Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Short stature (usually defined as height >2 standard deviations [SD] below the mean for age) and growth failure (a subnormal height velocity that leads to decline in growth percentiles, usually height velocity >1.5 SD below the mean for age) are symptoms, not diseases . Of the two, short stature is more likely to be noticed, but growth failure is more likely to be pathologic .
Short stature may represent a normal variant or it may be a signal of serious physical or emotional illness. Because linear growth is a crucial component of childhood, growth is in many ways an index of childhood well-being. Illnesses, even those not involving aberrations of growth-regulating hormones, often interfere with growth. Therefore, the measurement and charting of heights sequentially on standardized growth charts constitute a central part of a child’s medical evaluation, and the finding of short stature, particularly if associated with a subnormal height velocity, deserves close attention and appropriate evaluation.
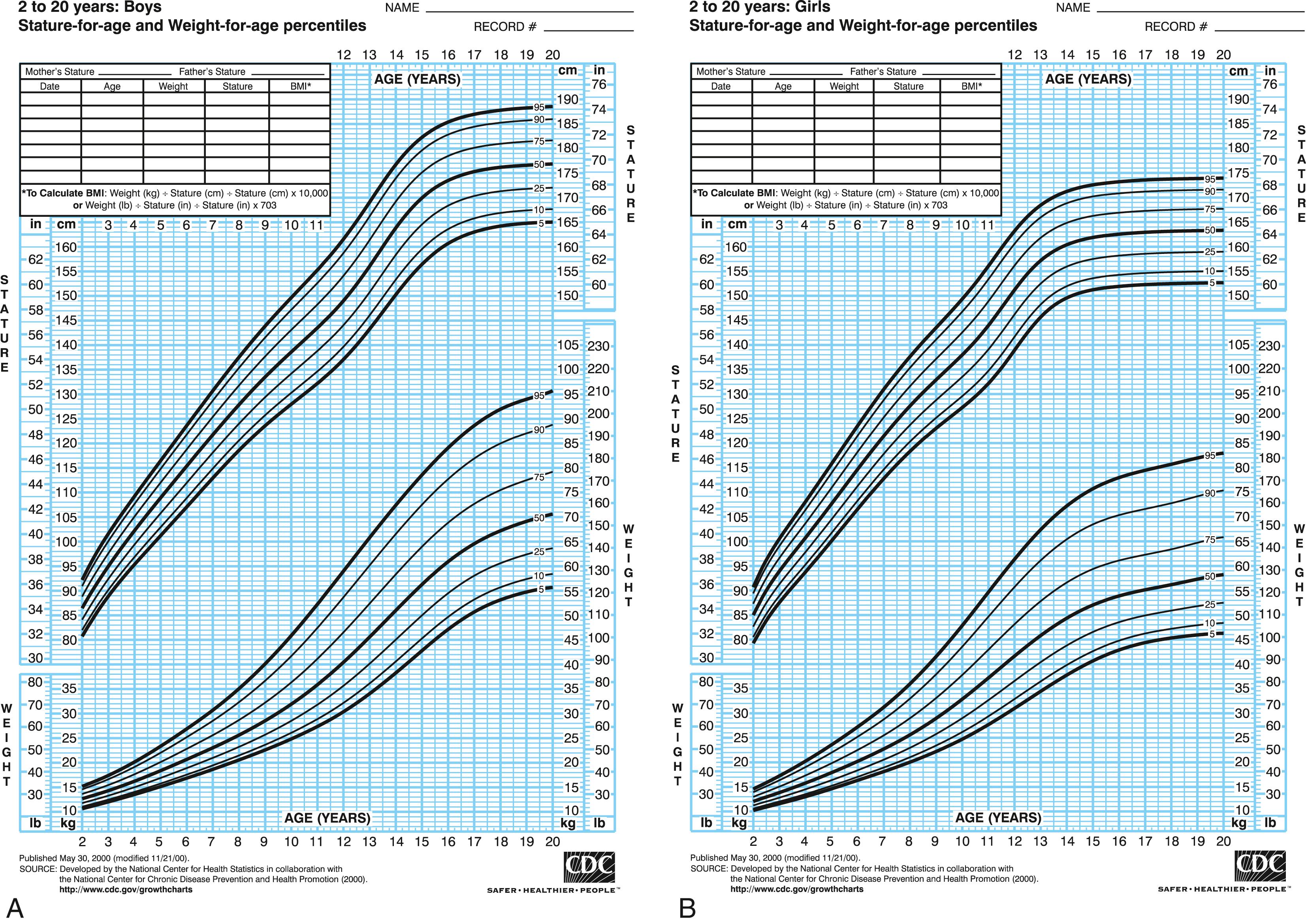
Short stature is defined as height more than 2 SD below the mean for age, which is at the 2.3 percentile ( Fig. 56.1 A , B ). On growth charts (and in clinical practice) it is more common to define short stature as height below the 3rd percentile for age, which is −1.88 SD. Implicit in this definition is the understanding that height is a normally distributed characteristic; therefore, a proportion of normal individuals have heights more than 2 SD above or below the mean. Since height is strongly heritable (i.e., a large proportion of the observed variation between individuals is hereditary), stature that is inappropriately low for the child’s genetic endowment may also be a cause for concern.
Growth failure is defined as a subnormal rate of growth, a height velocity that is below the norm for that age. Since sustained height velocity below the 25th percentile is insufficient to maintain a child’s position on the growth chart, growth failure is sometimes defined as height velocity below the 25th percentile for age. Other authorities prefer to use >1.5 SD below the average height velocity for that age as the cut-off (see Chapter 12 ).
Short stature should be distinguished from “failure to thrive.” The latter term refers primarily to poor weight gain in infants and young children (although linear growth may be secondarily affected), whereas short stature refers primarily to subnormal linear growth in childhood and adolescence (see Chapter 12 ).
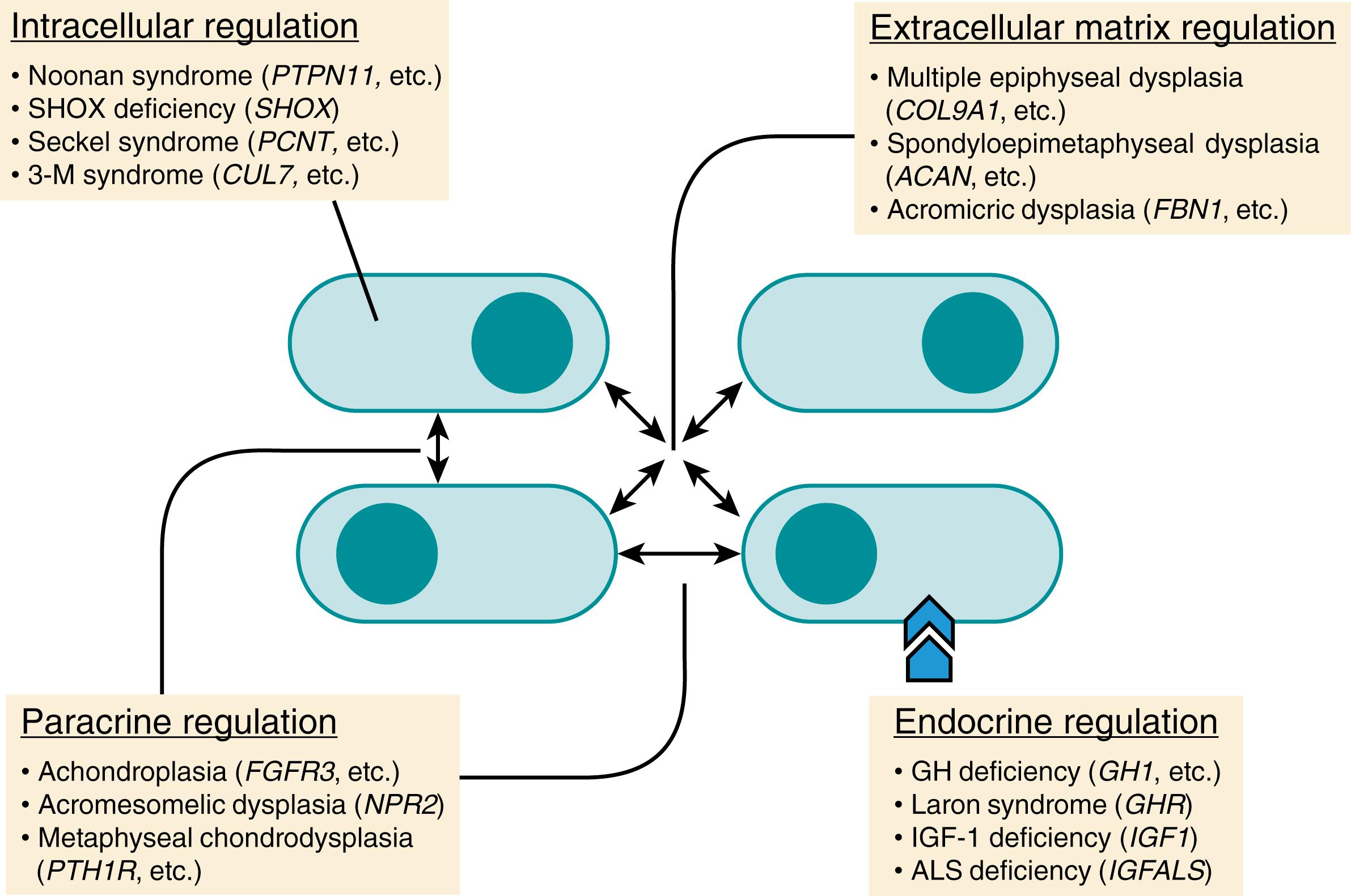
Height is highly heritable, but the genetic program underlying this regulation is not fully understood. The growth of individual organs as well as the growth of the skeleton as a whole is regulated by negative feedback mechanisms that slow and eventually stop growth as organs and the organism reach their final size. This gradual deceleration of growth with age does not appear to be driven primarily by changes in the endocrine system, since levels of growth hormone (GH) and the insulin-like growth factors remain elevated even as growth slows in the latter part of puberty. The final height and growth pattern of any given individual are affected by subtle variations in many genes, while more significant variants in individual - specific genes cause the various genetic forms of short stature and skeletal dysplasias ( Fig. 56.2 ).
A human being experiences the most rapid linear growth in the prenatal period (growing from near-zero to about 50 cm in length in just 9 months). While genetic factors play a major role in postnatal growth, fetal growth and birth size mainly reflect maternal and placental factors, including maternal or uterine size, parity, multiparity, nutrition, and placental function. Many congenital disorders such as Turner syndrome and congenital GH deficiency that markedly stunt postnatal growth have only minimal effects on prenatal growth and birth size. Birth size is generally a poor predictor of the eventual growth pattern in most children. An exception is the neonate who is small for gestational age as a result of intrauterine growth restriction (IUGR) ( Table 56.1 ). While most infants with IUGR (caused by nutritional problems or poor placental function) show catch-up growth (a period of rapid growth that occurs spontaneously after relief from the adverse intrauterine condition that had suppressed the rate of growth), 10–20% remain shorter than expected beyond infancy and early childhood, making IUGR one of the causes of childhood short stature.
FETAL
PLACENTAL
MATERNAL/PATERNAL
|
Infancy is also a period of relatively rapid growth (faster than at any other time in postnatal life). Growth then gradually slows as the infant gets older, declining to its lowest point just before puberty, before accelerating again during the pubertal growth spurt and finally ending with the completion of linear growth about 5 years after the onset of puberty ( Table 56.2 and Fig. 56.3 ).
| Age Interval | Average Height Velocity |
|---|---|
| Prenatal | 66 cm/yr |
| 0–1 yr | 25 cm/yr |
| 1–2 yr | 12 cm/yr |
| 2–3 yr | 8 cm/yr |
| 3–5 yr | 7 cm/yr |
| 5 yr–onset of puberty | 5–6 cm/yr |
| Pubertal growth spurt | Girls 8–12 cm |
| Boys 10–14 cm |
Since birth length is mostly determined by maternal and placental factors, size at birth may not reflect the infant’s genetic growth potential . It is during the first 2 years of life that infants gradually transition from their birth size to their own genetically determined height potential. Therefore, it is normal for infants to shift linear growth percentiles during this period; 65% of infants will exhibit such shifts, moving up or down on the growth chart. By 24 months, these shifts are complete, and most children have entered a specific “growth channel” or linear growth percentile in relation to peers and any significant deviation from this channel should evince concern.
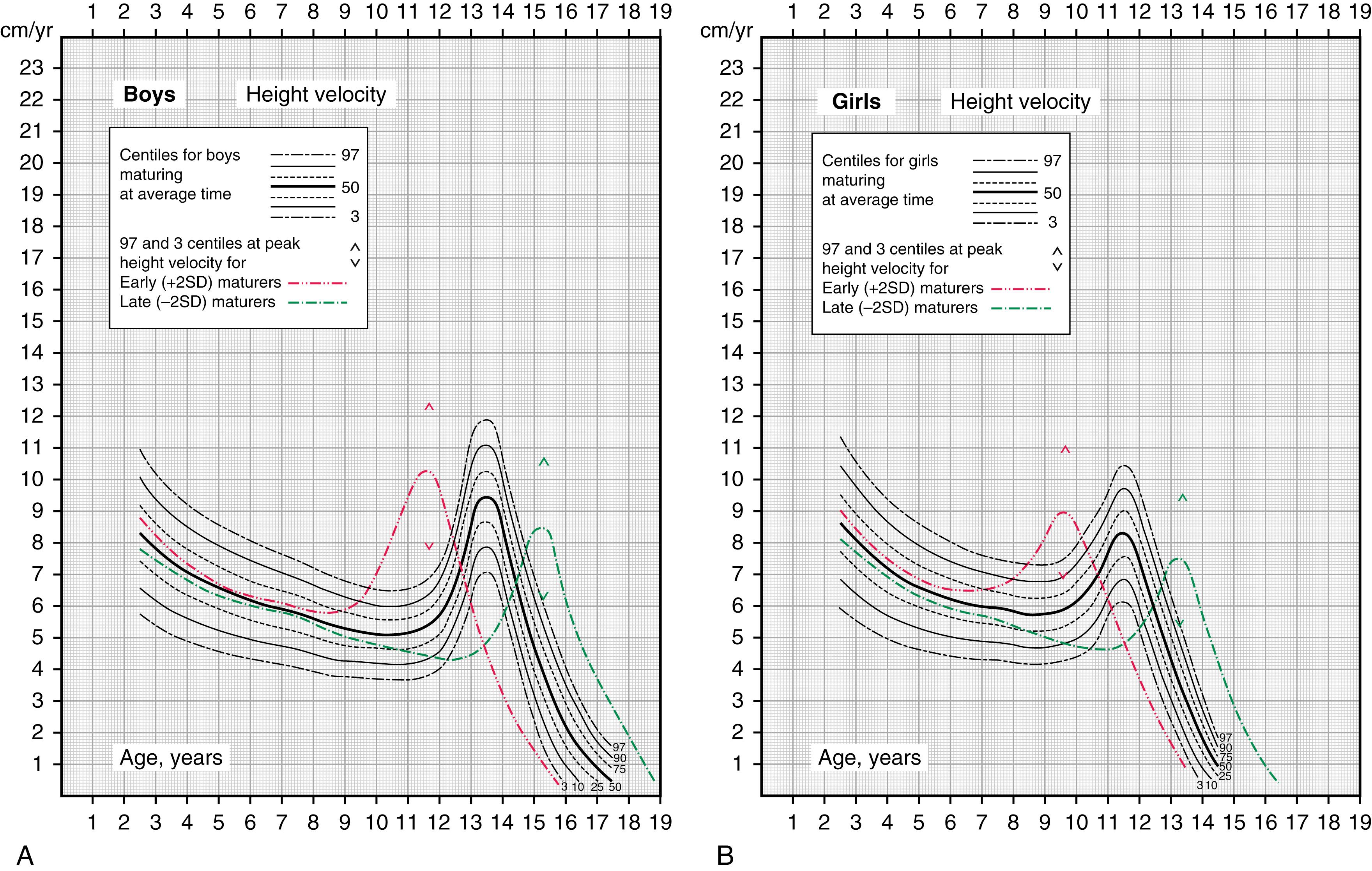
After a period of slow but steady growth during childhood, many children experience a further “prepubertal dip” in height velocity, reaching a nadir just before onset of puberty. Height velocity then accelerates once again as puberty advances. This acceleration of growth during puberty reaches a peak known as the “pubertal growth spurt.” The timing of the pubertal growth spurt differs between females and males. Females generally begin pubertal development at 10 to 11 years of age and their pubertal growth spurt starts coincidentally with breast development and peaks before menarche. For females with an average tempo of puberty, peak growth velocity (8–9 cm per year) is reached at 11–12 years of age. After menarche (which generally occurs 2–2.5 years after onset of puberty), the growth rate declines and growth finally stops about 2 years after menarche, with girls gaining an average of 7 cm in height after menarche.
In males, testicular enlargement is generally the first sign of puberty and occurs at approximately 11.5 years on average (range, 9–14.3 years). In males with an average tempo of pubertal development, peak growth velocity occurs about 2 years after onset of puberty (so it is later than girls in absolute terms, as well as in terms of stage of puberty) at approximately 13–14 years, with an average rate of 10.3 cm (4 inches) per year. It is worth noting that prepubertal males and females grow at very similar rates; the ultimate taller stature of males relative to females is mostly the result of a longer period of growth and a higher peak growth velocity during puberty.
These are the average timings of puberty and pubertal growth, but it should be kept in mind that there are large variations in the timing of puberty—and therefore in growth rates—among individuals of the same age during the period of adolescence.
Because of these characteristic patterns of growth during childhood and adolescence, the rate of growth (centimeters per year or inches per year) is a key variable in evaluating a short child. Growth rates may vary somewhat with season and can be affected by transient illness, but a child should maintain a relatively set growth channel on the linear growth percentile charts after 2–3 years of age. A persistently slow rate of growth in relation to age-appropriate norms is alarming and is likely to reflect an underlying medical disorder.
Stature is evaluated as supine length until 2 years of age and as standing height thereafter ( Fig. 56.4 ). For measurement of supine length, an infant lies on an inflexible ruled horizontal surface, at one end of which one person holds the infant’s head in contact with a fixed board; a second person extends the infant’s leg as much as possible and brings a movable plate in contact with the infant’s heel. Recumbent measurements average 1 cm (0.4 inch) more than standing height .
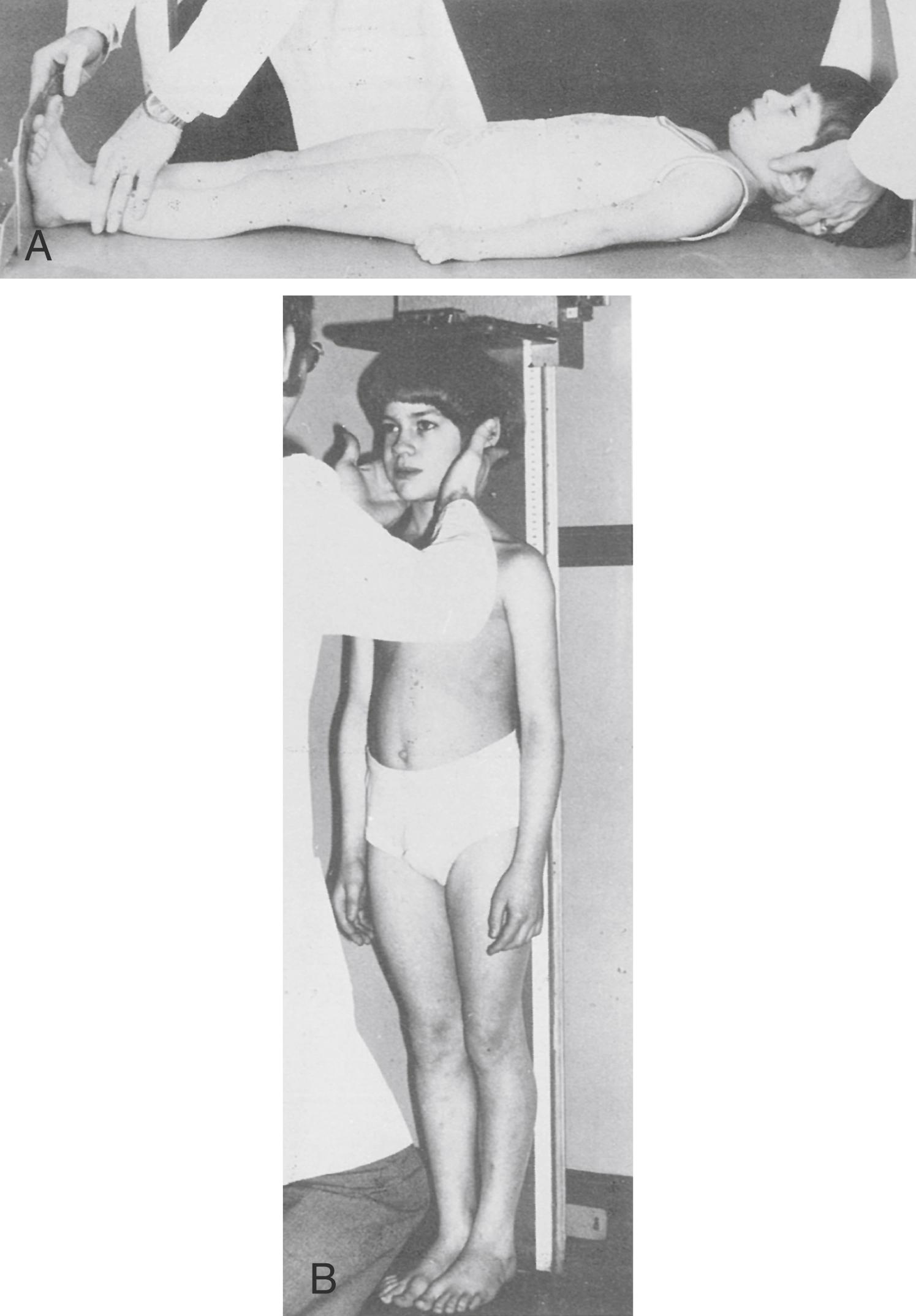
After 2 years of age, children are measured standing and barefoot with a device such as a Harpenden stadiometer; a vertical metal bar is affixed to an upright board or wall, and height is measured at the top of the head by a sliding perpendicular plate or block. Measurements of length using pen marks on the examining table at the head and foot of infants are often grossly inaccurate, as are height measurements using a flexible metal rod atop a standard weight scale.
With the use of optimal techniques, the variation in measurement among observers is <0.3 cm (0.1 inch). It is then possible to determine changes in height over 3- to 4-month intervals to estimate the annualized growth rate. However, because of normal seasonal variations in growth rates, a longer interval between measurements (6–12 months) is more reliable in the calculation of height velocity.
Measurements are then plotted on standard growth charts; it is recommended that World Health Organization (WHO) growth charts be used for infants from birth to 2 years of age and Centers for Disease Control and Prevention (CDC) growth charts for children from ages 2 to 18 years (see Fig. 56.1 ). These CDC and WHO growth charts are available at http://www.cdc.gov/growthcharts/ and https://www.who.int/childgrowth/standards/en/ .
For children with specific disorders, such as Turner syndrome, there are disorder-specific standardized growth curves for many of these disorders. Children with known disorders should be plotted on these curves to avoid unintentional evaluation for growth failure, as they may appear to be abnormally short on the standard growth charts. Calculated growth rates (centimeters per year or inches per year) should be evaluated in relation to age-related norms with growth velocity charts for North American children for children over 2 years of age (see Fig. 56.3 ).
The cephalo-caudal ratios change with age until adult height is attained ( Fig. 56.5 ). Thus, apart from linear height, it is also useful to assess the upper-to-lower segment ratio (U/L) ( Fig. 56.6 ) and the arm span. The U/L is determined by measuring the lower segment (vertical distance between the top of the symphysis pubis and the floor, with the child standing) and the upper segment (height minus lower segment). Arm span is the distance between the outstretched middle fingertips with the child standing against a flat board or wall. The U/L and arm span are used to determine whether the child is normally proportioned or not (in Europe it is more common to assess sitting height vs standing height for the same purpose).
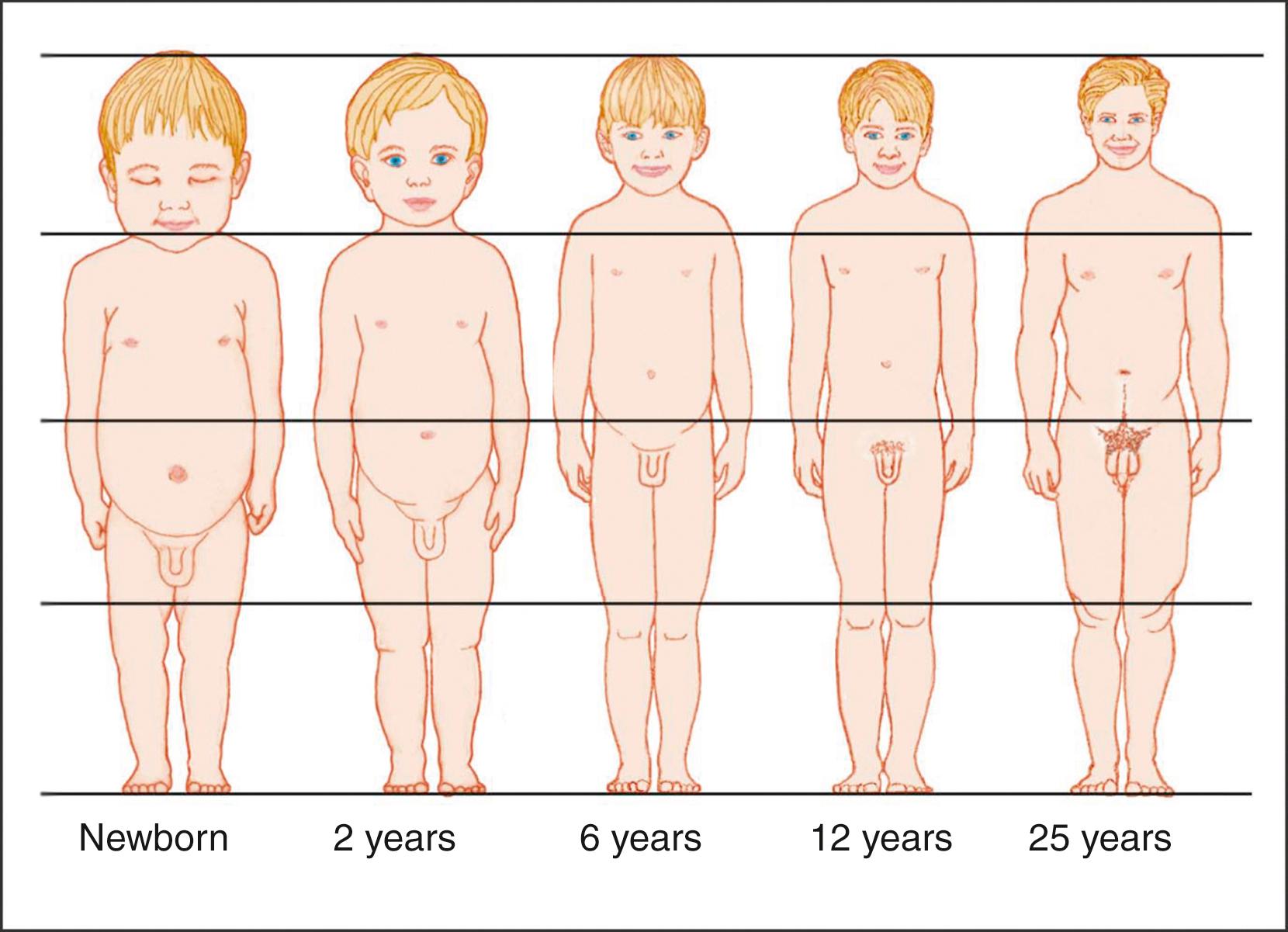
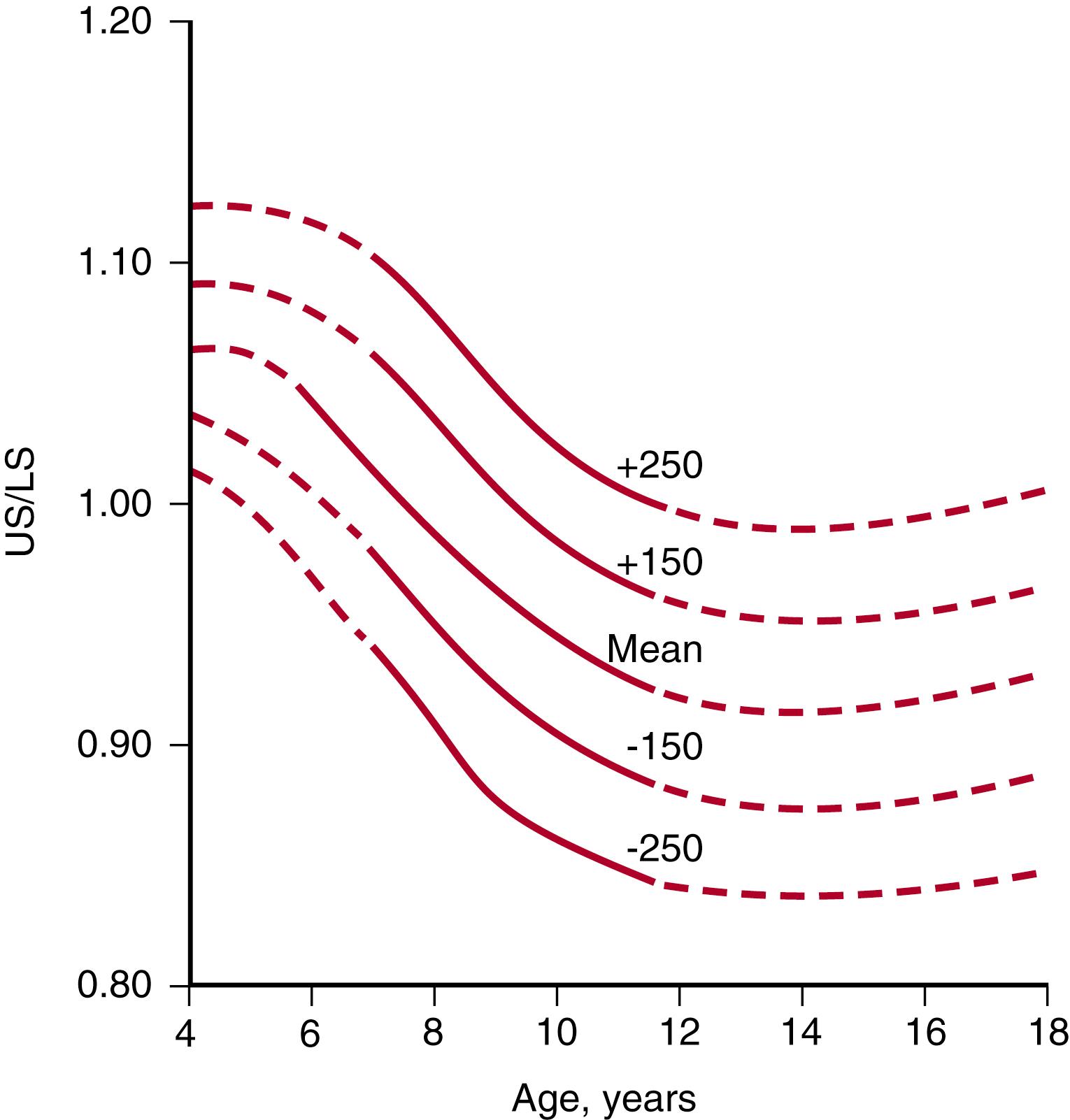
The U/L gradually declines with age throughout childhood (see Fig. 56.6 ). Since infants have relatively short legs, the U/L is high (an average of 1.7) at birth. It then declines throughout childhood as the legs increase in length relative to the upper body, decreasing to a mean of 0.9 by late puberty (in males; in females the ratio may be closer to 0.95). The arm span as compared to the height is another measure of body proportions and is normally shorter than the height in younger children and increases to become slightly longer than the height by late puberty (about 5 cm more than height in boys, 1.2 cm more than height in girls). Deviations from the norm in the U/L and the arm span may point to syndromic conditions such as skeletal dysplasias, Turner syndrome, or longstanding hypothyroidism (increased U/L, i.e., relatively short extremities) and radiation-induced spinal damage or genetic disorders like Klinefelter or Marfan syndrome and homocystinuria (low U/L, due to a relatively short trunk or unusually long extremities).
Weight should also be considered in relation to a child’s stature. Undernutrition is generally caused by nonendocrine factors (poor nutritional intake, malabsorption, systemic illness) and typically leads to a decrease in weight before a decrease in linear growth. Obesity in childhood is usually exogenous; exogenous obesity is generally associated with an accelerated growth rate. In contrast, endocrine disorders that cause poor growth and short stature are often associated with weight gain and obesity (Cushing syndrome, hypothyroidism, and, in some instances, GH deficiency). Therefore, the obese child who has a slow growth velocity is more likely to have an endocrine cause of short stature, while the undernourished child with short stature likely has short stature secondary to poor weight gain and is unlikely to have an endocrine disorder.
Both parental height and parental pattern of growth are key determinants of a child’s growth pattern. This strong familial influence on height is not detectable at birth but is manifested by 2–3 years of age. Final adult height is strongly heritable with heritability estimates ranging from 0.8 to 0.95 (i.e., 80–95% of the observed variation is explained by hereditary rather than environmental factors). The midparental height (MPH) is used as a measure of the child’s genetic growth potential and is an average of the height of both parents after correcting for gender . Because the average adult male is taller than the average adult female by 13 cm (5 inches), 13 cm is added to the mother’s height in the case of a male child, while 13 cm is subtracted from the father’s height in the case of a female child; the parental heights, after correction for gender, are then averaged to obtain the MPH.
MPH is determined in the following manner:
The MPH is a good index of the child’s genetic height potential but tends to become less accurate if one parent is unusually tall or short; that is, the offspring of an exceptionally tall parent or exceptionally short parent will be closer to the average height than is predicted by the MPH because of the phenomenon of regression to the mean .
In addition to the influence on final adult height, parents’ patterns of growth are also often repeated in their children. In particular, many males who were “late bloomers” with delayed onset of puberty and delayed but normal growth spurts have sons with a similar growth pattern.
The average height of various populations across the globe is not the same; Northern European populations are taller than many (but not all) other ethnic groups and it therefore seems to make sense to use population-specific standards of growth because a child from a generally short ethnic group may be labeled as “short stature” by Northern European standards, but may be normal for their own ethnic group. Two additional observations need to be kept in mind though:
Migrants from countries where the average height is lower tend to have children who are taller than their parents when they move to a country with a higher standard of living. This indicates that at least some of the observed height difference may be environmental (most likely related to nutrition and childhood disease burden) and that this height difference may shrink or disappear in subsequent generations.
The Northern European populations were themselves much shorter in the 18th and 19th centuries and average heights have steadily increased as living standards improved (reflecting improvements in nutrition and other public health measures). This “secular trend” in height slows down and plateaus over time (most likely because a “genetic ceiling” is reached) but is much more marked in populations that have recently seen an improvement in living standards.
Practitioners should make allowances for ethnic differences in height when evaluating children from different ethnic backgrounds but should not automatically assume such differences as the sole explanation for short stature in children from historically shorter populations. Growth velocity in particular should not be abnormal even in historically shorter populations, and a subnormal growth velocity should trigger an evaluation in the same way as it would in children from a Northern European background.
Because growth is a barometer of a child’s health and general well-being, freedom from serious illness is necessary for a child to achieve their genetic growth potential. Chronic illnesses that are not primarily problems of stature often interfere with growth secondarily, and short stature may be the presenting feature of such conditions as inflammatory bowel disease, celiac disease, and renal disease.
Under normal circumstances, emotional and psychological factors do not have a great effect on growth. However, in certain cases, emotional deprivation can lead to very significant growth failure (sometimes labeled “deprivation dwarfism” or “psychosocial dwarfism”) via mechanisms that are still poorly understood.
Growth hormone (GH) is produced by the anterior pituitary under the control of growth hormone–releasing hormone (GHRH) from the hypothalamus and is essential for normal growth in childhood and adolescence (though it appears to play a relatively small role in prenatal growth). GH is secreted in brief pulses and peak secretion occurs during sleep, so random serum levels have little utility in the evaluation of GH deficiency except in the immediate newborn period (when random GH levels are usually elevated and a level below 7 ng/mL is very likely to reflect GH deficiency).
GH exerts its growth-promoting effect mainly through stimulating the production of insulin-like growth factor 1 (IGF-1) (primarily in the liver, but also in some target tissues), though there is also some direct actions of GH on bone. Most circulating IGF-1 is bound to IGF-binding proteins, with the largest proportion being bound in a ternary complex with IGF-binding protein-3 (IGFBP3) and a protein named the acid-labile-subunit (ALS). Because IGF-1 levels in the blood are stable through the day and reflect the integrated effect of GH secretion, measurement of IGF-1 is often used as a surrogate measure of GH secretion.
Thyroid hormone also has a relatively limited role in prenatal growth but is essential for normal postnatal linear growth, both via direct actions on the epiphyseal growth plate and via a permissive effect on GH secretion. Hypothyroidism can therefore lead to very profound growth failure and an evaluation of thyroid hormone status is essential in the investigation of growth failure.
Glucocorticoids do not play any significant role in normal growth but are powerful inhibitors of growth when present in excess. Persistent exposure to excess corticosteroids (whether endogenous or exogenous) can lead to very severe growth failure (along with significant weight gain). Deficiency of glucocorticoids generally does not adversely affect growth if the child is otherwise healthy.
Sex steroids mediate the pubertal growth spurt (see Chapter 55 ). This involves direct effects of sex steroids on bone growth as well as steroid-induced amplification of GH secretion. Most of the bone-maturing action of sex steroids is mediated by estrogen in both sexes; while testosterone has some direct effect on bone strength and thickness, most of the effects of testosterone on linear growth and the maturation of growth plates in males also occur via the action of estrogen produced by the peripheral conversion of testosterone. Consequently, even in males, bone maturation can be affected by genetic defects in the production or action of estrogen, as well as by pharmacologic inhibition of this pathway (e.g., by using aromatase inhibitors that block the conversion of testosterone to estrogen). Sexual precocity (true precocious puberty, exogenous exposure, or congenital adrenal hyperplasia) tends to accelerate linear growth transiently as a result of premature or excessive production of sex steroids (see Chapter 55 ). But, if left untreated, these conditions eventually advance osseous maturation, leading to premature epiphyseal fusion and short final adult height. Absence of sex steroids ( hypogonadism ), in the absence of other abnormalities, blunts the pubertal growth spurt but tends not to limit final height as bone maturation and epiphyseal fusion are also delayed by the lack of estrogen in these patients.
Osseous maturation follows a very predictable pattern during the growth and development of the child and an X-ray of the nondominant hand can be used to assess bone age (i.e., the degree of maturation of the bones compared to age-matched standards). Bone age is usually estimated by comparing the child’s radiologic findings with a standard set of radiologic images, or by using various scoring algorithms. These readings are subject to observer bias and error (other methods that use artificial intelligence to automatically assess bone age may bypass this issue) but are still useful as long as this inherent subjectivity is kept in mind. Bone age is very closely correlated with pubertal maturation, and an assessment of bone age can be especially useful in cases of precocious puberty, delayed puberty, and constitutional growth delay. Except in cases of precocious puberty, bone age is rarely useful in the evaluation of short stature in a child younger than 5 years of age.
Understanding the factors that influence childhood growth leads directly to an understanding of the causes of short stature and to the differential diagnosis of short stature for an individual child ( Tables 56.3, 56.4, 56.5, 56.6, and 56.7 ). While a very large number of conditions can potentially lead to short stature and growth failure, the vast majority of children with short stature either are normal variants (familial short stature, constitutional delay of growth and puberty) or have no discernible cause (idiopathic short stature). It is also important to remember that the child with growth failure is far more likely to have an underlying pathology than a child who happens to be short but has a normal growth velocity.
|
|
The two most frequent causes of short stature in children are familial short stature and constitutional delay of growth and puberty . These are considered normal variants and their recognition can help avoid expensive and unnecessary testing and interventions.
The term familial short stature is usually reserved for familial forms of mild to moderate short stature that do not have a specific identifiable genetic defect; since hundreds of genes play a role in growth, this height potential reflects the influence of multiple loci of small effect or a few loci of relatively moderate effect. Genetic disorders that result in severe short stature, or that are associated with other abnormal physical findings (syndromes), or that are caused by known genetic defects (see Table 56.7 ), are conventionally treated separately from familial short stature. In familial short stature the child’s height is in keeping with their genetic endowment, and the child is otherwise healthy. Typically, one or both parents (and often other family members) are about 1.5 to 2 SD below the mean in height. This relatively short genetic potential is reflected in short MPH, and if the child continues to grow along the current percentile, the child would fall within 9 cm of this MPH (this is approximately 2 SD of the MPH); by age 2 the child is usually noted to be small in relation to peers. Although the growth channel is low, it should parallel the normal growth curve from that point onward. Continued deviation away from the normal growth curve (indicating a subnormal growth velocity) is not typical and should raise concerns about a disorder other than familial short stature. Stature that is extremely low (2.5–3 or more SD below the mean) raises concerns even if the parents are short, since this degree of short stature may reflect a specific genetic cause of short stature (e.g., a mild defect in GH secretion or action, or a form of hypochondroplasia) in the parents as well as the child.
The review of systems is generally negative in the otherwise healthy child, as are the physical examination findings (aside from short stature). The height-to-weight ratio, body proportions, muscularity, and pubertal development are normal for age. Abnormalities found on review of systems or physical examination should prompt consideration of other diagnoses.
Laboratory studies are not mandatory but if done are all normal. The bone age is also normal. Prediction of adult height can be made on the basis of bone age but the accuracy is variable; the predicted height is within 9 cm of the MPH.
Constitutional delay of growth and puberty is a growth pattern that is also considered a variant of normal. These children appear to have a slowed maturational pattern and their bone age is delayed relative to their peers. As part of this slowed maturation, they also enter puberty later than their peers. This variant occurs in both sexes but is more common in males and is recognized predominantly in them for this reason, as well as for cultural reasons (greater attention being paid to short stature in adolescent males than females).
These children often begin to show moderate short stature (height −1.5 to −2.5 SD) during early to middle childhood but are otherwise healthy. They have delayed onset of puberty and therefore a delayed growth spurt. One or both parents (or other family members) may have a history of delayed puberty and a late adolescent growth spurt, with eventual cessation of growth during late adolescence or even into the third decade of life. The affected parent usually is of normal adult stature. Otherwise, the history and review of systems are negative. The physical examination findings are normal except for delayed onset of puberty in children of an appropriate age.
Laboratory tests are not mandatory but are normal if performed, with the important exception of a delayed bone age (bone age < chronologic age). It is important to note that IGF-1 levels normally increase with onset of puberty and should be compared with standards for the child’s pubertal stage and bone age rather than the chronologic age. The delayed bone age suggests that the child has more “room to grow” than the average age-matched child and that the child is likely to reach an adult height taller than that suggested by the current height percentile. Some children have mixed familial short stature and constitutional delay in growth and development; they tend to have both delayed puberty and a short-predicted adult height.
Chronic illness may mimic constitutional delay in growth and development and should be considered in the differential diagnosis. It is sometimes difficult to distinguish children with constitutional delay in growth and development from the more unusual condition of central (hypothalamic/pituitary) hypogonadism; a positive family history of delayed but normal puberty and growth, a normal sense of smell (to exclude Kallmann syndrome), and normal neurologic findings favor constitutional delay. Evaluation for permanent central hypogonadism is indicated if puberty fails to begin by age 13 in females or 14 in males, particularly if there is no family history of constitutional growth delay.
Females with delayed puberty are more likely to have an underlying pathologic cause, so the threshold for investigation is lower in females. On the other hand, benign constitutional delay is common in males and in the presence of a positive family history further evaluation may be delayed even beyond the age of 14. The possibility of central hypogonadism becomes stronger the longer the onset of puberty is delayed, but it should be noted that cases of spontaneous development of normal puberty can occur up to and even beyond the age of 18.
In most cases only reassurance and an explanation of the growth pattern is required, but if the parents and the child are eager to hasten pubertal development (usually because of involvement in sports or because of bullying and other psychosocial considerations), then a short course of low-dose sex steroids can accelerate the onset of puberty. Such intervention does not change the final height but hastens the child’s progression to that height. Treatment options are discussed in detail later in this chapter.
Idiopathic short stature (ISS) is a clinical description rather than a disease. ISS is generally considered a normal variant of growth (since pathologic causes are excluded, by definition). The exact definition varies from country to country; in the United States, the Food and Drug Administration (FDA) used a definition of height >2.25 SD below the age mean (which is the 1st percentile for height) in children in whom no specific cause of short stature has been identified after a thorough evaluation. This corresponds to an adult height <160 cm (5 feet 3 inches) for males and <150 cm (4 feet 11 inches) for females.
In many cases these children have familial short stature and their height is not inappropriate for parental height. But in most definitions they are included in the category of ISS because the purpose of the definition was to identify children who may not have any pathology, but whose short stature per se constitutes a possible reason to treat them with GH. This notion remains controversial because there is no universal agreement about the psychosocial or economic impact of ISS. Those who support treatment of these children argue that such a degree of short stature may have adverse psychological, social, and economic consequences and deserves to be treated even if no underlying pathology is identified. Opponents argue that there is no convincing evidence that short stature of this degree itself leads to any significant handicaps that would justify prolonged invasive therapy with an extremely expensive medication that may itself have future adverse health consequences. Third-party payers often regard such treatment as cosmetic and may not approve requests for therapy. It should be noted that in most cases there are no consistent adverse effects of short stature on quality of life in children at or just below the 1st percentile; however, some studies do indicate that practical and psychosocial difficulties increase in those with extreme degrees of short stature.
ISS is a diagnosis of exclusion; history, physical examination, laboratory studies, and imaging studies do not reveal any specific cause of short stature in an otherwise normal child. How much investigation is needed before a child can be labeled as having ISS remains a matter of controversy and clinical practice can vary significantly within and between different countries. Treatment with GH was approved by the FDA in 2003 but remains controversial and may not be approved by third-party payers. Treatment is more likely to be beneficial in those with more extreme degrees of short stature. A predicted adult height <136 cm (about 4 feet 6 inches) in females and less than 149 cm (about 4 feet 11 inches) in males is very likely to be associated with quality-of-life issues and deserves treatment even in the absence of any underlying cause.
Children who are born small for gestational age (SGA) usually catch up with their peers by 2–3 years of age, but 10–20% of children born SGA will fail to catch up and will continue to be below the 3rd percentile. This is more likely in children who are born premature as well as SGA and in those most severely affected at birth. The mechanism underlying this failure of catch-up growth is not well understood. Children with IUGR also have an increased future risk of metabolic disorders like type 2 diabetes. Children who were SGA typically have normal proportions and no other physical findings; in some cases, the IUGR is just one component of a genetic syndrome (see Tables 56.3, 56.6, and 56.7 ) and other features of the syndrome are present.
Children who were born SGA with poor postnatal growth typically have normal or even elevated GH levels but lower average values of IGF-1 and IGFBP3 (though most are still in the normal range), indicating some degree of GH resistance in at least some of these children. GH treatment increases final height by about 1 SD on average (about 6 cm) if started early in life and continued for at least 7 years. In the United States, treatment is approved by the FDA for children who were born SGA and whose height remains >2 SD below the mean at age 2. In Europe, treatment is approved for children whose height is >2.5 SD below the mean by age 4. These children can be mildly GH resistant and may therefore require GH doses at the higher end of the dose range. Since individual response varies, GH treatment can be started at the usual dose (30–50 μg/kg/day) and then increased if needed based on growth response and IGF-1 levels.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here