Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Procedural skills are an important component of care in the neonatal intensive care unit (NICU). Procedures have inherent risks and can be the source of significant morbidity and mortality if the proper techniques are not applied. Historically, the acquisition of these competencies required that trainees observed a procedure and then attempted to perform it under close supervision. Obviously, although this practice served a laudable purpose, it is fraught with significant limitations, and therefore most training programs abandoned it. The traditional practice of “see one, do one, and teach one” has been put to rest because of the introduction of highly sophisticated simulation laboratories where trainees can learn the proper techniques of most procedures without subjecting patients to potentially painful and harmful unsuccessful attempts. Also, this novel learning modality takes the pressure away from the learner, who can now hone in on the skills and acquire the confidence to perform successfully in a real-life situation at bedside. The introduction of point-of-care ultrasonography is another important advancement in the procedural arena in neonatology. Introduction of a central vascular catheter, or performing a paracentesis under ultrasonographic guidance, is standard in adult and pediatric intensive care units, and that practice is quickly gaining momentum in neonatal units as well.
Before any procedure is performed in a newborn, a thorough and thoughtful consideration of the indications, contraindications, benefits, and potential complications is required. A discussion with the family and obtaining parental informed consent are mandatory, except in emergency situations where a delay may pose a life-threatening risk. Gathering the appropriate clinical information and patient identification, preparation of the supplies, and making sure that all caregivers involved are aware of the procedure to be performed is fundamental. Utilization of checklists and procedural bundles as well as a huddle or “time out” have significantly facilitated these processes and decreased errors.
In this chapter, a few select procedures are discussed; however, the information presented here is meant to provide the reader with only a general overview and should not be considered enough to achieve competence.
Use of central catheters requires careful consideration of the risks involved ( Box D.1 ). The relatively easy access to the umbilical vessels makes these vessels a very good option for central access in emergency situations. A central catheter inserted into the aorta via an umbilical artery may be required in the management of the sick neonate for monitoring of blood pressure, intermittent blood sampling to check acid–base status, and, while in place, infusion of parenteral fluids and medications.
Hemorrhage
Perforation into
Peritoneal cavity
Urachus
Pericardium
Hepatic laceration
Thrombi and emboli
Splenic vein thrombus or embolus
Displacement of thrombus in ductus venosus
Pulmonary infarction (pulmonary vein thrombus)
Retained broken-off catheter fragment
Calcification of portal vein or umbilical vein
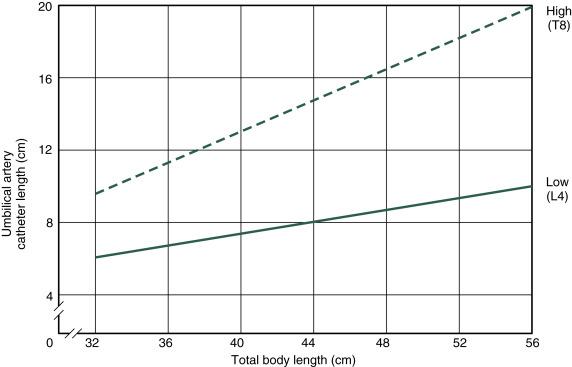
Umbilical artery catheters must be precisely located. A major objective is to avoid the origin of the renal arteries because a catheter may occlude a renal artery, and catheters in the area may produce thrombosis. Both situations can result in renal infarction. Some prefer to position the catheter in the midthoracic aorta (high); others prefer to locate the catheter tip between L3 and L4 (low). Thrombotic complications are reported with both high and low placement. Resolution of the thrombus or development of collateral circulation generally occurs, even when extensive thrombosis (e.g., aorta distal to renal arteries, common iliac) has been documented. Occasionally, a neonatal death is considered a direct consequence of complications related to umbilical vessel catheterization. Hypertension in the neonate following use of high umbilical artery catheters has been described. However, in a prospective study, the incidence of hypertension was similar with low and high catheters.
Hemorrhage, either resulting from loose connections or careless use of the stopcocks or occurring at the time of removal, is a major complication of arterial catheters. Another major complication is thrombus formation with release of microemboli into the systemic circulation. It is speculated that the catheter tip can traumatize the vessel wall, which may release tissue thromboplastin and activate intravascular coagulation. Alternatively, the presence of the catheter itself may produce clot formation. More rare complications include intimal flap formation and aneurysmatic dilatation of the abdominal artery. Ultrasonographic examination of the abdominal aorta and its branches is indicated when any of these complications are suspected.
Arterial blood samples may be obtained by multiple arterial punctures (of the radial artery) or an indwelling radial artery cannula as the method of choice or in cases in which umbilical artery catheterization is unsuccessful.
In general, umbilical vein catheterization is technically easier. However, it should be avoided except when immediate access to a vein is needed because of an unexpected emergency (e.g., the need for delivery room resuscitation) because complications may be serious and difficult to avoid. An umbilical vein catheter tip may locate in a branch of the portal vein and lead to areas of liver necrosis without perforation of the vein wall following infusions of hypertonic solutions such as sodium bicarbonate and hypertonic glucose. Portal vein thrombosis and aseptic abscess formation have also occurred with and without infection. In addition, spontaneous perforation of the colon after exchange transfusion via an umbilical vein catheter has been reported. Radiographic verification of catheter tip location was not performed in any of these cases; most likely, the catheter tip was in the portal vein, and the cause of perforation was local necrosis of the bowel wall following hemorrhagic infarction as a result of retrograde microemboli or obstructive hemodynamic changes. Air embolism in the portal system has also been observed rarely.
In the first hour or so of life in a normal term infant, or for many hours and occasionally for many days in a sick or preterm infant, an umbilical vein catheter may be passed through the ductus venosus into the inferior vena cava.
Depending on the circumstances and preference of the physician, exchange transfusions can be done using either vessel or both, but not by infusing into the artery.
The umbilical vessel catheter should be removed as soon as possible and a peripheral intravenous line substituted, if necessary. In a nondistressed newborn infant requiring parenteral fluids, under no circumstances should an umbilical vessel catheter be used when a peripheral intravenous line could be started via a scalp vein or an extremity vein. In the extremely low-birth-weight group, in whom parenteral nutrition is essential until enteral feeds are established, common practice is to routinely place percutaneous indwelling central catheters and remove umbilical lines as soon as possible.
In the small premature infant, the entire catheterization should be done as an operating room procedure with the infant in an incubator or under a radiant warmer to prevent hypothermia. In the delivery room, a radiant heater should be used.
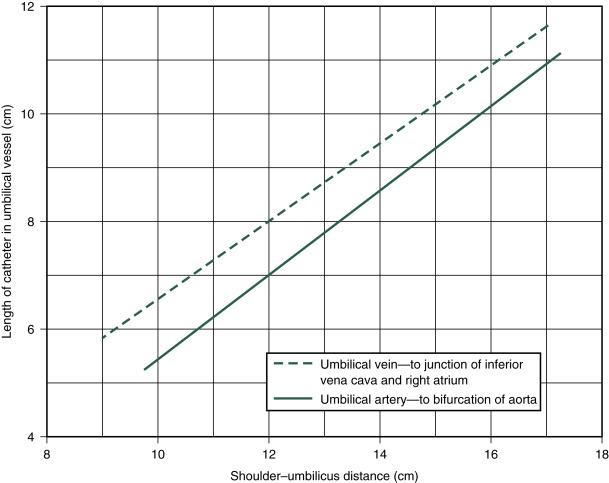
When not precluded by an emergency (e.g., acute asphyxia), the following protocol should be followed. The operator carefully scrubs hands and arms to the elbows and puts on sterile gloves. A 3.5F or 4F (for infants weighing <1500 g) or a 5F catheter with rounded tip, which has a radiopaque line and end hole (Argyle umbilical artery catheter, Coridien, Mansfield, MA), is attached to a syringe by a three-way stopcock. The system is filled with heparinized saline solution (1 U heparin per milliliter of normal or half-normal saline). ( Box D.2 lists the equipment found on the catheterization tray.) Before the procedure is begun, the length of the catheter to be inserted should be marked according to the location desired ( Figs. D.1 and D.2 ). After the umbilical stump and surrounding abdominal wall are carefully prepared with an antiseptic solution, sterile towels are placed around the stump, and a circumcision drape is placed with the hole over the stump. The base of the cord is loosely tied with umbilical tape, with care taken to avoid the skin. The cord stump is then grasped and cut perpendicular to its axis to within approximately 1.5 cm of the abdominal wall with a surgical blade. The exposed vessels are identified—thin-walled oval vein and two smaller thick-walled round arteries with tightly constricted lumens. Occasionally, only one artery is present. The cord is stabilized by grasping the Wharton jelly with one or two Kelly clamps.
2 Iris dressing forceps, 4 inch curved
1 Iris dressing forceps, 4 inch straight
2 Halsted mosquito forceps, 4 inch curved
2 Halsted mosquito forceps, 4 inch straight
1 Derf needle holder, 4.75 inches
1 Iris scissors, 4.5 inches
1 Operating scissors, 5.5 inches
1 Medicine cup
4 Gauze preparation sponges, 3 × 3 inch
1 Huck towel, folded
1 Steam chemical integrator
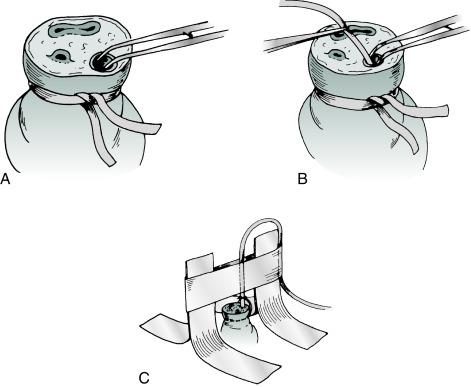
The lumen of the vessel to be used is gently dilated with curved Iris dressing forceps or a small obturator ( Fig. D.3A,B ). The catheter is then inserted and gently advanced. Obstruction at the level of the abdominal wall may be relieved by applying gentle traction on the umbilical cord stump accompanied by steady but gentle pressure for about 30 seconds. During umbilical artery catheterization, obstruction may also occur at the level of the bladder. It may be overcome by application of gentle, steady pressure for 30 seconds. Alternatively, marked resistance may be found where the umbilical artery meets the internal iliac artery (usually at 5 cm). The operator should avoid applying undue pressure to overcome this point of resistance because of the possibility of perforation of the vessel and severe hemorrhage. If continued resistance is met, the other artery should be used. If at any point during or after the line placement persistent blanching or cyanosis of the ipsilateral or contralateral extremity is observed, the catheter should be promptly removed. Cyanosis involving the toes or part of the foot on the side of the catheter may be relieved by warming the contralateral foot; if this is not successful, the catheter should be removed. Both lower extremities and buttocks should be carefully watched for alterations in blood supply when an umbilical artery catheter is in place.
If an umbilical vein catheterization is performed, the next site of obstruction after the abdominal wall is the portal system. (The catheter meets resistance several centimeters before the distance marked on the catheter is reached.) The catheter should be withdrawn several centimeters, gently rotated, and reinserted in an attempt to get the tip through the ductus venosus into the inferior vena cava. Gentle application of caudal traction to the umbilical cord stump sometimes facilitates the introduction of an umbilical venous line. Occasionally, it is not possible to get the catheter into the inferior vena cava for anatomic reasons, and vigorous attempts to advance the catheter are to be avoided.
An umbilical vessel catheter should be tied in place with a silk suture around the vessel and catheter and sutured to the umbilical stump or taped to the abdominal wall. Disastrous hemorrhage can occur if the catheter is inadvertently pulled out or the stopcocks are disconnected by the activity of the infant. The position of the catheter must be identified by radiography immediately after insertion. It is important that the umbilical vein catheter tip be at least well into the ductus venosus ( Fig. D.4 ) to protect the portal system from receiving hypertonic solutions.
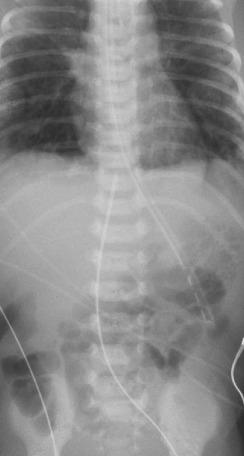
If the radiograph obtained after umbilical vessel catheterization indicates that the catheter has been inserted too far, it may be gently withdrawn an estimated amount for appropriate placement. If the catheter is not in far enough, it must be completely withdrawn and a new sterile one inserted after the area is appropriately prepared again.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here