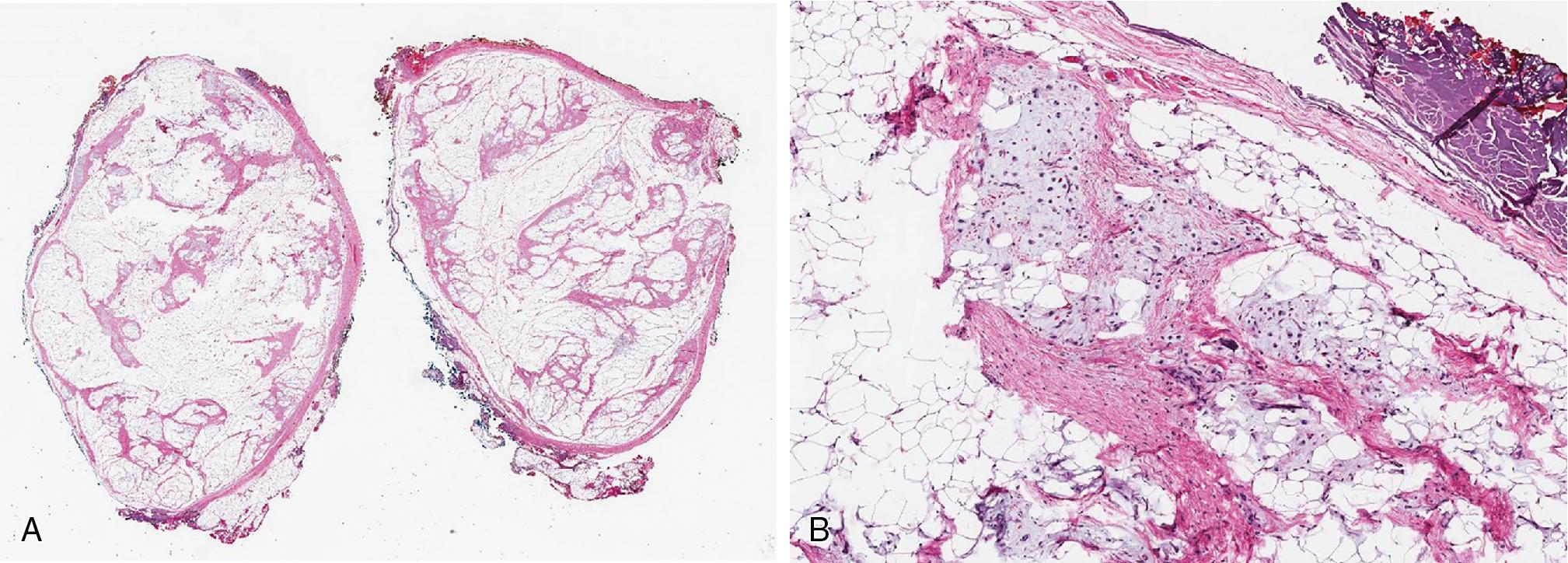
Lipomas are well-circumscribed fatty lesions with white-yellow discoloration, commonly encapsulated by a thin glistening fibrous membrane. Within skeletal muscle, intramuscular lipomas are infiltrative, entrapping skeletal muscle fibers within the tumor proper. Depending on their location and potential to remain undetected, lipomas may become quite large, exceeding 20 cm. Angiolipomas produce small, subcutaneous tan-yellow to red nodules. Chondroid lipomas are very well defined, with yellow-tan to mucinous cut surfaces. Gross inspection can help identify the “myo” component of myolipomas, especially when prominent, as tan whorled nodules admixed with yellow-tan adipose tissue. These tumors may become quite large (>10 cm), particularly when deep.
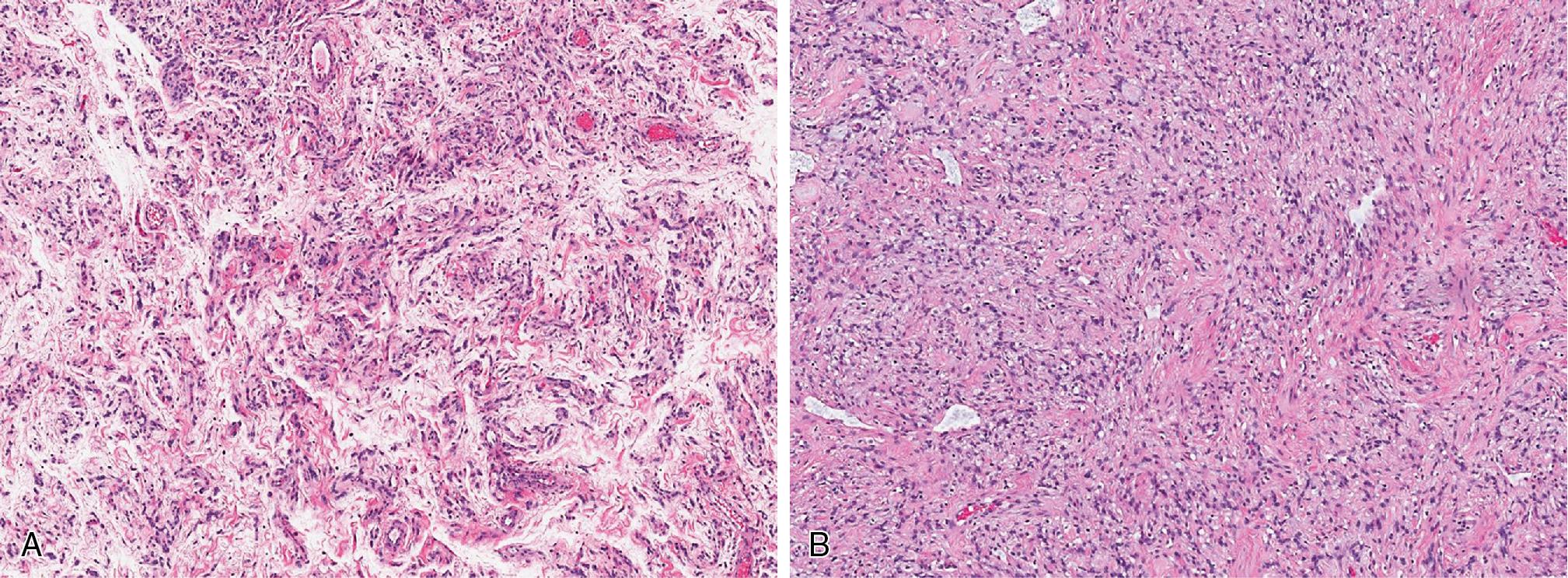
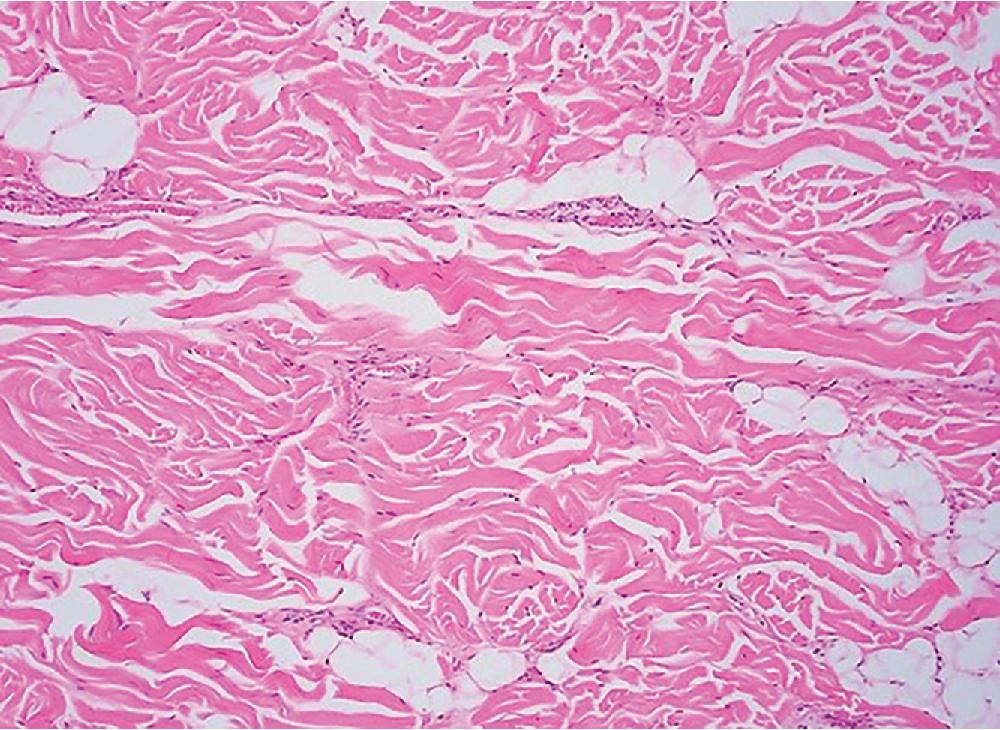
Lipomas are benign tumors composed of mature adipocytes, with little to no variation in size and shape ( Fig. 8.1 ). Some have thin fibrous septa dissecting the tumor proper. There is no to minimal cytologic atypia, and mitotic activity is virtually non-existent. Because of their infiltrative nature, intramuscular lipomas commonly entrap skeletal muscle fibers ( Fig. 8.2 ). Variable amounts of fat necrosis may occur.
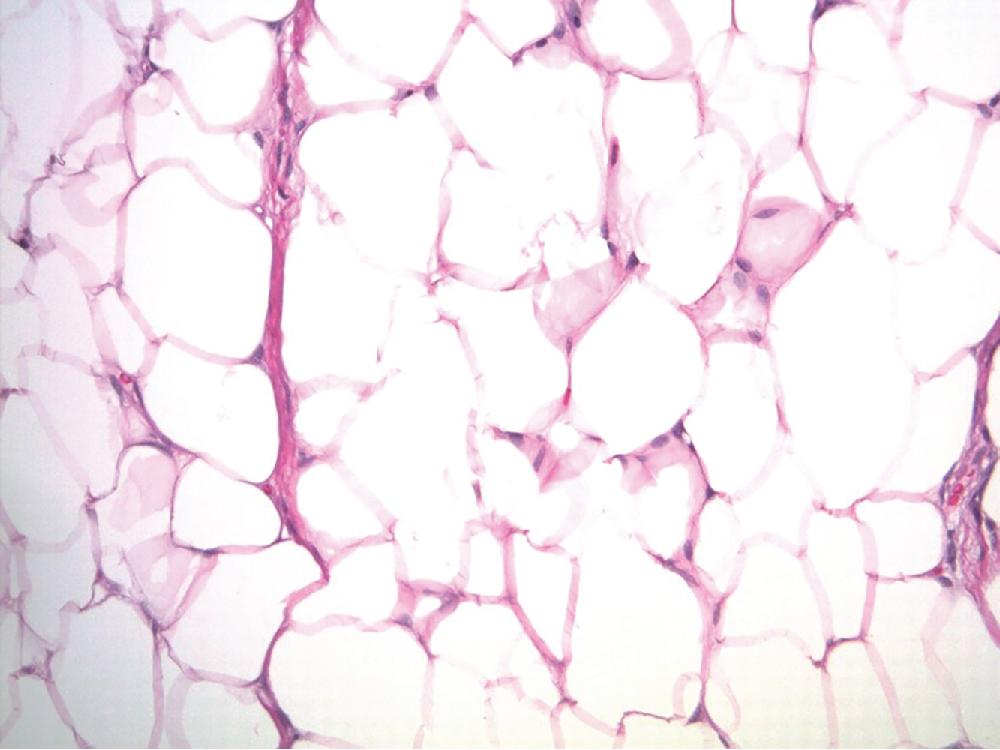
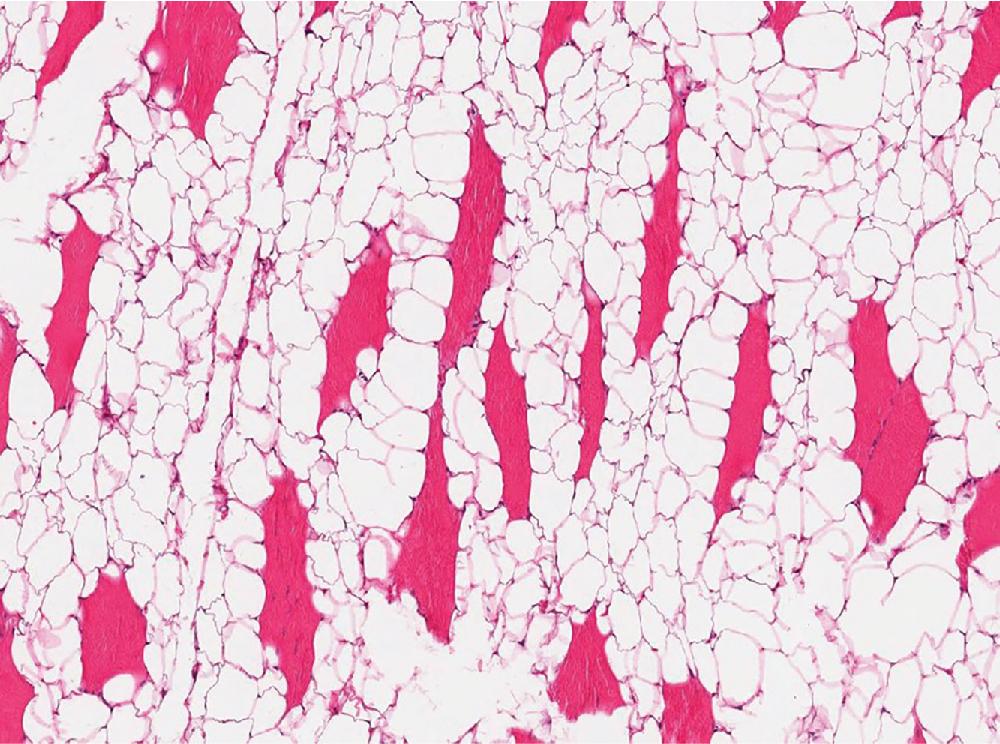
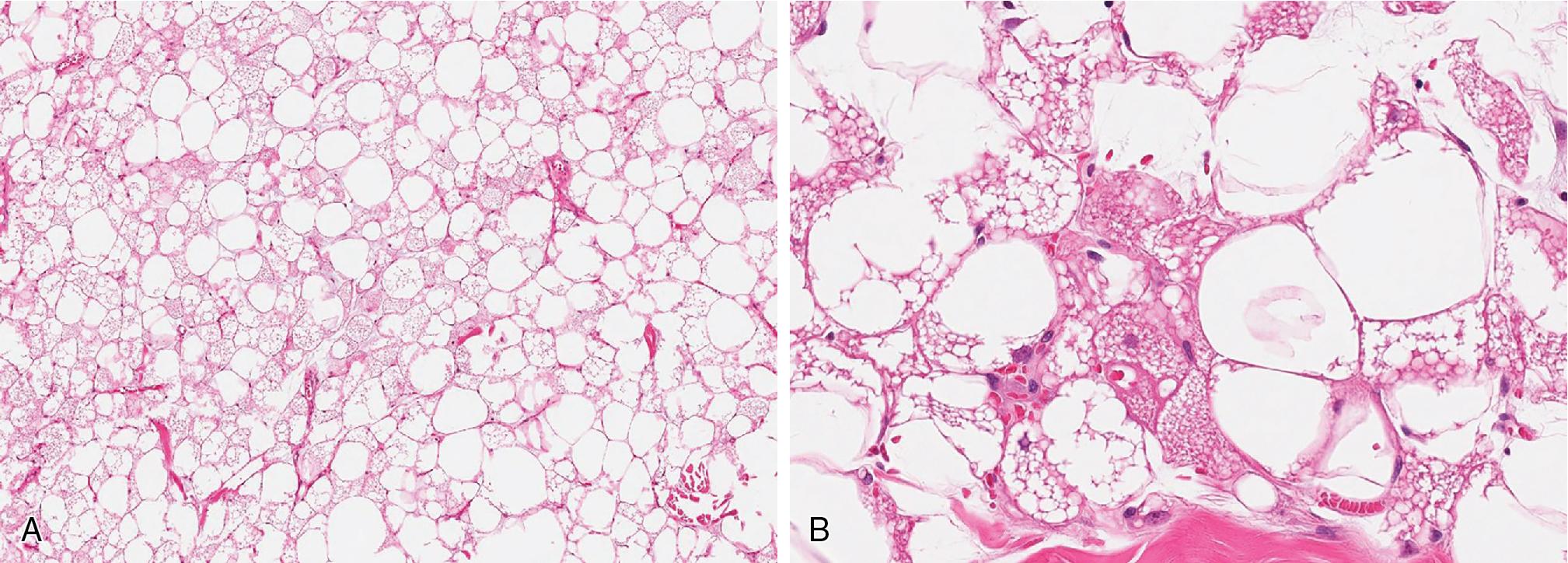
Angiolipomas ( Fig. 8.3 ) are well circumscribed and characterized by variable numbers and aggregates of small caliber, thin-walled capillary vessels, often containing microthrombi ( Fig. 8.3 B). These vessels are lined by bland endothelial cells and surrounded by a pericytic layer. Thick, muscular-walled blood vessels are not a feature of angiolipoma.
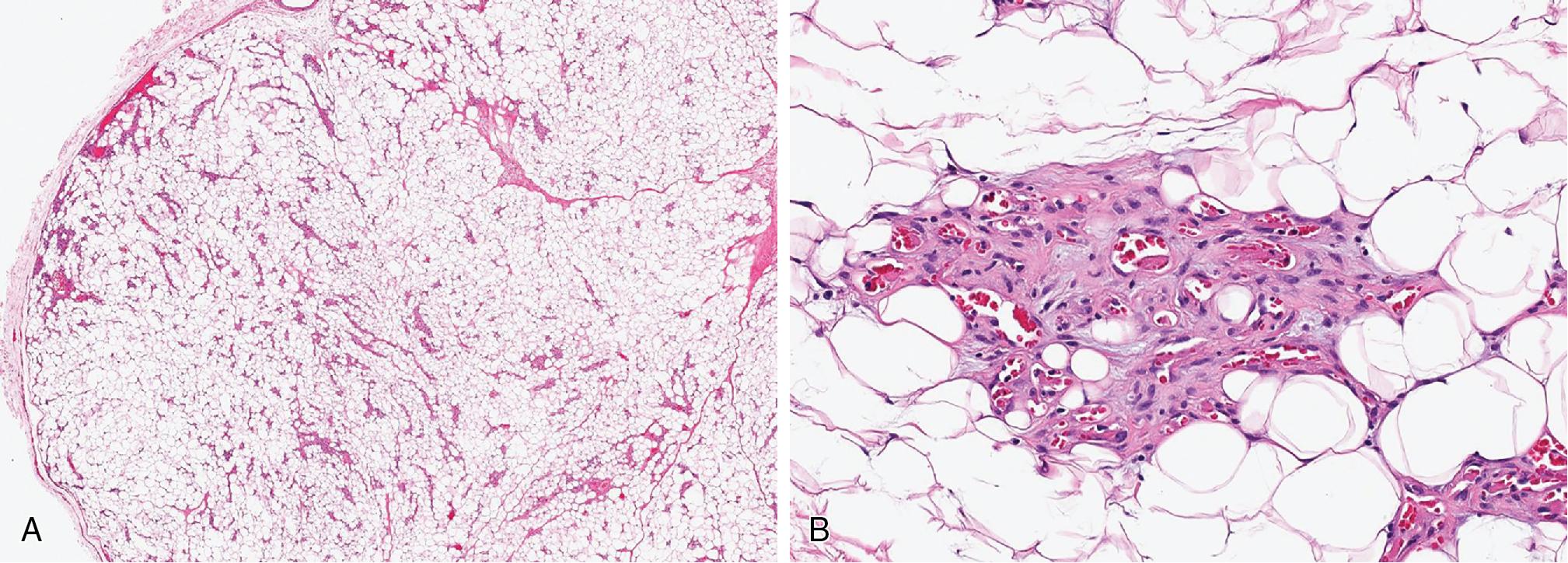
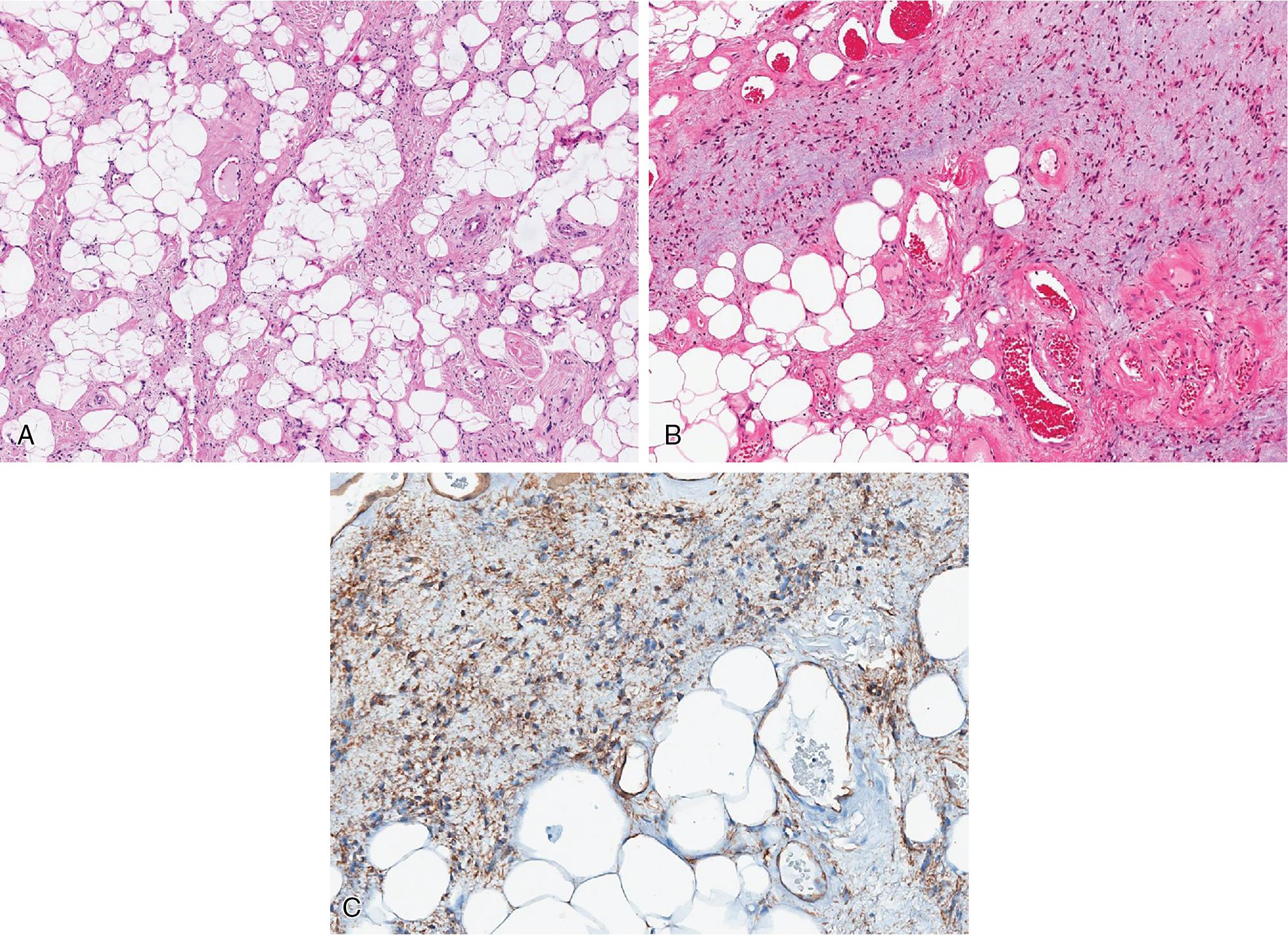
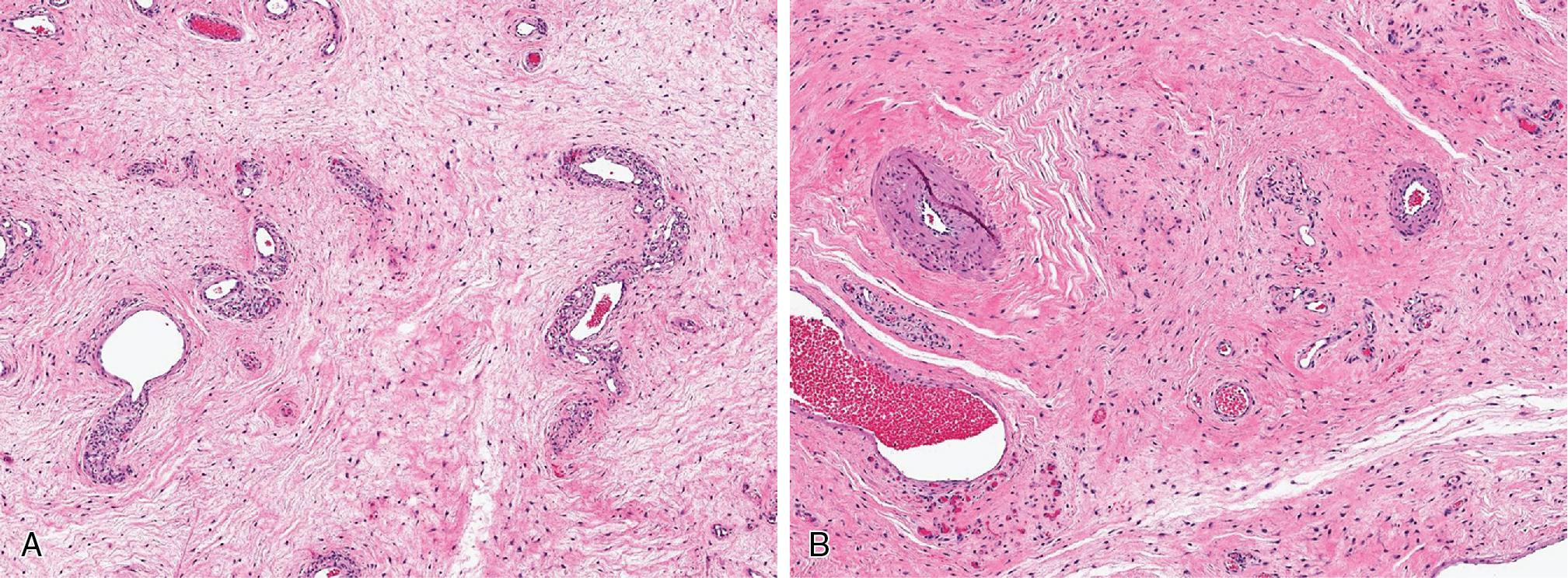
Myolipomas are mature adipocytic neoplasms that contain variable amounts of well-differentiated smooth muscle. There is no cytologic atypia or increased mitotic activity.
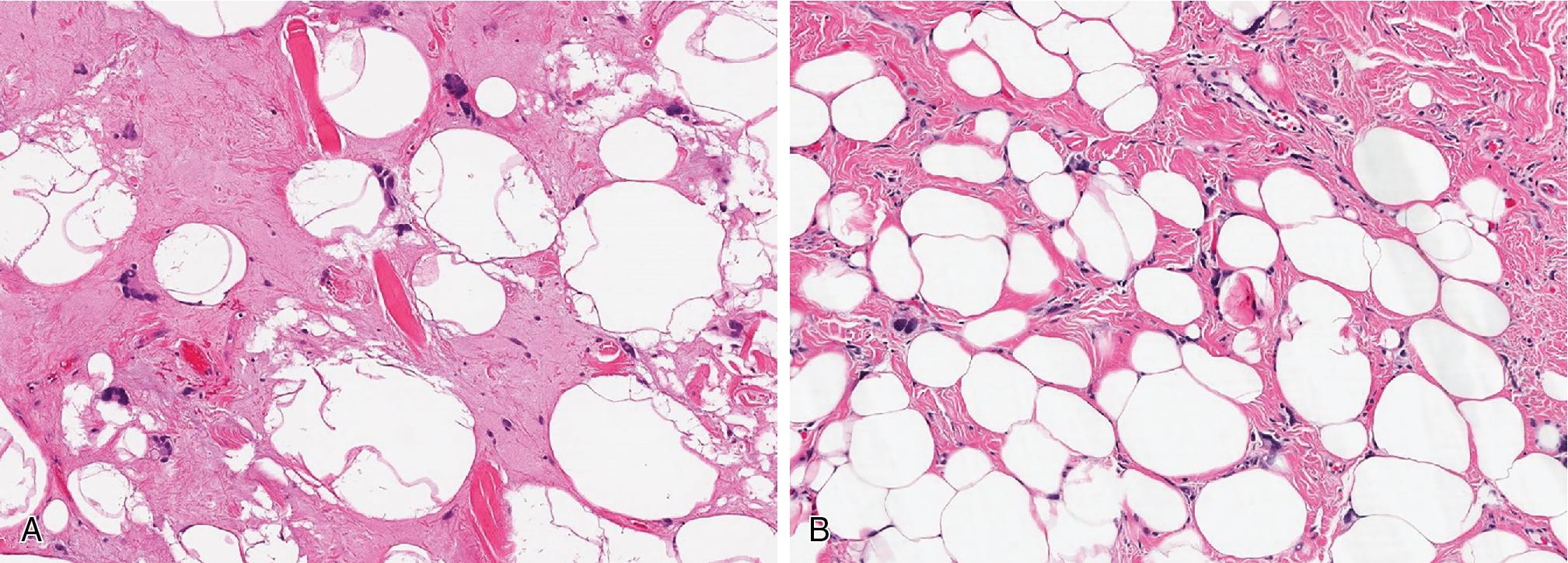
Chondroid lipomas ( Fig. 8.4 ) are lobulated, well-encapsulated masses with a characteristic histologic presentation of mature adipose tissue admixed with cords and nests of vacuolated cells (lipoblasts), embedded in a chondromyxoid to hyalinized matrix.
The adipocytes are positive for S100. The smooth muscle component in a myolipoma is diffusely positive for SMA, desmin, and caldesmon. In addition to S100 protein, keratins are rarely expressed in chondroid lipoma, but EMA is negative.
FISH analysis for MDM2 gene amplification is negative among all lipomas and variants. Angiolipomas usually have a normal karyotype.
Chondroid lipomas have a recurrent t(11;16)(q13;p130), associated with the C11orf95-MKL2 gene rearrangement.
The most important differential diagnosis, especially among deeply-seated benign adipocytic neoplasms, is atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDL). The presence of large and atypical, often multinucleated hyperchromatic cells is diagnostic of the latter, most frequently seen in the non-lipogenic fibrous tissue. FISH analysis for MDM2 gene amplification may be necessary in difficult cases, deep-seated tumors, especially in the abdominal and retroperitoneal regions, and among small needle biopsy specimens, primarily composed of normal-appearing adipose tissue.
In angiolipomas with a dominating “angio” component, the differential may include hemangioma. The presence of circumscription and interspersed adipose tissue will help establish the diagnosis of angiolipoma.
The extrauterine location of a myolipoma supports the diagnosis of myolipoma over leiomyoma with increased intralesional adipose tissue.
The differential diagnosis of chondroid lipoma includes myxoid liposarcoma (ML). Circumscription and encapsulation and absence of ML vascular pattern, accompanied by the characteristic epithelioid cells, lipoblasts, and mature fat, helps establish the diagnosis of chondroid lipoma.
Overall, local recurrences are rare in lipomas (<5%) and mainly seen in incompletely excised deeply seated lesion. In the absence of more obvious diagnostic features, locally recurrent “lipomas” probably should be evaluated for MDM2 gene amplification, to fully exclude an under-sampled ALT/WDL. Like ordinary lipomas, chondroid lipomas rarely locally recur. Surgical excision is curative for angiolipomas and myolipomas.
Lipoma is a benign tumor composed almost exclusively of mature adipocytes
Angiolipoma is a subcutaneous tumor composed of mature adipocytes intermingled with small, thin-walled capillaries, some of which contain fibrin microthrombi
Myolipoma is a benign extrauterine tumor composed of mature adipose tissue and variably amounts of well-differentiated smooth muscle
Chondroid lipoma is a benign adipose tissue tumor composed of variable numbers of lipoblasts, mature adipocytes, and epithelioid cells, embedded in a myxohyaline to chondroid matrix
Lipoma is the most common mesenchymal neoplasm in adults, seen in the upper back, proximal extremities, and abdomen, ranging from cutaneous to deep
Angiolipomas are relatively common, mostly seen in the subcutis of the forearm, followed by the trunk
Myolipoma, rare tumor, typically retroperitoneum, abdominal cavity, and pelvis, less commonly trunk or extremities
Chondroid lipoma, rare tumor, usually deep-seated involving skeletal muscle of proximal extremities and limb girdles
Lipoma, fifth to seventh decade of life
Angiolipoma, second to fourth decades of life
Myolipoma, adulthood
Chondroid lipoma, adulthood
Lipoma, commonly painless mass, symptomatic if associated with nerves or vital structures, occurs virtually anywhere
Angiolipoma, painful, often multiple, extremity subcutaneous masses
Myolipoma, retroperitoneal and extrauterine, incidental
Chondroid lipoma, painless, well-defined, deep-seated, extremity
Surgical excision is curative. Rare local recurrences if incomplete excision.
Lipomas are well-circumscribed, white-yellow encapsulated fatty lesions. Intramuscular lipomas are not well circumscribed and entrap skeletal muscle within the tumor proper
Angiolipoma, tan-yellow to red circumscribed nodules
Myolipoma, white-yellow adipose tissue with tan whorled nodules
Chondroid lipoma typically very well defined, fatty to glistening myxoid
Lipomas are exclusively mature adipocytes without cytologic atypia
Angiolipoma, mature adipocytes admixed with small thin-walled capillary vessels with occasional fibrin microthrombi
Myolipoma, extrauterine, mature adipocytes admixed with variable amounts of smooth muscle
Chondroid lipoma, lipoblasts, mature adipocytes, and occasional epithelioid cells, embedded in a chondromyxoid matrix
Atypical lipomatous tumor/well-differentiated liposarcoma, hemangioma (angiolipoma), uterine or parasitic leiomyoma (myolipoma), and myxoid liposarcoma (chondroid lipoma)
Lipoblastoma/lipoblastomatosis is a benign neoplasm of embryonal white fat that may present as a lobulated and localized, well-circumscribed mass (lipoblastoma) or, alternatively, a diffuse and infiltrative lesion (lipoblastomatosis). Most examples present in infancy and early childhood, before 5 years of age. There appears to be a slightly male predilection. The extremities and the trunk are the most common anatomic sites.
Usually lipoblastomas are <5 cm, although lesions larger than 10 cm have been described. They are typically well circumscribed and lobulated, and the cut surface is white-yellow often with prominent fibrous trabeculae separating myxoid areas, cystic spaces, or fat nodules.
Lipoblastomas are characterized by nodules of adipocytes of variable maturation embedded in a lightly myxoid stroma and separated by variably thick fibrous septa ( Fig. 8.5 ). The adipocytic maturation may represent a somewhat zonal maturation, ranging from primitive mesenchymal cells, to univacuolated (signet-ring like) lipoblasts, multivacuolated lipoblasts to mature adipocytes. The background myxoid stroma often exhibits thin-walled plexiform vessels, mimicking ML ( Fig. 8.5 B). Occasionally, heterologous elements can be seen, such as chondroid metaplasia, extramedullary hematopoiesis, and chronic inflammation. There is no significant cytologic atypia or mitotic activity.
As with ordinary adult lipomas, the adipocytes in lipoblastoma also are positive for S100. CD34 reactivity is seen in the more spindled cells in myxoid and fibrous regions, and desmin positivity is sometimes observed. Lipoblastomas typically contain structural alterations or gene fusions involving 8q12 ( PLAG1 ). One or more extra copies of chromosome 8 may occur with or without the associated gene fusion.
The most important differential diagnosis is ML. However, the presence of lobulation and more mature non-myxoid areas, especially when associated with a child, helps establish the diagnosis of lipoblastoma.
Surgical resection of the localized form (lipoblastoma) generally results in complete cure. Local recurrences are more likely to be associated with the diffuse form (lipoblastomatosis), ranging up to 50% of cases, when incompletely excised. Dedifferentiation does not occur, and there is no risk of distant metastases.
Benign tumor of embryonal white fat which may be localized or diffuse (lipoblastomatosis)
Rare neoplasm most commonly arising in the trunk and extremities
Children, <3 years, rarely adolescents and adults
Painless soft tissue swelling
Surgical excision is generally curative
Local recurrences are more likely to occur in the diffuse form of the disease
Lobulated, yellow to white, typically less than 5 cm in greatest dimension, focal myxoid changes and rarely cystic
Localized form typically lobulated with prominent fibrous septa enclosing mixtures of mature to immature adipocytes, often with foci of myxoid changes and hypervascularity.
Diffuse form may contain entrapped skeletal muscle fibers
Myxoid liposarcoma
Hibernomas are benign, slowly growing, painless neoplasms, most often arising in young to middle-aged adults, with a slight male predominance. The most common anatomic location is the thigh, followed by the trunk and upper extremity. Radiographically, many will display a signal intensity within the tumor proper described as intermediate between mature adipose tissue and skeletal muscle.
Most are well circumscribed and lobular adipocytic lesions, yellow to brown.
Hibernomas display varying proportions of brown fat admixed with mature, white adipose tissue. At low power, a distinct lobulation is evident. Cytologically, the brown fat cells exhibit a centrally placed nucleus and a prominent nucleolus, surrounded by a cytoplasm ranging from granular and eosinophilic to uniformly multi-vacuolated ( Fig. 8.6 ). Rarely, myxoid change and spindled cell areas occur. Mitotic figures are absent, and significant nuclear atypia is not a feature.
S100 protein is strongly and diffusely positive within both brown and white fat cells. CD10 has recently been noted to be expressed and may be helpful in distinguishing hibernoma from potential fatty lesion mimics. Desmin and SMA are negative.
Cytogenetic analysis reveals structural rearrangements, including translocations and deletions, involving chromosome 11q13. MDM2 gene amplification is not present.
The most important differential diagnosis is ALT/WDL. The enlarged atypical and often multi-nucleated cells and lipoblasts associated with it are not seen in hibernoma. When hibernomas are composed of a predominance of brown fat with eosinophilic cells, rhabdomyoma may also be considered within the differential diagnosis. Desmin positivity excludes hibernoma.
Surgical resection is curative.
Benign lobular adipocytic neoplasm with multivacuolated brown fat differentiation
Rare, thigh, trunk, chest, and upper extremity
Young to middle-aged adults
Slow-growing, painless, mobile and usually subcutaneous, less commonly intramuscular
MRI: Hibernomas are T1 iso- to hypointense and T2 iso- to hyperintense compared with the normal fat; often described as intermediate between normal subcutaneous fat and skeletal muscle
Benign neoplasm, complete excision is curative
Well-circumscribed, lobulated, brown-yellow fatty mass
Circumscribed and lobulated, fibrous septa, mixture of varying proportions of mature white and brown adipocytes, with extensive cytoplasmic multi-vacuolation to eosinophilic granular change, centrally placed nuclei, prominent nucleoli
Atypical lipomatous tumor/well-differentiated liposarcoma and adult rhabdomyoma, especially if predominantly eosinophilic brown fat cells
Most common presentation for spindle cell/pleomorphic lipoma is the posterior neck, back, and shoulders of elderly males. In women, these lipomas are frequently found outside the shawl region, often at younger ages and with a wider anatomic distribution, such as the extremities and the face.
Well-circumscribed, ovoid mass with yellow-to-white and myxoid cut surface. Based on the proportion of the adipose and fibrous tissue, it may be firmer than a lipoma.
Spindle cell lipomas and pleomorphic lipomas represent spectrums of a similar neoplasm, both being well circumscribed and often encapsulated, usually subcutaneous, less commonly dermal or intramuscular. “Spindle” cell lesions are composed of mature adipocytes and various amounts of intermingled fibrous to myxoid tissue ( Fig. 8.7 ), within which are bland spindle cells, admixed with scattered variably thick, eosinophilic collagen fibers (“ropy collagen”) and mast cells. At the opposite end, “pleomorphic” forms are further characterized by the additional presence of randomly distributed multinucleated, “floret-like” giant cells ( Fig. 8.8 ).
Occasionally, there may be little to no adipocytes, so-called “fat-poor” spindle cell/pleomorphic lipoma. Rare examples display branching and dilated vascular-like spaces that separate the tumor to form pseudopapillary projections, designated as the pseudoangiomatous subtype.
The spindled and pleomorphic cells are diffusely and strongly positive for CD34 ( Fig. 8.7 C) and usually have loss of nuclear RB1 protein expression. As expected, the adipocytes express S100 protein.
The recently described atypical spindle cell/pleomorphic lipomatous tumor represents a benign adipocytic neoplasm, characterized by ill-defined tumor margins, mild to moderate nuclear atypia, enlarged and bizarre lipoblasts, and occasional presence of pleomorphic, multinucleated cells. Admittedly, some examples may be indistinguishable from spindle cell/pleomorphic lipoma. There is no MDM2 gene amplification, low tendency to locally recur (10%–15%) if incompletely excised, but no risk for dedifferentiation. CD34 positivity and RB1 loss can be shared by both atypical spindle cell/pleomorphic lipomatous tumor and spindle cell/pleomorphic lipoma.
ALT/WDL is only rarely seen in the superficial soft tissues of the head and neck and shoulder regions; more often arises in deeply seated extremities, abdominal cavity, or retroperitoneum; and is characterized by MDM2 gene amplification. The atypical and enlarged, multinucleated cells in the latter exhibit some morphologic overlap with the floret cells seen in spindle cell/pleomorphic lipoma.
Local recurrences are rare, even with incomplete resection.
Benign adipocytic neoplasm composed of varying proportions of adipocytes and spindled cells embedded in fibromyxoid stroma with scattered ropy collagen and mast cells
Pleomorphic, multinucleated “floret-like” giant cells in addition to the abode histology, may be present
Elderly men, back, shoulders, and neck. <10% of cases occur in females, often in unusual locations (such as extremities and face)
Males, 50–75 years
Subcutaneous, rarely intramuscular, well-circumscribed lesion
Benign lesion, surgical excision is curative
Well-circumscribed nodule, yellow to white, can present as myxoid and firmer than conventional lipoma
Various proportions of adipocytes and spindled cells embedded in myxoid to fibromyxoid stroma with scattered ropy collagen and mast cells
Atypical spindle cell/pleomorphic lipomatous tumor and atypical lipomatous tumor/well-differentiated liposarcoma
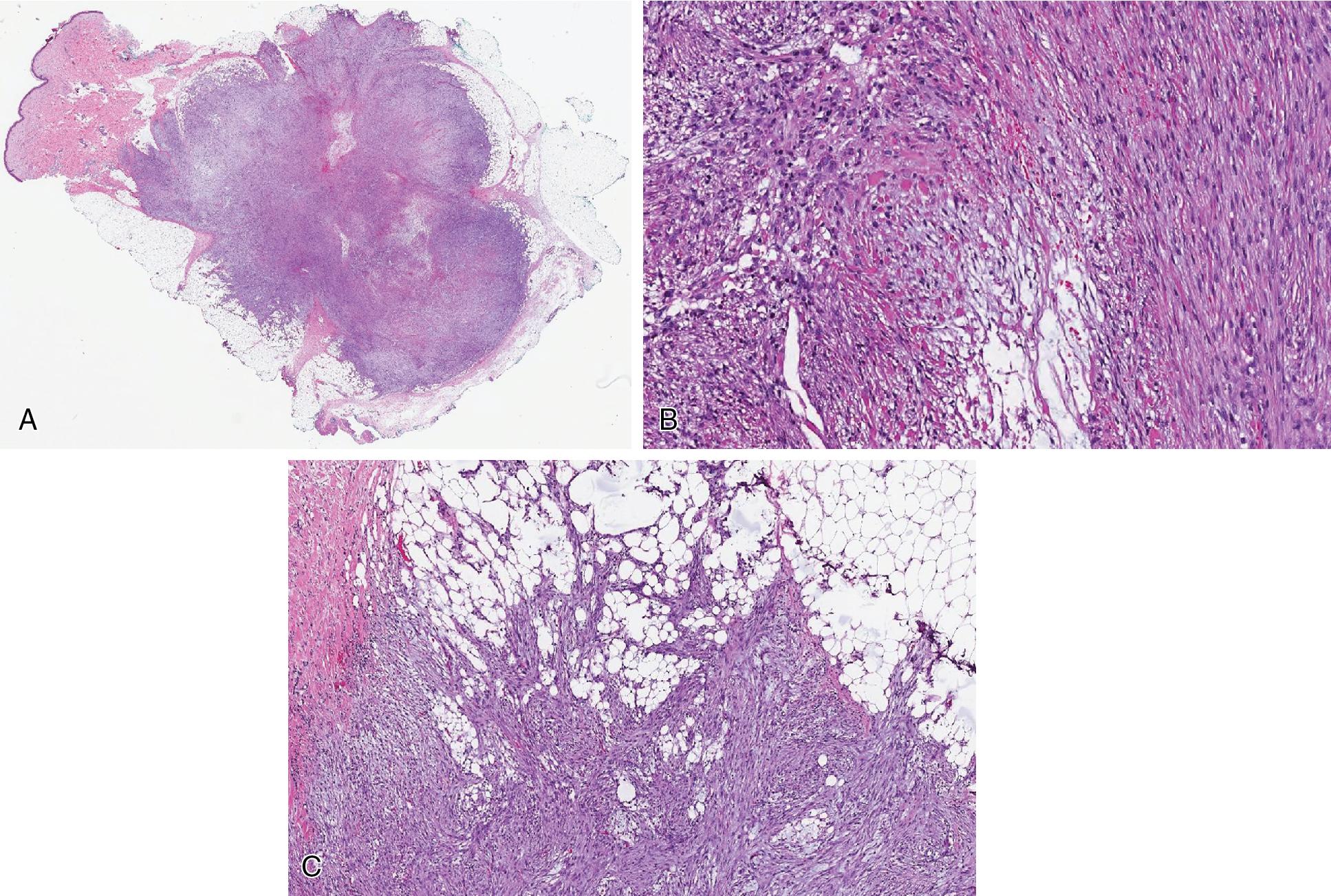
ALT/WDL is a potentially locally aggressive mature adipocytic neoplasm characterized by variably thick fibrous septa and enlarged, atypical and often multinucleated cells, predominantly within fibrous tissue and occasionally mature adipocytes. Intersecting adipocytic proliferation. It most commonly is deeply seated, arising bellow the fascia of the extremities. The retroperitoneum and the paratesticular soft tissues are common areas where ALT/WDL arises. Less common anatomic sites include the mediastinum, distal extremities, and rarely the head and neck region.
Well-circumscribed and lobulated yellow fatty mass. Some areas of myxoid changes and, rarely, typically within retroperitoneum, areas of infarct-like necrosis may be focally observed.
The adipocytes show heterogeneity in size and shape, usually separated in lobules by variably thick fibrous septa ( Fig. 8.9 ). The scattered atypical and often multinucleated stromal cells are significantly pleomorphic (even bizarre), characterized by nuclei with irregular nuclear membranes and dense hyperchromatic chromatin pattern, giving the impression of “smudged” cells ( Fig. 8.9 B). Most are seen within the fibrous tissue, less commonly mature adipocytes and muscular walls of blood vessels. Lipoblasts are less commonly observed ( Fig. 8.9 C). Fat necrosis rarely dominates.
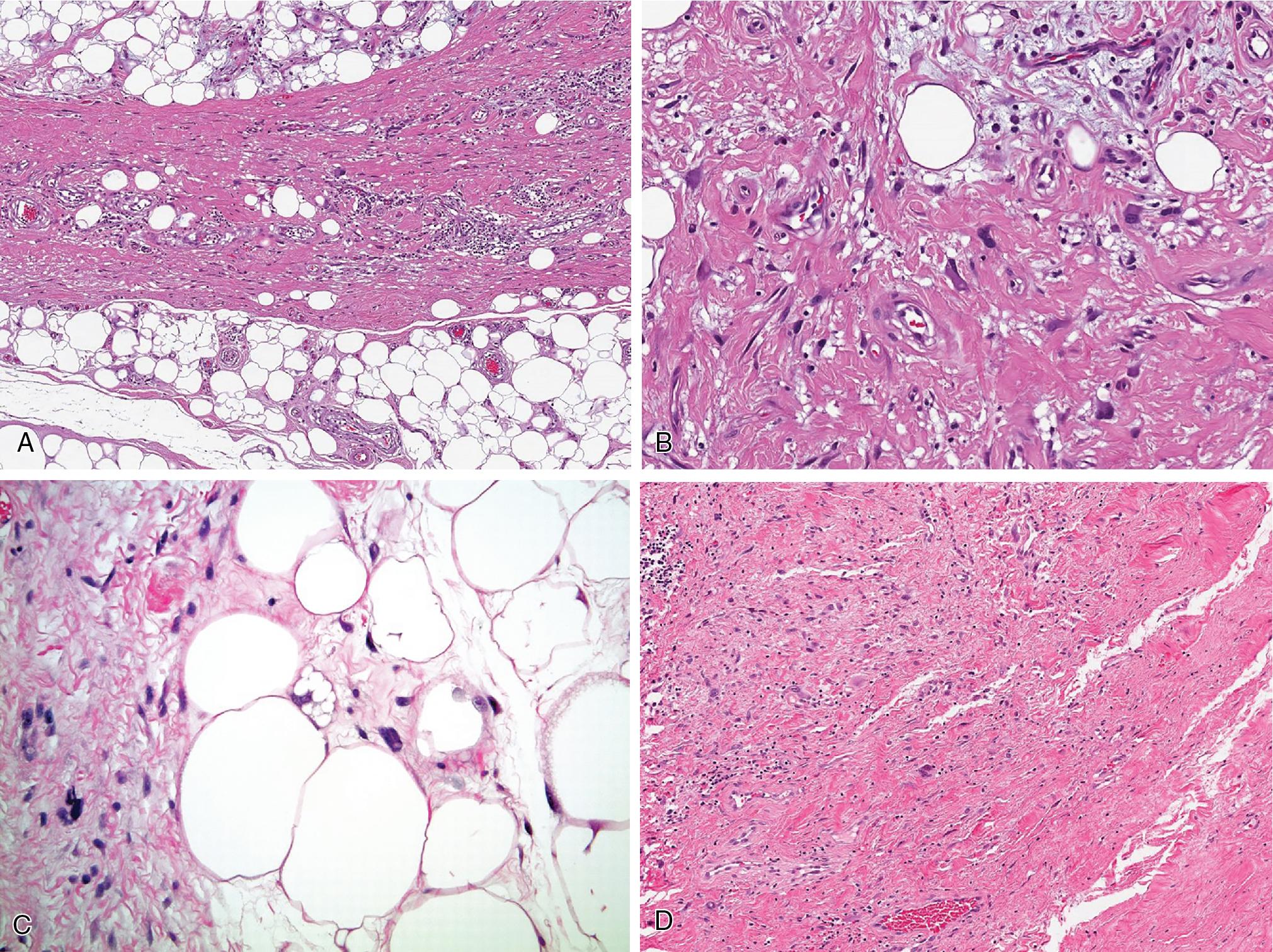
When fibrous tissue predominates, some designate these as “sclerosing” WDL ( Fig. 8.9 D). Even more rare, abundant lymphocytes and plasma cells within the fibrous tissue may obscure the background atypical cells/lipoblasts; this form has been referred to as “inflammatory” WDL.
Mature heterologous features, usually in the form of osseous, cartilaginous, or myogenic differentiation, are rare but should not be confused with dedifferentiation.
Mitotic figures tend to be rare, but, in more cellular examples, should never exceed 4 mitoses/10 high-power fields (HPF).
Immunohistochemistry usually shows MDM2 nuclear expression, particularly with the larger atypical cells. More sensitive, especially in lipoma-like WDL, and more specific is confirmation of MDM2 (12q15) gene amplification via FISH analysis. On cytogenetic analysis, karyotyping of ALT/WDL is characterized by supernumerary ring chromosomes and/or giant marker chromosomes, involving chromosome 12.
The differential diagnosis includes most commonly lipoma, hibernoma, and spindle cell/pleomorphic lipoma. The deeper location, especially when seen in the retroperitoneum, presence of enlarged, atypical and hyperchromatic cells, less commonly lipoblasts, and, when necessary, MDM2 gene amplification help establish the diagnosis.
Lesions that arise in the retroperitoneum, paratesticular soft tissue, mediastinum, and similar places where complete excision is challenging, the term WDL is better used given the higher potential for local recurrence and progression compared with lesions arising within the extremities for which the term atypical lipomatous neoplasm is better applied. ALT/WDL has no metastatic potential, unless it has undergone dedifferentiation.
The risk for dedifferentiation varies depending on location, exceeding 20% for retroperitoneal forms but less than 2% when arising in the extremities. Consequently, the risk of mortality and metastases is greater for retroperitoneal and paratesticular lesions than for those localized to the extremities.
Locally aggressive adipocytic neoplasm, usually predominantly fatty and characterized by variably thick fibrous septa with atypical, enlarged to multinucleated cells and, less commonly, lipoblasts
Rare, deep soft tissues of the extremities, retroperitoneum, and paratesticular regions
Middle-aged to older adults
Deep-seated, painless, slowly enlarging mass
Often asymptomatic, large, when arising in retroperitoneum and abdominal cavity
Complete resection, when possible, potentially lowers the risk for local recurrence and potential for dedifferentiation, representing transformation to a higher-grade neoplasm
Metastases only occur with dedifferentiation
Well-circumscribed, lobulated fatty lesion, yellow to firm white and gelatinous
Areas of myxoid changes, fat necrosis, and sclerosis may be seen
Adipocytes of varying size and shapes (heterogeneity) separated by variably thickened fibrous septa
Randomly distributed, atypical, enlarged and often multinucleated cells and, less common, multivacuolated lipoblasts
Lipomas and variants
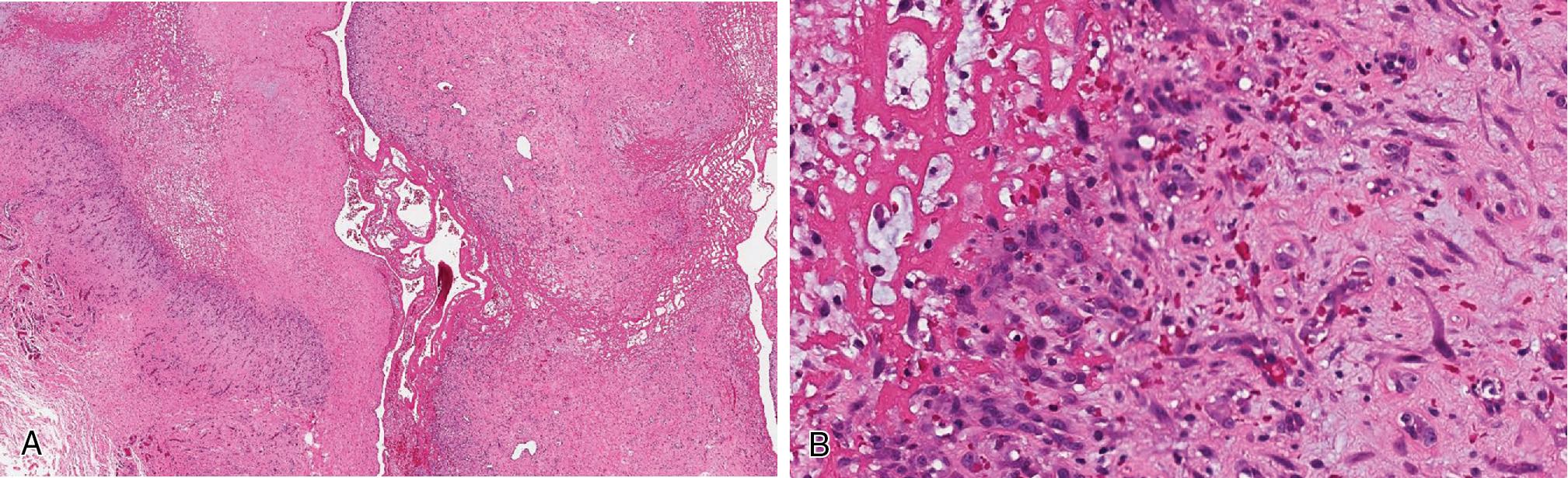
Dedifferentiated liposarcoma (DL) is defined as a high-grade sarcoma arising concomitantly from an ALT/WDL (most common) or occurring in the same anatomic site as a previously resected ALT/WDL. In cases where the precursor ALT/WDL is not present, the diagnosis can be confirmed molecularly, namely amplification of MDM2 via FISH analysis. The vast majority are found in older adults, especially in the deep soft tissues of the proximal extremities, intra-abdominal, and retroperitoneal locations.
DL usually presents as a large fleshy mass, juxtaposed to a smaller yellow-tan lipoma or lipoma-like lesion. Calcifications and even ossification are rarely present. Necrosis and hemorrhage are commonly seen.
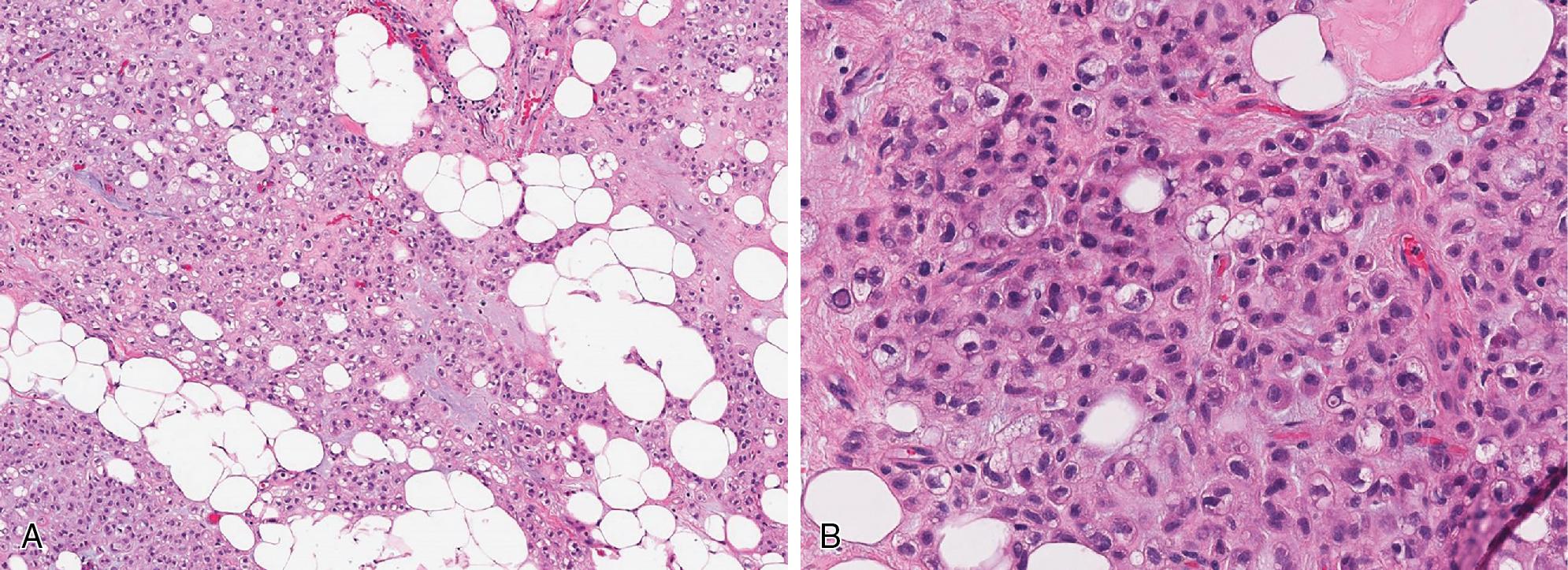
Most commonly, the transition of the ALT/WDL and DL components is sharply demarcated ( Fig. 8.10 A), but a gradual transition from the well-differentiated component to the high-grade, non-lipogenic areas is rarely present. Usually, the hypercellular dedifferentiated component is composed of atypical and pleomorphic spindled cells, arranged in fascicles and whorls ( Fig. 8.10 B), resembling undifferentiated pleomorphic sarcoma (UPS), without further defining features. However, in about 5% to 10% of cases, the dedifferentiated component exhibits varying degrees of heterologous differentiation including osteosarcoma/chondrosarcoma, leiomyosarcoma, myxofibrosarcoma, and rhabdomyosarcoma. So-called “homologous” lipoblastic differentiation, mimicking pleomorphic liposarcoma (PLS), is characterized by the presence of isolated and/or groups of lipoblasts. By definition, the dedifferentiated component should have at least ≥5 mitoses/10 HPF to qualify as “dedifferentiated,” separating it morphologically and prognostically from cellular forms of ALT/WDL.
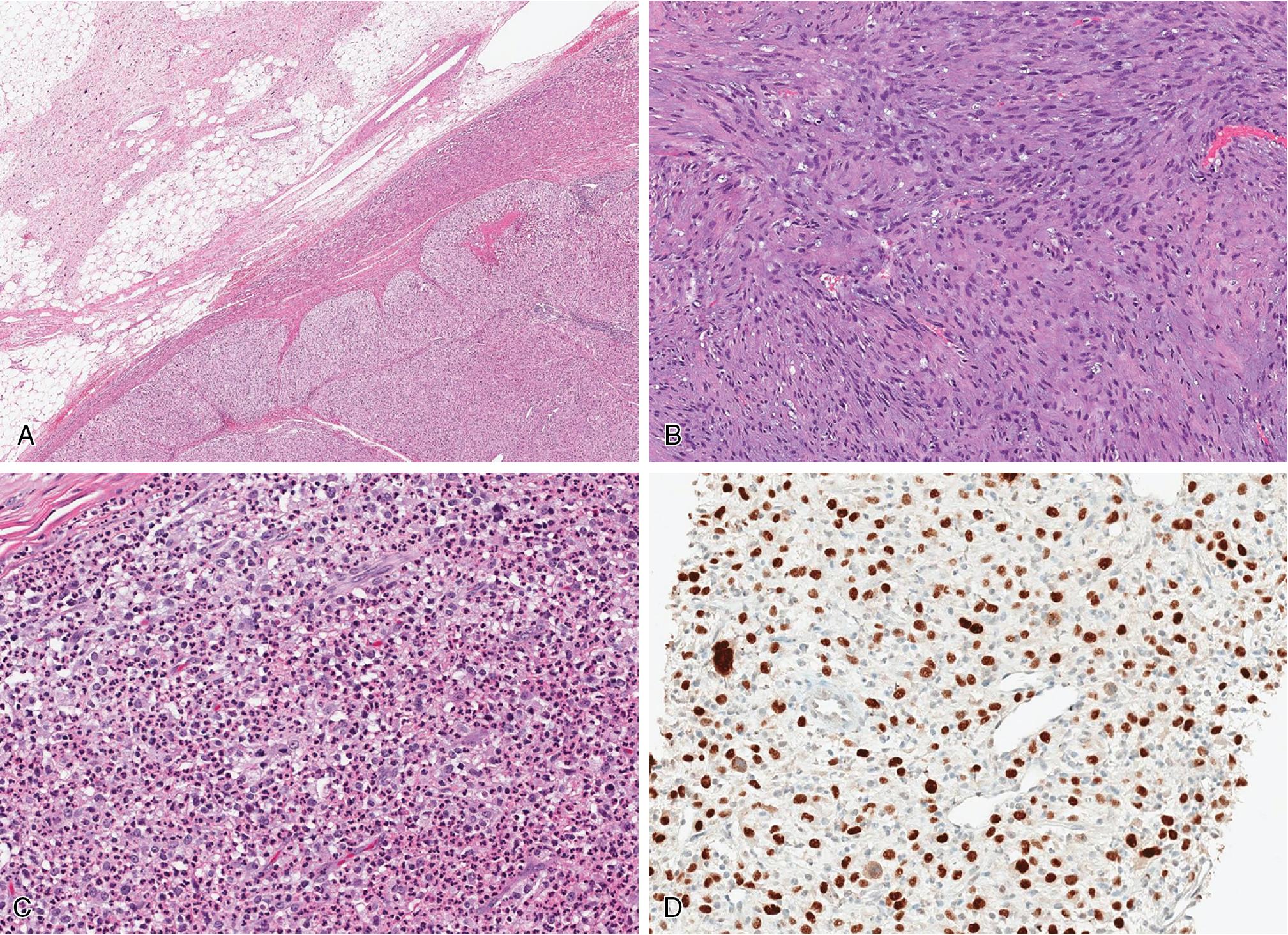
MDM2 immunohistochemistry may be helpful ( Fig. 8.10 D), especially in small biopsy specimen and in instances where the low-grade component is not obviously present. Confirmation of MDM2 gene amplification by FISH analysis represents the gold standard.
UPS is a diagnosis of exclusion, does not have MDM2 gene amplification, or is evidence of a concomitant or pre-existing ALT/WDL.
Primary PLS, leiomyosarcoma, rhabdomyosarcoma, and extraskeletal osteosarcoma can be excluded if the low-grade ALT/WDL component is confirmed or in the presence of MDM2 immunoexpression and/or MDM2 gene amplification via FISH.
Prognosis is significantly related to anatomic site, with retroperitoneal/intra-abdominal and paratesticular origins correlated with a worse survival than those localized to the deep soft tissues of the extremities. Local recurrence rates range up to 40%, while distant metastasis occurs in approximately 15% to 20% of cases. Overall mortality is estimated at 28% to 30% at 5 years.
There appears to be no association between the extent of the dedifferentiated component and survival, although data is limited. However, the presence of myogenic differentiation is associated with a worse clinical outcome.
High-grade spindled to epithelioid sarcoma, with at least 5 mitoses/10 HPF, arising concomitantly or in the setting of a previously resected ALT/WDL, or, in the absence of a known low-grade component, confirmed by molecular evidence of MDM2 gene amplification, with or without evidence of heterologous differentiation
Retroperitoneum, deep soft tissues of extremities, paratesticular, mediastinum
Adults, typically over 50 years of age
Presence of lipomatous and non-lipomatous components in the deep soft tissues or retroperitoneum, best seen radiologically, painless swelling/mass
Local recurrence in up to 40%, distant metastasis in 15%–20%
The overall mortality is estimated at 28%–30% at 5 years
Retroperitoneal and paratesticular origin predicts a worse survival
Complete resection with negative margins, for both high- and low-grade components, is necessary
Large fleshy brown-tan mass, usually juxtaposed to a generally smaller, yellow and fatty lesion
Pleomorphic, high-grade, usually non-lipogenic neoplasm with abrupt transition from well-differentiated to dedifferentiated component
Sometimes the well-differentiated lipogenic component is not identified, and molecular confirmation of MDM2 gene amplification is required to establish the diagnosis
Homologous “lipoblastic” and heterologous elements rarely seen
Mitotic activity in the dedifferentiated component should be at least 5 mitoses/10 HPF
Undifferentiated pleomorphic sarcoma, extraskeletal osteosarcoma, and pleomorphic forms of liposarcoma, leiomyosarcoma, and rhabdomyosarcoma
ML presents as a large soft tissue mass, usually involving the deep soft tissues of the extremities. The most common anatomic location is the posterior thigh. Retroperitoneal ML most often represents either metastasis or evidence of a DL.
MLs are large (>10 cm), typically well-demarcated, myxoid, and multinodular neoplasms.
ML presents as a lobulated neoplasm, variably cellular, with often noticeable increased cellularity at the periphery of the nodules. The background stroma is abundant and myxoid accompanied by a delicate network of plexiform and arborizing vessels ( Fig. 8.11 ). Sometimes foci of extracellular mucinous matrix appear extremely paucicellular, giving a pulmonary edema appearance. The uniform neoplastic cells have small, bland round to oval nuclei with minimal amounts of eosinophilic cytoplasm. Signet ring cell univacuolated lipoblasts are often present, at least focally ( Fig. 8.11 B).
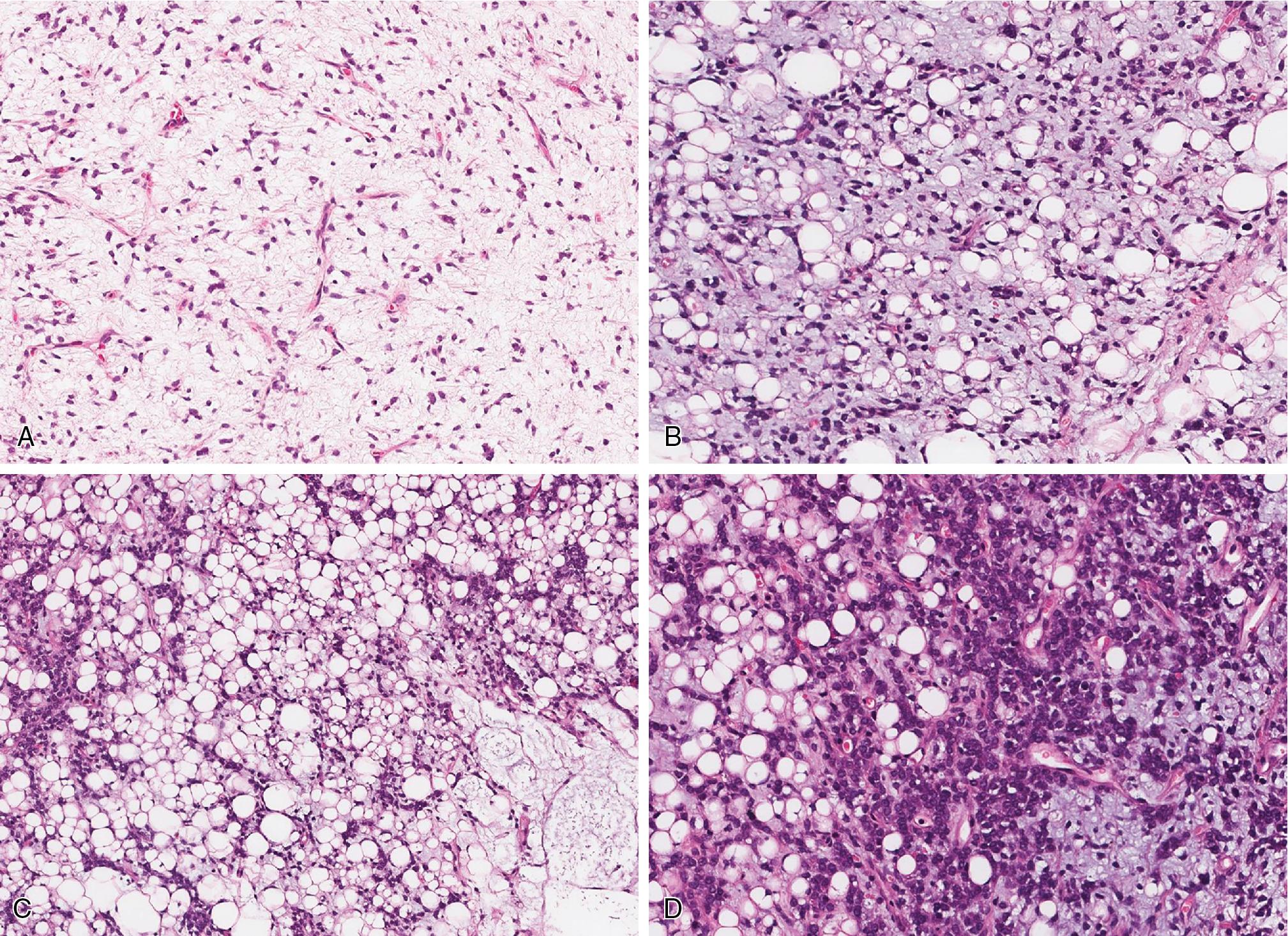
High-grade ML (also known as round cell liposarcoma) is defined as ML with more than 5% of round cell component ( Fig. 8.11 C). The histologic features of the round cell component are characterized by marked hypercellularity, diminished background myxoid stroma, and a population of larger, round cells with prominent nucleoli and increased mitotic activity. Pure high-grade ML (round cell liposarcoma), especially in small biopsy specimens, may lack an obvious myxoid stroma, making the diagnosis difficult to establish by routine microscopy alone ( Fig. 8.11 D).
Immunohistochemistry is not generally helpful. In difficult cases, demonstration of a DDIT3 gene rearrangement, most often combined with FUS , associated with the t(12;16)(q13;p11) translocation, and, less often with EWSR1 , t(12;22)(q13;q12), is helpful in establishing the diagnosis. Generally, the diagnosis of ML, especially when low grade, does not require further ancillary studies.
Intramuscular myxoma may be confused with ML, but is typically less cellular, lacks the characteristic vascular network, and does not display signet ring cell lipoblasts.
Myxofibrosarcoma is a relatively common sarcoma, most commonly arising in the superficial soft tissues of the extremities in elderly patients. The tumors are highly infiltrative and show significant nuclear pleomorphism and cytologic atypia, background curvilinear blood vessels, and an absence of DDIT3 gene fusions.
Lipoblastoma, commonly seen in infants and children, may focally mimic ML but is usually easily distinguished if attention is paid to the age of the patient and the presence of extensive mature fat.
ML-like lesions arising in the retroperitoneum should be considered ALT/WDL or, if higher grade, DL until proven otherwise.
Local recurrence is commonly seen (up to 25% per some studies) as well as distal metastasis (up to 60%). Distant metastasis can be seen many years after the initial diagnosis. The most common location for the distant metastases is bone, followed by the lung. The presence of a round cell component (hypercellularity) >5% and/or tumor necrosis correlates with increased risk of metastases and poor survival. Mainstay of treatment is surgical excision, but the tumor is very radiosensitive, responding well to radiation therapy.
Myxoid liposarcoma is malignant neoplasm arising in the deep soft tissues of the extremities, composed of nodules of uniform, small oval to round, bland cells embedded in abundant myxoid matrix with variable amounts of univacuolated/bivacuolated lipoblasts admixed with a prominent and delicate branching vascular network.
Rare, deep soft tissues of the extremities, most commonly in the posterior thigh, rarely in subcutaneous locations and retroperitoneum
Peak age fourth to fifth decades
Large soft tissue mass involving the deep soft tissues of the proximal extremities
Local recurrences and distant metastases are commonly seen, especially if a round cell component is present
Surgical excision is the optimal treatment, but tumors also respond to radiation therapy
Large, well-circumscribed and multinodular mass with gelatinous/myxoid cut surface
Lobulated neoplasm with tendency toward increased cellularity at the periphery of the lobules, composed of remarkably uniform, round to ovoid cells
The background stroma is myxoid and associated with a network of delicate and arborizing blood vessels, accompanied by scattered uni- to multivacuolated lipoblasts
High-grade ML (round cell liposarcoma) is characterized by larger, round cells with irregular nuclear membranes, significant hypercellularity, prominent nucleoli, resulting in a diminished background myxoid matrix
Intramuscular myxoma, myxofibrosarcoma, and lipoblastoma
If retroperitoneal or intra-abdominal, atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma
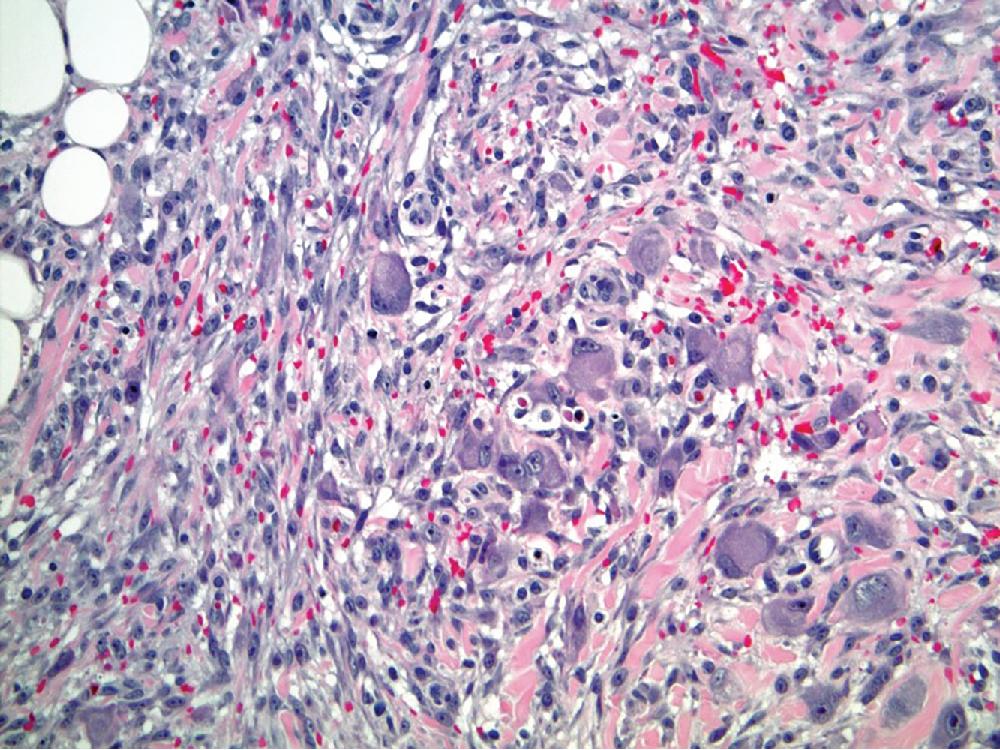
PLS presents as a rapidly growing, often painless mass, most commonly involving the deep soft tissues of the extremities, followed by the trunk, retroperitoneum, and spermatic cord. Up to 25% of cases arise in the subcutaneous tissues. By definition, there are no areas of concomitant ALT/WDL.
PLS are typically large neoplasms, non-encapsulated, often with ill-defined and infiltrative borders. Myxoid change and necrosis are commonly seen.
Histologically, PLS is a high-grade sarcoma, predominantly pleomorphic undifferentiated-appearing spindle to epithelioid cells with variable numbers of diagnostic multivacuolated lipoblasts ( Fig. 8.12 ). There is usually significantly increased mitotic activity with many atypical mitotic forms. Necrosis and myxoid stromal change, sometimes mimicking myxofibrosarcoma, are commonly seen. The lipoblasts are frequently quite large with multiple, variably sized intracytoplasmic vacuoles, usually indenting the large, central to para-centrally placed nucleus. Dominating epithelioid features, resembling a carcinoma, are seen in a minority of cases. The presence of at least focal lipoblasts is absolutely necessary for establishing the diagnosis.
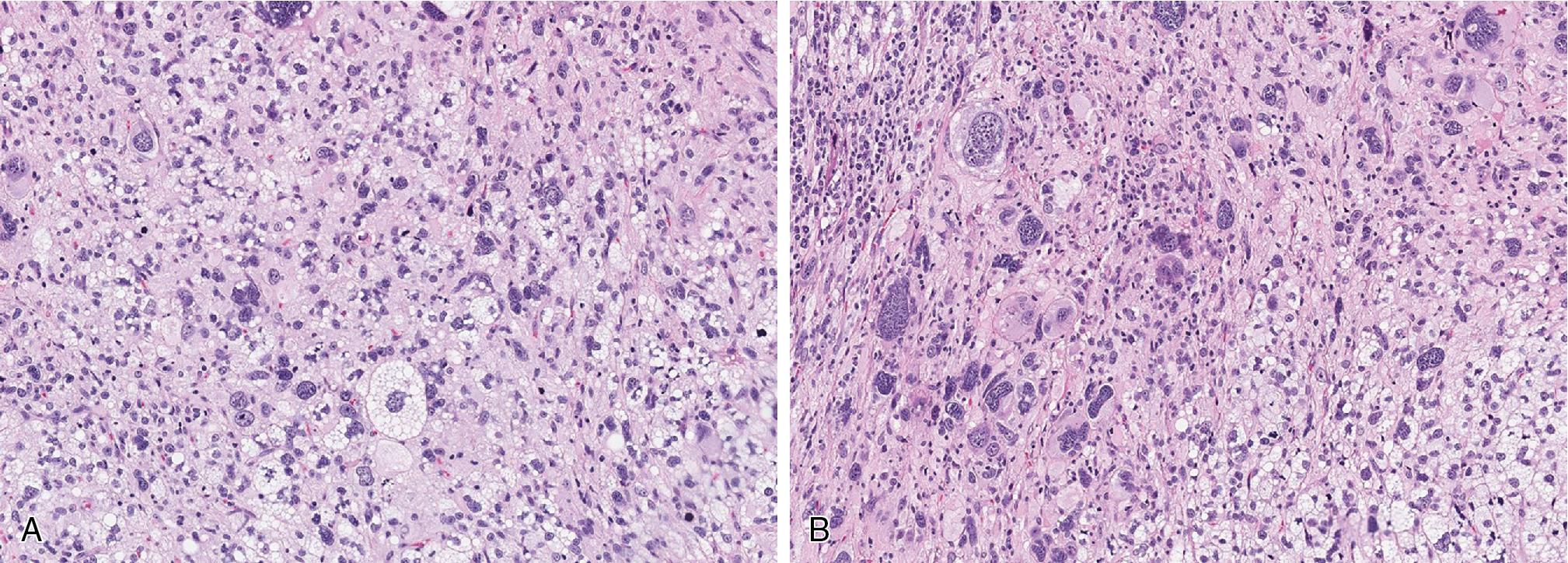
There are no immunohistochemical or molecular features pathognomonic for PLS. In the absence of an obvious ALT/WDL liposarcomatous component, evaluation for MDM2 gene amplification may be useful to fully exclude DL with a homologous PLS component, especially among intra-abdominal and retroperitoneal tumors.
DL should have a concomitant, often juxtaposed low-grade component, ALT/WDL, and MDM2 gene amplification. PLS with epithelioid features may express keratins, potentially confounding the diagnosis with “carcinoma.” The presence of lipoblasts helps establish the diagnosis of PLS.
UPS is a diagnosis of exclusion, by definition lacking lipoblastic differentiation.
PLS are aggressive sarcomas, commonly exhibiting local recurrence and metastatic events; overall 5-year survival rates approach 60%. Distant metastases are mostly seen in the lungs and pleura.
High grade pleomorphic, usually spindled to epithelioid sarcoma, with at least focal lipoblastic differentiation and an absence of an associated ALT/WDL
Rare sarcoma, deep soft tissues of the extremities, followed by the trunk/chest wall, retroperitoneum, and spermatic cord
Adults, peak incidence in seventh decade
Rapidly growing, commonly painless mass with symptoms related to anatomic location
Aggressive sarcoma with common local recurrences and distant metastases to lung and pleura
Surgical resection with negative margins is the therapy of choice
Large, well-demarcated to infiltrative, fleshy to white-yellow neoplasm, with necrosis
High-grade sarcoma, resembling undifferentiated pleomorphic sarcoma, composed at least focally of multi-vacuolated lipoblasts, and often necrosis
Dedifferentiated liposarcoma, undifferentiated pleomorphic sarcoma, rarely poorly differentiated carcinoma
Nodular fasciitis is a benign, rapidly growing, and often self-limiting, typically subcutaneous lesion in the upper extremities, trunk, and head and neck, almost never exceeding 5 cm. Most patients are between 20 and 40 years of age. Occasionally, it localizes to intramuscular areas, but intradermal lesions tend to be rare. Head and neck localization is often seen in children.
Well-circumscribed, usually subcutaneous white to slightly myxoid lesion, typically 1 to 2 cm.
Nodular fasciitis is a circumscribed, but non-encapsulated, neoplasm, composed of plump, uniform, and proliferating spindled cells, arranged loosely in short fascicles ( Fig. 8.13 ) and storiform patterns, creating a “tissue culture” appearance. The stroma is variably collagenous to myxoid with microcysts and frequent extravasated red blood cells and lymphocytes. The borders of nodular fasciitis can be infiltrative ( Fig. 8.13 C). Mitotic activity may be prominent; however, atypical mitotic figures are never seen.
Immunohistochemistry is generally not required for the diagnosis. The spindled cells display a myofibroblastic phenotype, virtually always diffuse smooth muscle actin positivity, variable to negative desmin expression, but negativity for S100, cytokeratin, EMA, and CD34. At the molecular level, most examples harbor a USP6 (17p13.2) gene rearrangement, most commonly t(17;22)(p13.2;q12.3) with the USP6-MYH9 gene fusion, supporting the concept of a neoplasm.
Various spindle cell sarcomas, typically dermatofibrosarcoma protuberans (DFSP) and low-grade fibromyxoid sarcoma (LGFS), represent the most important differential diagnoses for nodular fasciitis. Nevertheless, the characteristic superficial location and circumscription, loose tissue culture-like growth pattern, uniformity of the tumor cells without significant atypia, and the presence of SMA but absence of CD34 expression is diagnostic of nodular fasciitis. MUC-4 positivity, characteristic of LGFS, is not seen in nodular fasciitis, and diffuse CD34 expression is characteristic of DFSP.
Intravascular fasciitis has virtually identical histologic features to nodular fasciitis but, as the name implies, involves blood vessels, typically intravascular and muscular walled veins.
Cranial fasciitis refers to a rapidly growing, nodular fasciitis-like lesion, involving the skull outer table of infants within the first year of life. It is commonly associated with myxoid stroma and foci of osseous metaplasia.
Self-limiting and spontaneous resolution, even following incomplete excision, is typical, leading to the designation of “transient neoplasia.” Local recurrence (or persistence) is very rare, less than 1%.
Well-circumscribed, subcutaneous, <2 cm, cellular myofibroblastic lesion with a tissue culture-like growth pattern, microcysts, and often myxoid stroma, arising in the extremities of young adults
Subcutaneous, occasionally intramuscular, upper extremities, trunk, and, especially in children, head and neck
Young adults, but may occur at any age
Rapidly growing, superficial, commonly painless soft tissue swelling, <2 cm
Benign, often spontaneous resolution, local recurrences rare
Circumscribed to slightly infiltrative, fibrous to myxoid, <2 cm, subcutaneous
Unencapsulated but sharply demarcated, cellular myofibroblastic proliferation, uniform spindled cells, arranged in sheets and fascicles with a tissue culture-like growth pattern, microcysts, extravasated lymphocytes and red blood cells, and collagenous to myxoid stroma
Cranial fasciitis (newborns), intravascular fasciitis, low-grade fibromyxoid sarcoma, and dermatofibrosarcoma protuberans
Ischemic fasciitis, also known as atypical decubital fibroplasia, is a reactive pseudosarcomatous fibroblastic/myofibroblastic proliferation commonly arising in areas of constant pressure and/or injury, frequently associated with immobilization (sacral region, limb girdles, greater trochanter). Most cases are elderly patients, over 60 years of age, and it tends to be more common in males.
White fibrous to yellow ill-defined lesion with central necrosis and often cystic changes. May be associated with an overlying ulcer.
It is poorly circumscribed but there is a distinct zonal appearance. Central hypocellular areas are characterized by fibrinoid degeneration/necrosis and pseudocyst formation ( Fig. 8.14 ), transitioning to a granulation tissue-like vascular proliferation with large ganglion-like reactive fibroblasts ( Fig. 8.14 B), similar to that seen in proliferative fasciitis and myositis.
Sometimes SMA and less often desmin are observed in the lesional cells. S100 is negative.
Proliferative fasciitis and myositis, although associated with similar ganglion-like fibroblasts, lack the distinct zoning appearance and are found exclusively in subcutaneous fat and skeletal muscle, respectively. Proliferative fasciitis, typically seen in the upper extremities of middle-aged to older adults, forms poorly circumscribed lesions in subcutaneous tissue, resembling nodular fasciitis but associated with ganglion-like fibroblasts. Proliferative myositis shows virtually identical morphologic features in the same age group but more frequently involves the trunk and shoulder girdles and is typically larger, and the ganglion-like fibroblasts are more prominent ( Fig. 8.15 ). Neither are associated with overlying ulceration of the skin. FOS/FOSB gene rearrangements have been documented in proliferative fasciitis and myositis.
Local excision is usually curative but may persist if underlying cause is not addressed.
Reactive pseudosarcomatous fibroblastic/myofibroblastic proliferation, characterized by zonal fibrinoid necrosis/cystic changes, ganglion-like fibroblasts, and commonly found in areas of pressure, associated with immobilization
Tends to occur in the deep subcutis in areas subjected to trauma or constant pressure (sacral region, limb girdles, and greater trochanter)
Elderly, between seventh and ninth decades
Often bed-ridden, frequently but not always associated with debilitation, ulcerated, painless mass
Local excision is usually curative
May persist in disabled patients if underlying cause is not addressed
Ill-defined, deep-seated lesion, sometimes underlying an ulcer
Distinct zonal appearance with more central hypocellular areas characterized by fibrinoid degeneration, necrosis, and/or pseudocyst formation, transitioning to a granulation tissue-like proliferation with large ganglion-like reactive fibroblasts
Proliferative fasciitis and myositis
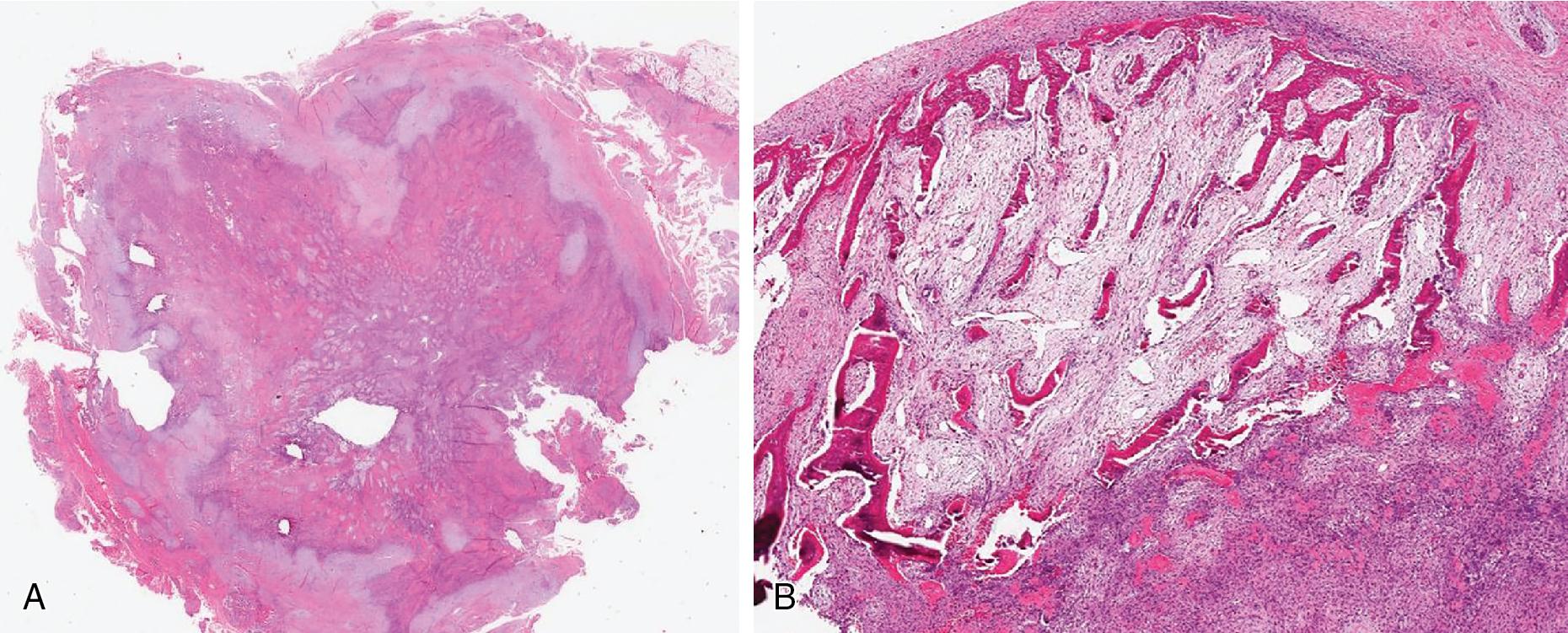
Myositis ossificans (MO) is a benign, rapidly growing but self-limited neoplasm, usually occurring in areas susceptible to trauma. It may develop anytime from early childhood and until late adulthood, but most arise in active young adults. On radiology, the early phases are characterized by well-demarcated, soft tissue fullness and edema. Focal (flocculent) mineralization in the periphery of the neoplasm begins after 2–6 weeks, evolving into an eggshell-like mineralized layer of bone with a radiolucent center. In the latest stages, MO becomes circumscribed, entirely mineralized, and hard.
Fibro-osseous pseudotumor of digits (FOPD), also known in the literature as florid reactive periostitis, is related to MO but represents a benign neoplasm specifically of the subcutaneous tissues of the phalanges and metatarsals/metacarpals of the fingers and, less frequently, the toes. Formation of a peripheral eggshell-like bone does not generally occur. Radiologically, a characteristic fusiform swelling is associated with juxtacortical calcifications and a periosteal reaction. The mineralization tends to be randomly distributed.
MO and FOPD are usually well-circumscribed, round to ovoid, hard masses with variable amounts of bone, depending on the age of the neoplasm. The center of MO is usually soft, consistent with a zonal growth pattern.
A typical zonal appearance is characteristic of in MO ( Fig. 8.16 ). The periphery is composed of at least a partial rim of osteoid to woven bone trabeculae, rimmed by osteoblasts, merging centrally with a densely cellular but uniform spindled to ganglion-like cell proliferation, often devoid of osteoid/bone, closely resembling nodular/proliferative fasciitis. These latter cells often are haphazardly arranged, have eosinophilic cytoplasm, plump to ovoid nuclei with open chromatin, and may be quite mitotically active. The background stroma is variably fibrous to edematous with increased vascularity. Extravasated erythrocytes, lymphocytes, and osteoclast-type giant cells are frequent. Rarely, MO undergoes central cystic degeneration, resembling an aneurysmal bone cyst. In FOPD, the bone does not usually form a peripheral outer layer, but instead, the bone and fibrous tissue are admixed and randomly distributed ( Fig. 8.17 ).
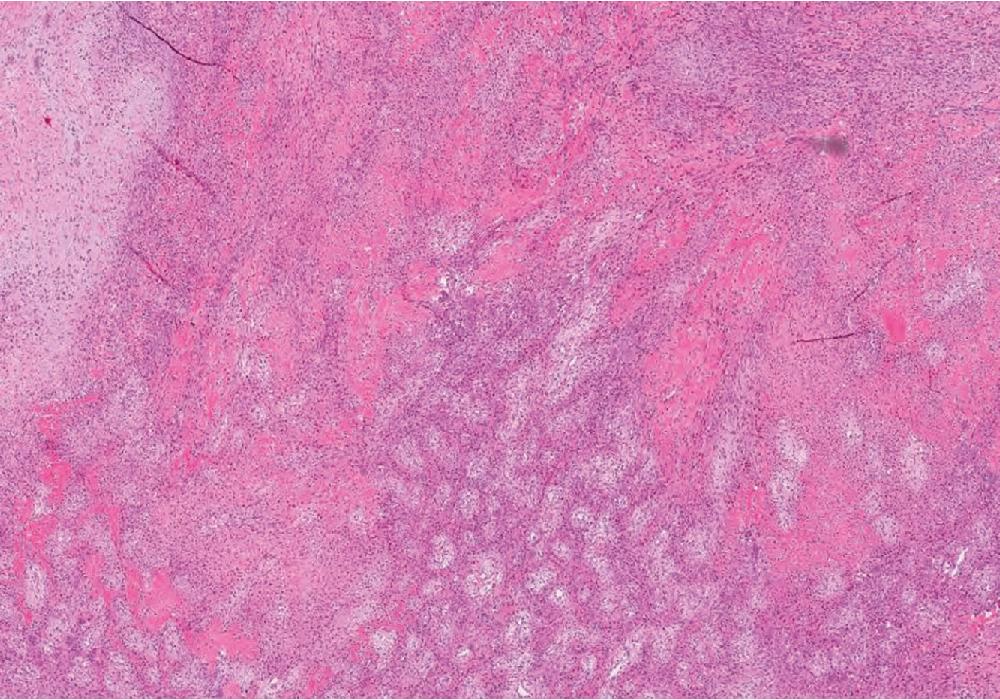
Immunohistochemically, the spindled cells often express SMA but are generally negative for desmin and CD34. As in nodular fasciitis, USP6 gene (17p13) arrangements are characteristic, but most commonly associated with the COL1A1 (17q21) gene.
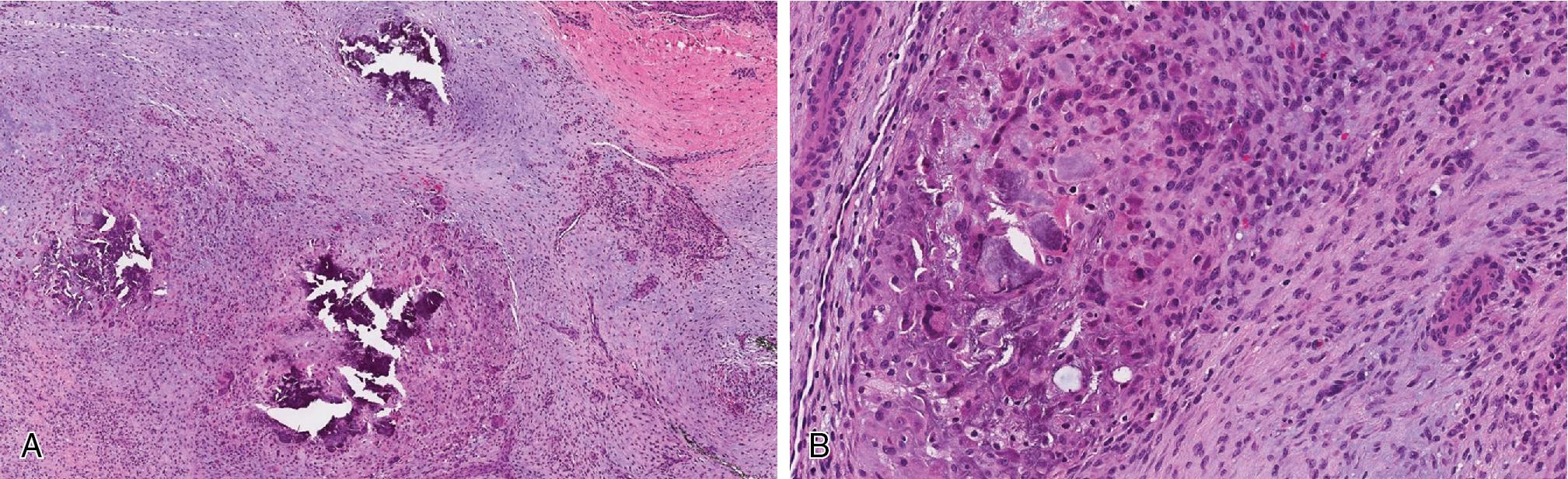
Calcifying aponeurotic fibroma (CAF) may be mistaken for FOPD. CAF is a rare benign neoplasm, most commonly seen in children, between 5 and 15 years of age, arising in association with fibro-tendinous tissues on the distal extremities, palmar, and plantar surfaces. There is no involvement of underlying bone or periosteal reaction. It has high risk of local recurrence (50%) if incompletely excised. The tumors are variably cellular with infiltrative fibromatosis-like spindled cell areas admixed with less cellular zones, characterized by plump epithelioid fibroblasts admixed with zones of calcification, sometimes appearing hyalinized to chondroid ( Fig. 8.18 ). CAF is not associated with USP6 gene rearrangements.
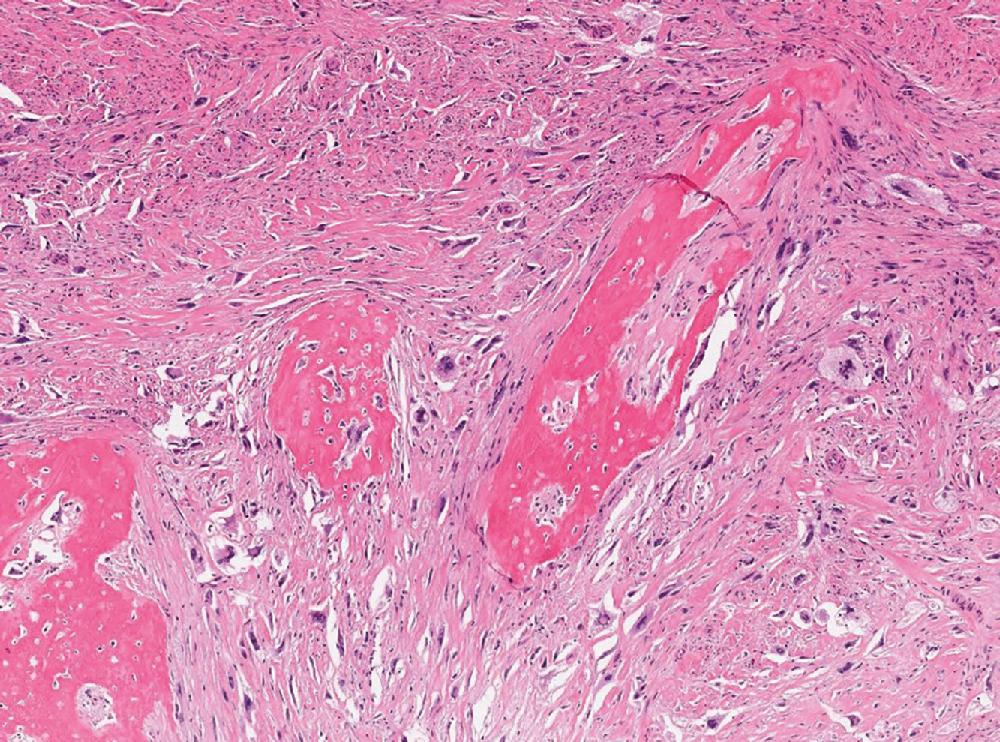
Extraskeletal osteosarcomas form deeply seated masses, arising in older adults, lacking the characteristic zonal pattern of MO. Microscopically, they appear obviously malignant, with significant cytologic atypia and pleomorphism along with other signs of malignancy (increased mitotic activity, necrosis, infiltration) ( Fig. 8.19 ). There is no characteristic gene fusion associated with extraskeletal osteosarcoma.
Although clinical follow-up without further therapy is a reasonable decision, the preferred treatment for MO and FOPD is simple excision, and both entities have an excellent prognosis. Local recurrence rarely occurs in FOPD.
MO is a rapidly growing and self-limited neoplasm, sometimes associated with trauma, characterized by a zonation, with a more mature, ossified periphery and a less mature, often non-ossified center
FOPD is a benign neoplasm in the subcutaneous tissues/periosteum, arising as a fusiform lesion of the small tubular bones of the digits, characterized as a non-zonal fibro-osseous process
Extremities, typically proximal (MO) and acral (FOPD)
Wide age distribution, but most commonly adolescents and young adults
Well circumscribed, rapidly growing
Zonation may be radiographically evident with MO, while a periosteal reaction and fusiform mass swelling are classical for FOPD
Excellent prognosis, simple excision generally curative
Well circumscribed, firm masses
MO, well circumscribed peripheral osteoid/bone shell, transitioning to less mature, hypercellular nodular/proliferative fasciitis-like centrally
FOPD, well-demarcated fusiform swelling/periosteal reaction from small tubular bones of the digits, associated with a benign-appearing fibro-osseous proliferation, without zonation
CAF, especially for FOPD, and extraskeletal osteosarcoma
Elastofibroma presents as an ill-defined, slowly growing, and painless solid tissue proliferation, which on MRI has a signal intensity similar to skeletal muscle with interspersed adipose tissue, commonly seen in the deep connective tissues between the lower scapula and the thoracic wall. Females older than 50 years of age are more commonly afflicted than males. Extrascapular localization has been described but is considered exceedingly rare.
Poorly defined rubbery fibrous tissue with interspersed fat is classically observed.
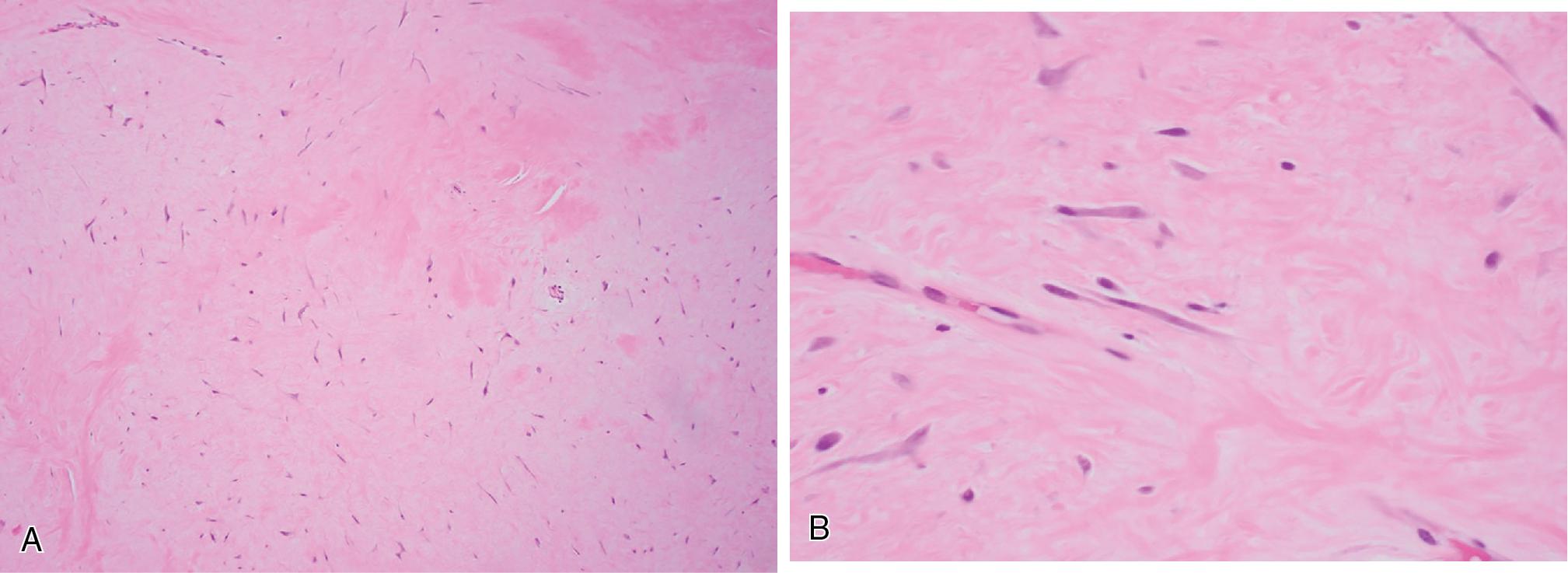
Elastofibromas form bland, ill-defined masses composed of mixtures of adipose and fibromyxoid tissue, with the latter containing haphazardly arranged abnormal and dystrophic elastic fibers ( Fig. 8.20 ). The background fibroblasts lack cytologic atypia, and mitotic activity is essentially non-existent.
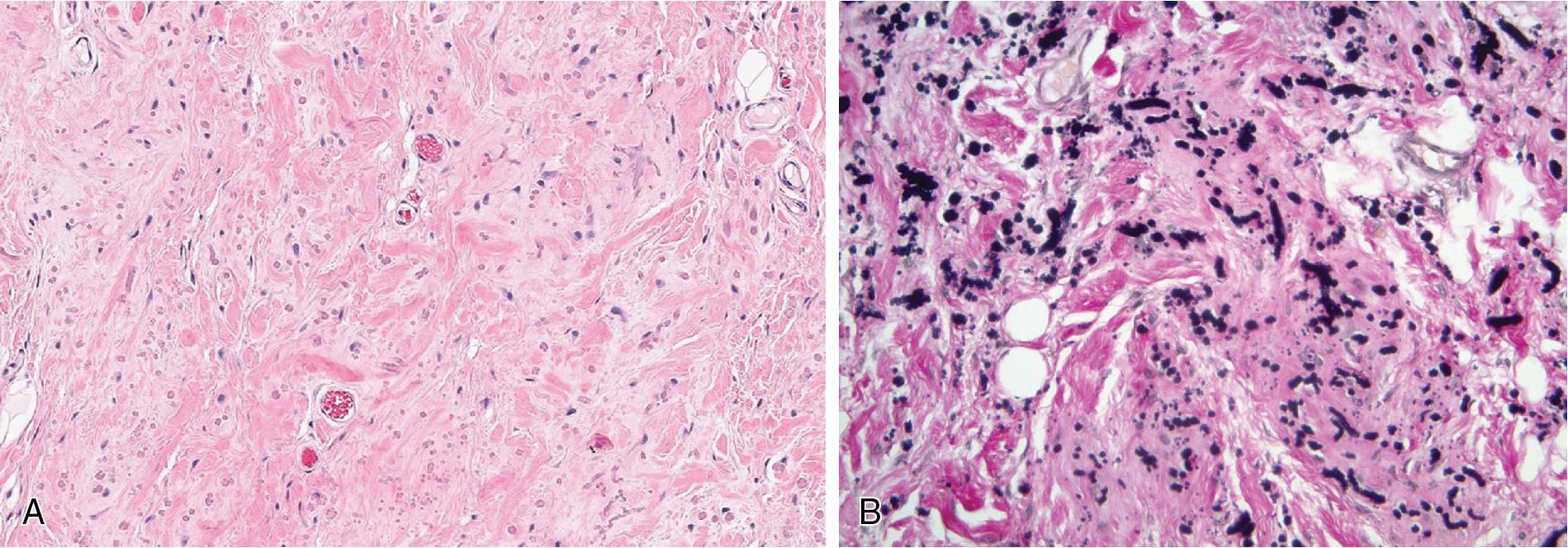
Elastin stains highlight the abnormal elastic fibers, described as coarse, deeply eosinophilic, and fragmented into linear beads of serrated discs. There is no characteristic gene fusion diagnostic of elastofibroma.
The most important differential diagnosis is desmoid-type fibromatosis, which is significantly more cellular and composed of sweeping fascicles of uniform spindled cells infiltrating fat and skeletal muscle.
Elastofibroma is an entirely benign lesion. Local recurrence is exceptionally rare after simple excision.
Benign, slowly growing fibroadipose tissue lesion, associated with abundant but abnormal elastic fibers, involving the connective tissues between the inferior scapula and thoracic wall of older females
Deep connective tissues between the scapula and thoracic wall
Elderly (almost all older than 50 years)
Benign, poorly defined and painless soft tissue mass, localized between the lower scapular and chest wall
Benign, local excision is curative
Fibrous hamartoma of infancy (FHI) typically presents as a small, superficial and mobile, painless mass, arising in the anterior and posterior axillary folds of infants and young children (<2 years old). Other common locations include the upper arm, trunk, inguinal region, and external genitalia.
Most are ill-defined with variably amounts of fibrous and adipose tissue.
FHI is characterized by a typical triphasic morphology with variable proportions of three distinct components: bland fibroblastic/myofibroblastic fascicles, primitive myxoid mesenchymal cells, and mature adipose tissue ( Fig. 8.21 ). The bland fibroblastic/myofibroblastic cell proliferation is composed of monomorphic spindled cells with dense eosinophilic collagenous background stroma. Hyalinization also may occur. The primitive mesenchymal component consists of primitive, rounded to stellate cells within myxoid stroma ( Fig. 8.21 B). Mitotic figures are rare.
SMA is variably positive in the fibroblastic areas and occasionally in the primitive mesenchymal zones, which also are positive for CD34. S100 is absent.
Lipofibromatosis also is composed of mixtures of fat and cellular fascicles of fibromatosis-like tissue, arising in subcutaneous tissues of children ( Fig. 8.22 ). However, it has a predilection for the hands and feet and lacks the classic triphasic organoid appearance of FHI.
Simple excision with potential for local recurrence if incompletely excised is usual for FHI.
Triphasic, hamartoma-like neoplasm composed of variably amounts of admixed bland fibroblastic/myofibroblastic fascicles, immature mesenchymal zones, and mature adipose tissue, arising in the superficial soft tissues of the trunk and extremities of infants and young children
Rare tumor, most commonly seen in the anterior/posterior axillary folds, trunk, extremities, and external genitalia
Infants and young children (<2 years of age)
Painless, mobile superficial nodule
Local recurrence may be seen in up to 15% of patients, but simple excision remains treatment of choice
Ill-defined rubbery fibrous tissue
Triphasic morphology of varying admixed amounts of bland fibroblastic/myofibroblastic fascicles, mature adipose tissue, and immature mesenchymal nests with lightly myxoid stroma
Lipofibromatosis
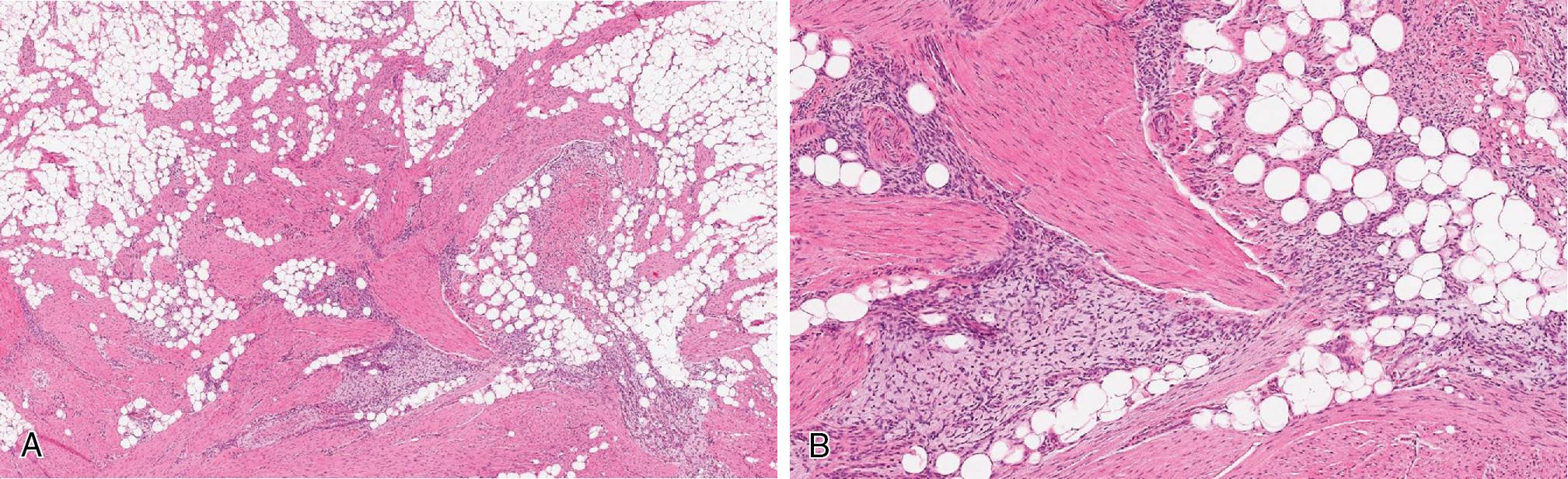
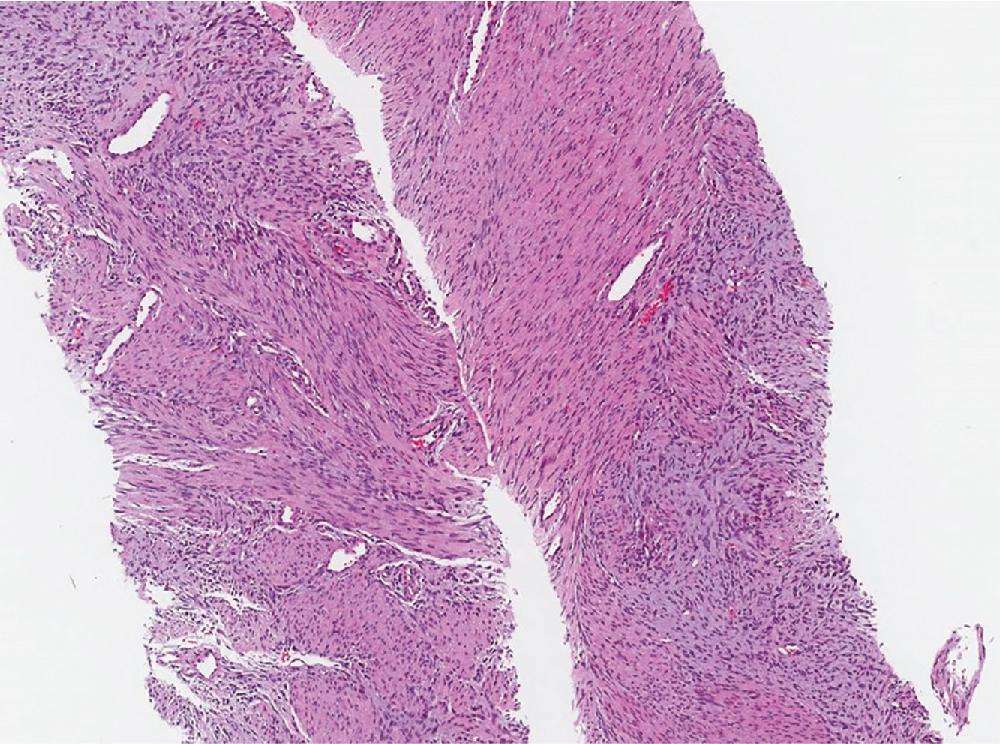
Desmoid fibromatosis presents as a deeply seated, painless but non-mobile mass typically arising in the extremities, trunk, retroperitoneum or abdominal cavity and, less commonly, in the head and neck, paraspinal and flank regions, of young to middle aged adults, especially females. It may be associated with prior trauma and/or surgery. A minority of patients with familial adenomatous polyposis (Gardner syndrome) develop intra-abdominal fibromatosis, usually multifocal, involving children and young adults.
Superficial fibromatoses represent a spectrum of similar nodular fibroblastic/myofibroblastic proliferations involving the hands (palmar fibromatosis, Dupuytren contracture.) and feet (plantar fibromatosis, Ledderhose disease). Palmar disease forms painless nodules and cords, causing contractures in the palms and fingers of older adults, usually males. The plantar form is more often seen in young to middle-aged adults, typically females. It is associated with painless swelling and no contractures.
Desmoid fibromatosis forms solid, usually large and infiltrative masses, with ill-defined margins and a fibrous, white to whorled cut surface. Superficial fibromatoses are small, rubbery fibrous nodules, intimately associated with fibro-tendinous tissues on the hands and feet.
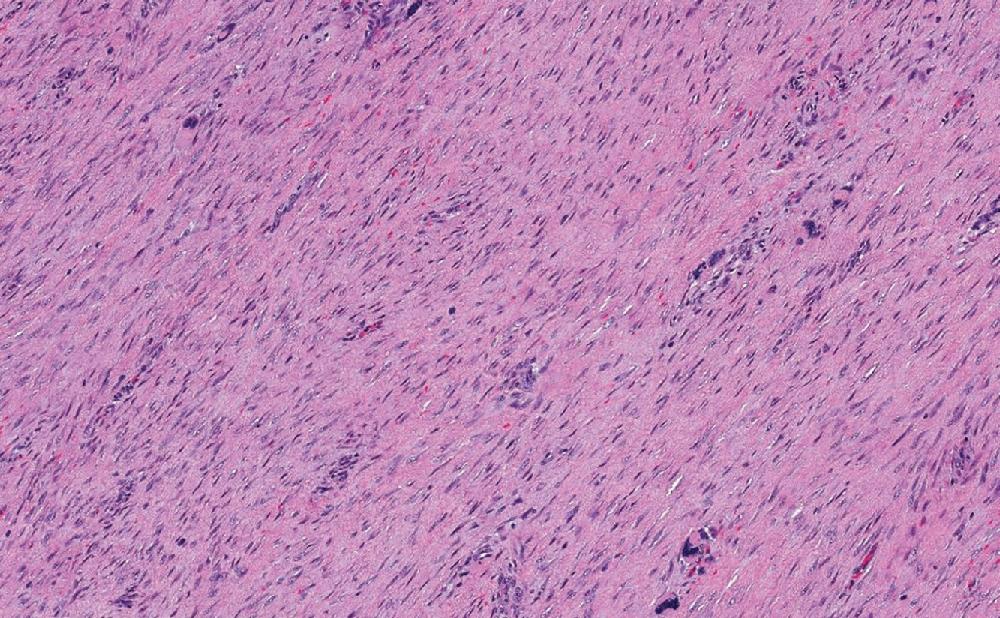
Both desmoid and superficial fibromatosis have overlapping morphologic features, characterized by long sweeping fascicles of elongated and uniform spindled cells. Cytologically, the cells have eosinophilic cytoplasm and elongated nuclei with open chromatin, without significant pleomorphism. The cellular proliferation in superficial fibromatoses, particularly the palmar form, often are confined to the fibro-tendinous and fascial tissues. Desmoid fibromatosis exhibits widespread infiltration, entrapping fat and skeletal muscle ( Fig. 8.23 A). At the periphery of the lesion, lymphoid aggregates often are seen ( Fig. 8.23 B). The background is commonly uniformly collagenous but rarely may exhibit keloid-like areas and myxoid changes, more common in intra-abdominal and mesenteric forms. The scattered background blood vessels have thin walls with characteristic perivascular edema ( Fig. 8.23 C). Mitotic figures tend to be uncommon, and tumor necrosis is not present.
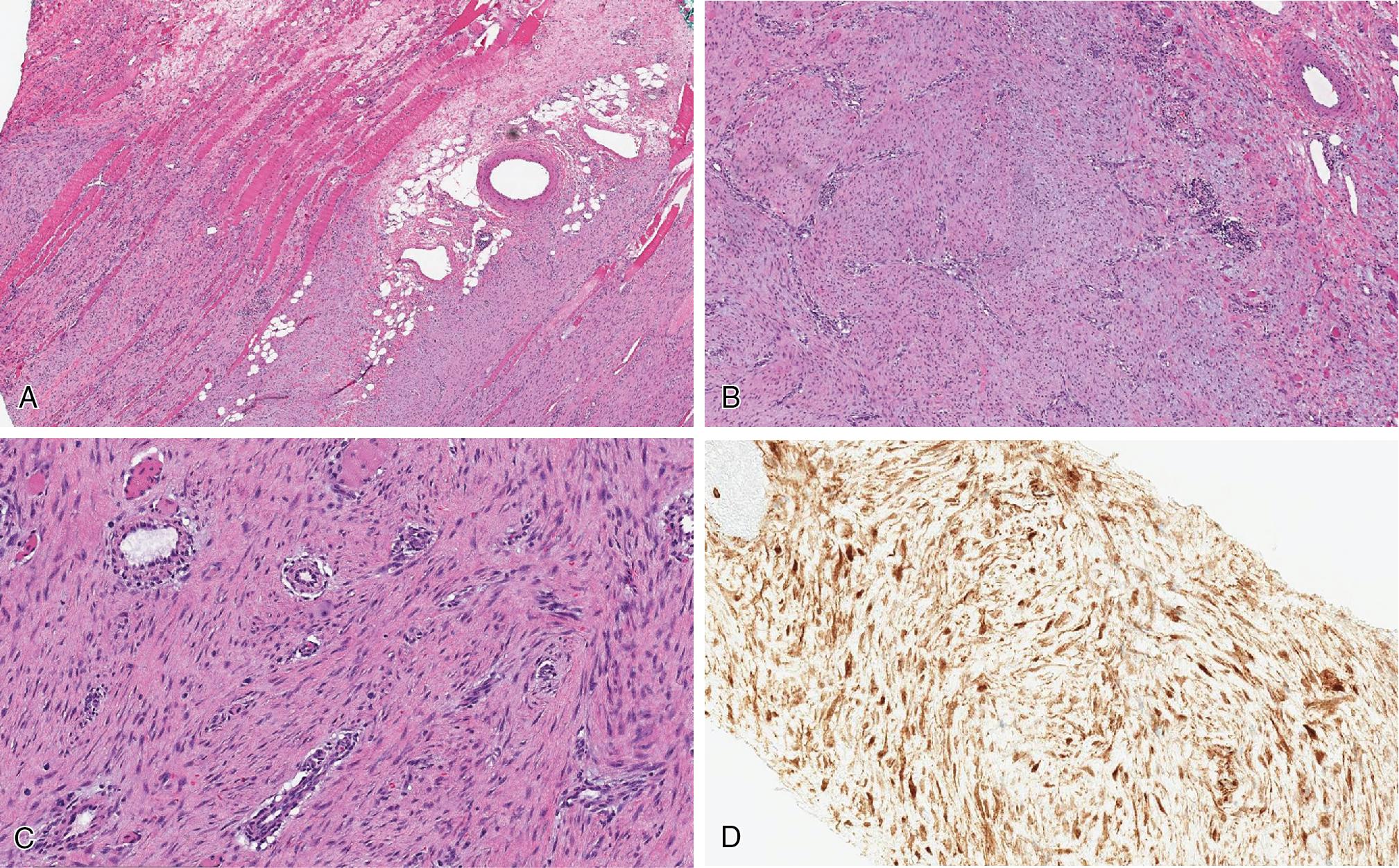
Immunohistochemical stains are not necessary for diagnosis. SMA is usually expressed by the lesional cells, but desmin positivity is variable. CD117 expression represents a potential diagnostic pitfall in intra-abdominal lesions, but cytokeratin, CD34, DOG1, and S100 are negative. Nuclear β-catenin positivity ( Fig. 8.23 D) is considered characteristic, but, in our experience, interpretation can be challenging. Somatic CTNNB1 mutations (β-catenin gene) and, in patients with Gardner syndrome, germline APC gene mutation are seen.
Superficial fibromatoses, despite morphologic overlap, have characteristic and distinct locations and presentations. Both plantar and palmar forms are commonly negative for β-catenin ( Fig. 8.24 ).
When involving viscera, sarcomatoid carcinoma should be considered; however, it is keratin positive and typically exhibits more significant cytologic atypia and pleomorphism.
DL may have fibromatosis-like areas, but the presence of a low-grade lipomatous component and, when necessary, confirmation of MDM2 gene amplification, helps to confirm this diagnosis.
The prognosis is variable, depending on the location, size, involvement of viscera, and resectability. Desmoid fibromatosis is an aggressive tumor, so wide surgical resection with negative margins is required. However, up to one third of the patients develop local recurrences, but distant metastases do not occur. The most important prognostic factor is adequacy of surgical excision. The goal of achieving negative margins should be balanced with a desire to preserve function.
Locally aggressive, recurring, and non-metastasizing deep-seated neoplasm, composed of long sweeping infiltrative fascicles of monotonous spindled cells within dense fibrous stroma, thin-walled blood vessels with perivascular edema, and peripheral lymphoid aggregates
Rare, deep soft tissues of the extremities, abdomen, retroperitoneum, and trunk
Young to middle-aged adults, usually female
Deep-seated and large mass, densely adherent/fixed to the surrounding tissues
Prior history of trauma and/or surgery is common
Aggressive and commonly locally recurs
Wide surgical excision with negative margins is the preferred approach
Solid, infiltrative mass, with white fibrous to whorled cut surface
Long sweeping fascicles composed of monomorphic spindled cells, with eosinophilic cytoplasm and elongated nuclei, infiltrating the surrounding adipose tissue and skeletal muscle
Peripheral lymphoid aggregates and perivascular edema
Collagenous to slightly myxoid stroma
Superficial (palmar or plantar) fibromatosis, sarcomatoid carcinoma, and dedifferentiated liposarcoma
Fibroma of tendon sheath presents as a slowly growing nodule, usually <3 cm and associated with tendon sheaths of fingers, involving patients, between the ages of 20 and 50 years.
Rounded, firm to lobular nodule, associated with tendons.
Well circumscribed and nodular, bland spindle cell proliferation ( Fig. 8.25 ), arranged in short fascicles within dense collagenous stroma, accompanied by thin-walled blood vessels with slit-like appearance ( Fig. 8.25 B). Cytologically the cells do not exhibit significant cytologic atypia and pleomorphism.
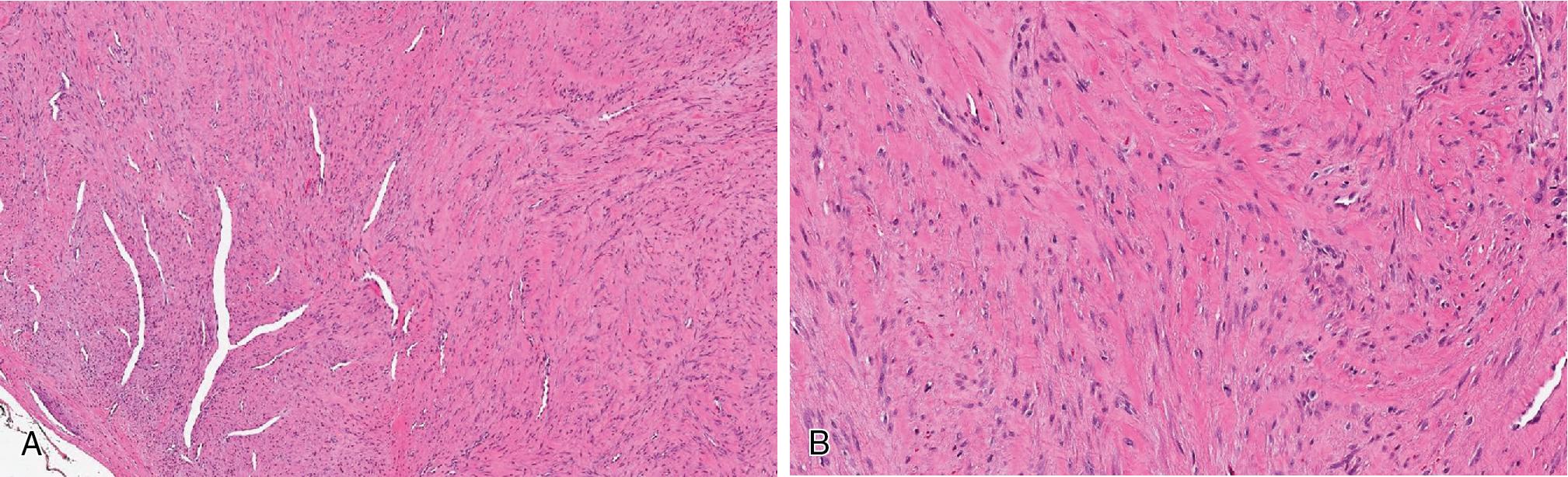
Immunohistochemically, most express SMA but are negative for S100 and desmin. A subset of more cellular examples, possibly representing a spectrum with nodular fasciitis, display USP6 gene rearrangements.
Nodular fasciitis is typically significantly more cellular, displaying a loose tissue culture appearance, variably collagenous stroma, extravasated red blood cells and lymphocytes, and predominantly occurs in the subcutis. More cellular forms of fibroma of tendon sheath, especially those with USP6 gene rearrangements, likely represent rare examples of tenosynovial nodular fasciitis.
Tenosynovial giant cell tumor (TGCT; localized type) may exhibit a virtually identical clinical presentation. However, TGCTs are not spindled cell lesions, being instead composed of mostly uniform epithelioid to histiocytoid mononuclear cells, scattered multinucleated osteoclast-type giant cells, and hemosiderin pigment.
Fibroma of tendon sheath is a benign tumor but may locally recur in up to 25% of cases. Surgical resection should be undertaken with the aim to preserve function.
Benign well circumscribed and lobular, usually hypocellular spindle cell proliferation associated with dense collagen and arising in tendons, especially fingers
Rare, associated with tendons of hands and, less commonly, feet
Young to middle-aged adults
Painless soft tissue mass, associated with finger tendons
Benign lesion, potential for local recurrences, with surgical excision
Lobulated firm and circumscribed nodule
Well-demarcated, lobulated lesion with spindled cells arranged in short fascicles within a densely collagenous background, containing slit-like blood vessels
Nodular fasciitis and tenosynovial giant cell tumor (localized type)
Myofibroblastoma is a benign neoplasm, originally described as arising in the mammary regions, within the superficial soft tissues of mostly males. However, mammary-type myofibroblastomas appear more common in extramammary sites, primarily the inguinal and groin areas, including the vulva/vaginal region and scrotum. Less common anatomic sites are extremities, viscera, and retroperitoneum. Most present as painless masses of older patients, although the age range is wide.
Myofibroblastoma forms well-circumscribed masses, white whorled to yellowish, with variable amounts of fat component.
Myofibroblastoma is a well-circumscribed but unencapsulated spindled cell neoplasm composed of short and stubby spindled cells with indistinct cell borders and eosinophilic cytoplasm. The cells are usually arranged in short fascicles and sheets ( Fig. 8.26 ). Their nuclei are oval with even chromatin and occasional pinpoint nucleoli. The background stroma is myxoid with scattered ropy to thick collagenous bundles and randomly distributed mast cells.
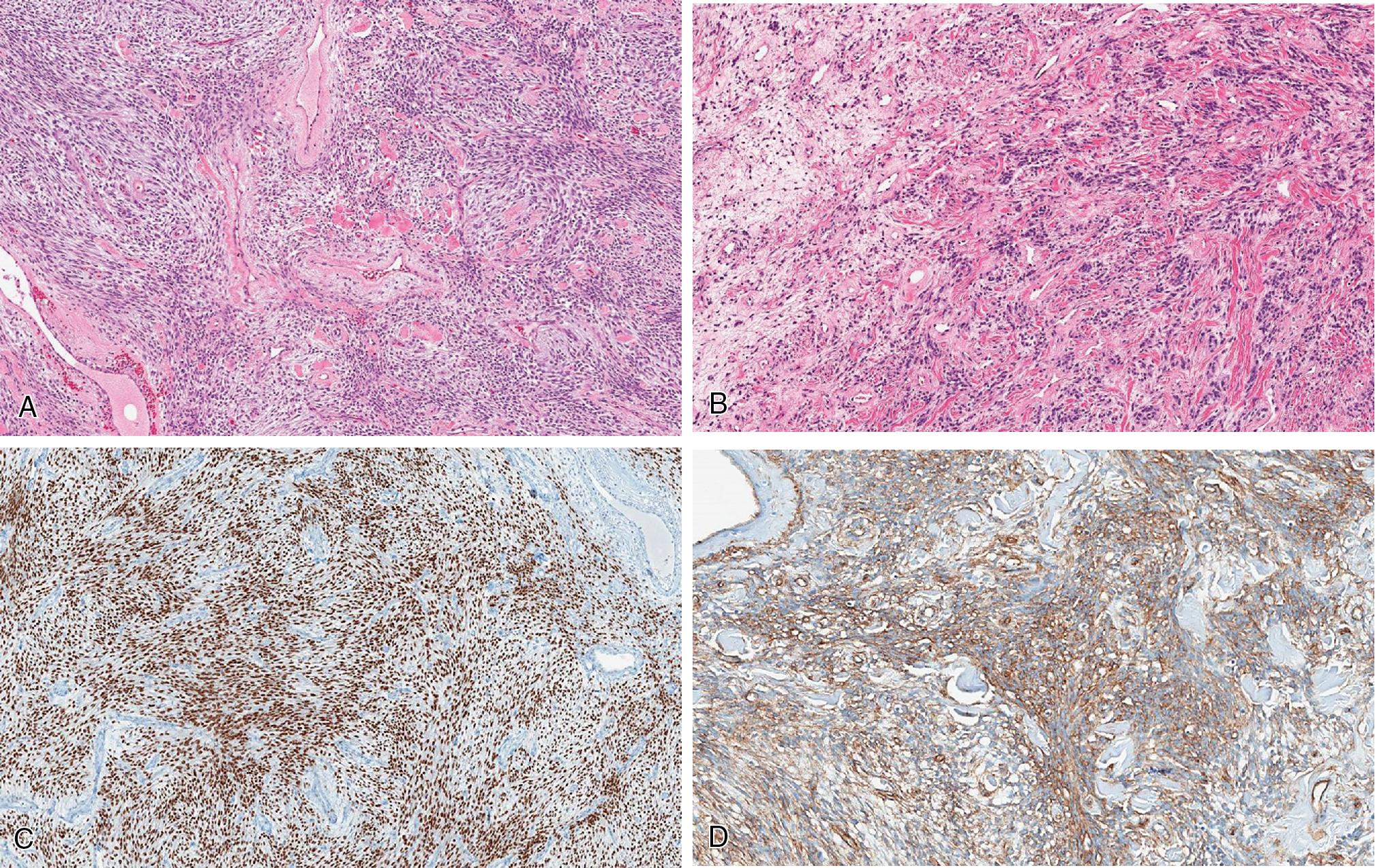
Immunohistochemically, the spindled cells are diffusely and strongly positive for ER ( Fig. 8.26 C), CD34 ( Fig. 8.26 D), and desmin, but usually negative for SMA. Loss of RB1 also is present. At the molecular level, myofibroblastomas have loss of 13q14 ( RB1 gene), a shared genetic abnormality with spindle cell/pleomorphic lipoma and cellular angiofibroma.
Spindle cell lipomas typically arise in the neck, shoulder, and upper back subcutis of elderly adults. Desmin always is negative.
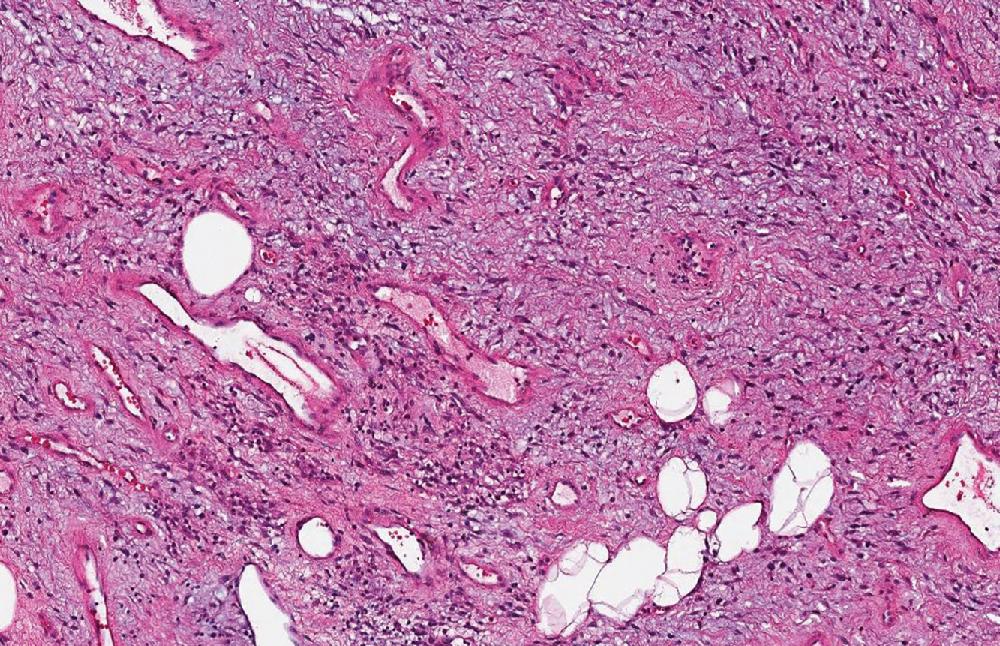
Cellular angiofibroma is a benign, hypercellular, and vascular neoplasm, located commonly in the superficial soft tissues of the scrotal and vulvar region, characterized by circumscription and uniform, short, spindled cells within an edematous stroma with admixed collagen bundles and medium-sized blood vessels with irregular branching lumina ( Fig. 8.27 ). Variable expression of SMA, desmin, as well as ER and PR, are described. CD34 may be positive in up to 30% of cases, but nuclear RB1 is lost.
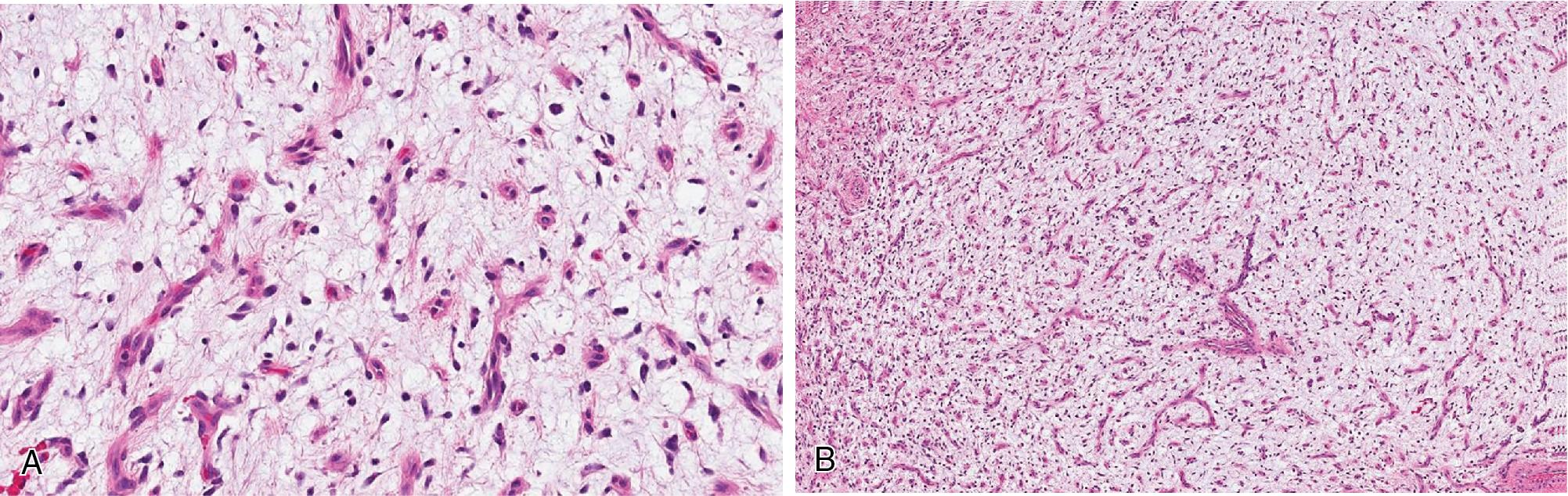
Angiofibroma of soft tissue also is a benign, slowly growing, and painless neoplasm, most commonly arising in the extremities (legs) of middle-aged males. Histologically, they form well-demarcated lesions composed of uniform spindled cells, abundant myxoid to fibromyxoid stroma, and a prominent network of innumerable branching, thin-walled blood vessels ( Fig. 8.28 ). These tumors have near-diploid karyotypes with a recurrent t(5;8) (p15;q13) and AHRR-NCOA2 gene fusion.
Myofibroblastomas are benign neoplasms with exceptionally rare recurrence potential, even after marginal excision.
Benign soft tissue neoplasm occurring in superficial tissues, less often deep, characterized by short stubby spindled cells, arranged in haphazard fascicles within myxoid stroma with admixed thick collagenous fibers and mast cells
Lesional cells show diffuse and strong expression of CD34, desmin, and ER, with loss of nuclear RB1
Rare, inguinal and groin
Wide age range, peak in middle-aged to older adults
Painless soft tissue nodule
Benign neoplasm, exceptionally rare potential for local recurrences, even after marginal excision
Well-delineated, nodular firm lesion with variable yellowish fat component
Well-circumscribed, non-encapsulated spindled cell neoplasm composed of short and stubby spindled cells, arranged in fascicles, within a lightly myxoid stroma with admixed scattered thick collagenous fibers and mast cells
Spindle cell lipoma, cellular angiofibroma, and angiofibroma of soft tissue
Angiomyofibroblastomas present as well-circumscribed and painless masses, arising in the subcutis of pelvic region, most commonly seen in the vulva and vagina of middle-aged females, typically premenopausal. A subset occurs in males, in the paratesticular region and scrotum.
Well-delineated, non-encapsulated soft tissue mass, red-tan and soft, are usual gross features.
Histologically, angiomyofibroblastomas are well-circumscribed lesions, composed of spindled to epithelioid cells, often concentrated around blood vessels, within an edematous to lightly myxoid stroma with prominent vascularity ( Fig. 8.29 ). A minority display an adipocytic component. The epithelioid cells are frequently multi-nucleated and plasmacytoid. The background can be loose and edematous with scattered small vessels that can have hyalinized walls.
Immunohistochemically, strong and diffuse desmin and ER co-expression is typical, often accompanied by CD34 positivity. SMA may be focally positive.
Deep angiomyxoma is the most important differential diagnosis. Although benign, deep angiomyxomas are “aggressive” infiltrative neoplasms, arising within the deep soft tissues of the pelvis/perineum of young to middle-aged females. Histologically they are relatively hypocellular spindled cell neoplasms with abundant edematous to myxoid stroma, accompanied by medium to large muscular-walled blood vessels ( Fig. 8.30 ). The cells are most often randomly distributed, uniform, and may be associated with entrapment of fat. Diffuse co-expression of desmin and ER is typical, with positivity for CD34 and SMA less consistent. HMGA2 (12q14) gene rearrangements have been confirmed by FISH analysis.
Marginal excision is rarely associated with local recurrence.
Uncommon, benign and circumscribed neoplasm arising from the subcutis of the pelvic/perineal region, composed of spindled to epithelioid cells, often aggregating around blood vessels, within an edematous to lightly myxoid stroma
Pelvic/inguinal region, most commonly vulvar/vaginal
Females of reproductive age, rarely postmenopausal females and males
Pelvic/perineal subcutaneous nodule, circumscribed and painless
Benign neoplasm, rare local recurrences following excision
Circumscribed, soft to slightly gelatinous
Well-circumscribed edematous to lightly myxoid hypervascular neoplasms, associated with spindled to epithelioid cells, often concentrated around vessels, sometimes with hyalinized walls
An adipocytic component is seen in a minority of cases
Deep (aggressive) angiomyxoma
Nuchal-type fibroma is a benign, ill-defined neoplasm, usually occurring in the subcutaneous tissues of the posterior neck of middle aged to older males, often with diabetes mellitus. Extra-nuchal sites include the upper back and extremities.
Firm white, ill-defined nodule, between 2 and 5 cm is usually observed.
Nuchal-type fibromas are paucicellular and poorly circumscribed lesions, composed of monomorphic spindled cells, within a densely collagenous background stroma, entrapping fat and peripheral nerve bundles ( Fig. 8.31 ).
The spindled fibroblasts are positive for CD34 negative, variably positive for SMA, but negative for desmin and β-catenin.
Gardner fibroma, histologically indistinguishable from nuchal type fibroma, but is associated with familial adenomatous polyposis and germline APC mutations. They are most commonly seen in children < 10 years of age. The spindled cells are positive for CD34 but typically negative for SMA.
Desmoplastic fibroblastoma (collagenous fibroma) is a relatively circumscribed, benign, hypocellular neoplasm with an abundantly collagenous to lightly myxoid stroma, composed of bland stellate to spindled fibroblasts ( Fig. 8.32 ). Most arise in the subcutaneous tissues of the extremities of adults. SMA is variably expressed, but S100, EMA, and CD34 are usually negative. Simple excision is curative.
Nuchal-type fibroma is a benign tumor with potential for local recurrence.
Benign and ill-defined, hypocellular collagenous lesion, entrapping fat, arising in the subcutis of the posterior neck of middle-aged to older males
Rare, posterior neck most common, followed by upper back
Middle-aged males
Firm subcutaneous but painless nodule, often in patients with diabetes mellitus
Benign neoplasm with potential for local recurrence
Poorly circumscribed, firm white soft tissue nodule
Paucicellular, poorly defined soft tissue neoplasm composed of bland spindled cells and abundant and thick collagenous fibers, entrapping adipose tissue
Gardner fibroma and desmoplastic fibroblastoma
DFSP is a locally aggressive and rarely metastasizing neoplasm, arising as a solitary to multinodular, painless, and superficial mass of the trunk and proximal extremities/limb girdle, in young to middle-aged adults. It is rarely seen involving the head and neck, acral distal extremities, or genital area.
DFSP displays a nodular to indurated plaque-like appearance with overlying skin, with ill-defined margins and extensive involvement of the subcutis.
DFSP is characteristically hypercellular and diffusely infiltrates the dermis and subcutis ( Fig. 8.33 ), often associated with trails of adipocytes even up into the superficial dermis. The spindled neoplastic cells are remarkably monotonous, arranged in tight storiform patterns and short fascicles. Infiltration within the subcutaneous tissue often creates a characteristic honeycomb-like appearance ( Fig. 8.33 C). Mitotic activity tends to be low, not exceeding 5 mitoses/10 HPF. Admixed inflammatory cells are not a feature of DFPS. Myxoid stromal changes rarely occurs.
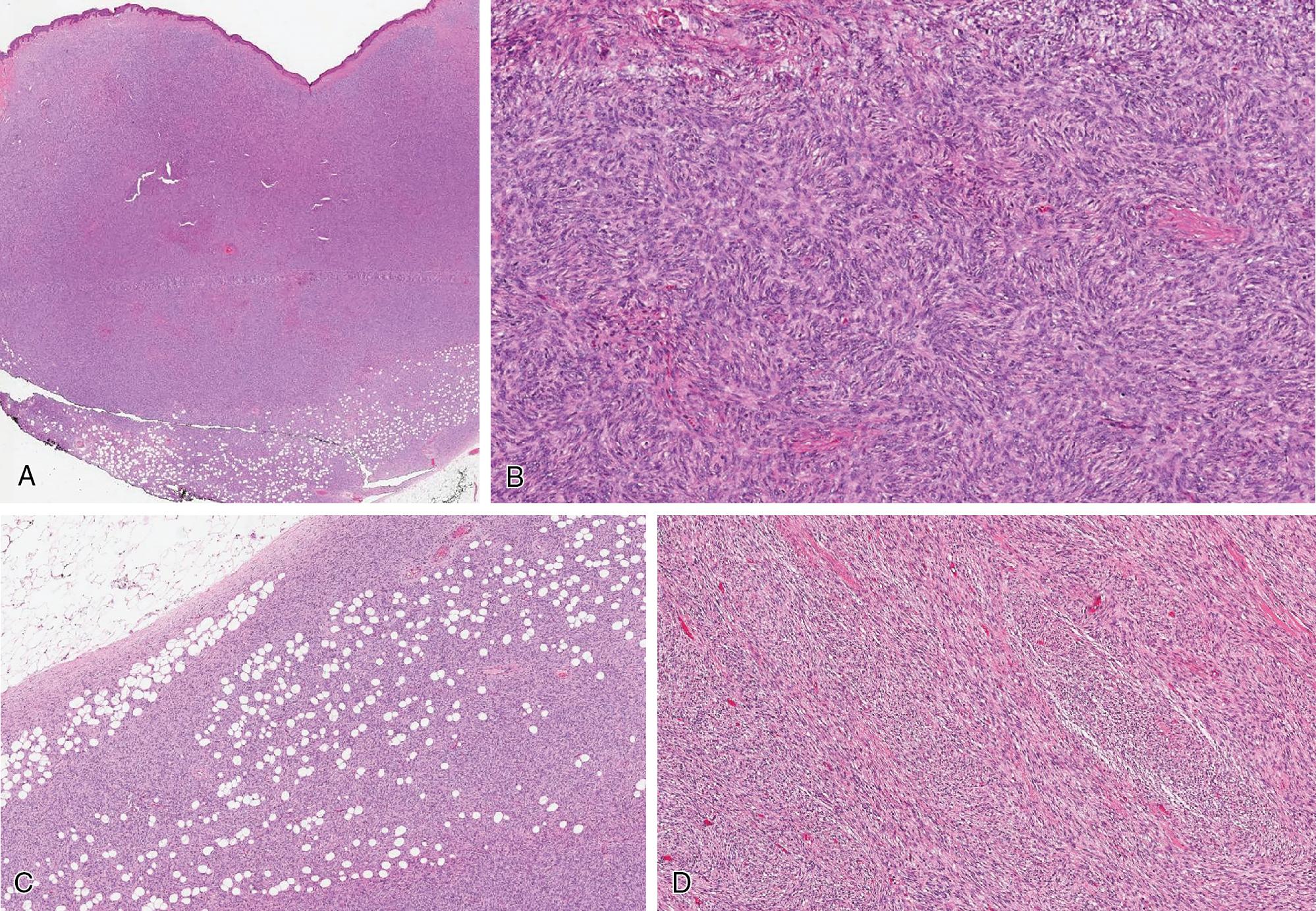
In fibrosarcomatous transformation, the cells exhibit a morphologic progression toward greater cytologic atypia, forming intersecting fascicles, often with a herringbone appearance ( Fig. 8.33 D). Mitotic activity inherently becomes more prominent, frequently exceeding 10 mitoses/10 HPF.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here