Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Seizures commonly arise in persons without epilepsy as a symptom of various general medical disorders, and they can have important implications for the treatment and prognosis of the primary disease. The occurrence and management of seizures in common medical conditions are discussed in this chapter. Attention is also directed at certain less common medical diseases in which seizures are a relatively frequent complication, and at the treatment of pre-existing epilepsy in patients with medical conditions that might complicate management. Selected therapeutic agents and recreational drugs that may cause seizures are reviewed.
Several basic points deserve emphasis. First, in persons with pre-existing epilepsy, seizures frequently worsen during an acute medical illness that typically would not result in seizures. Their occurrence may relate to a lowering of the seizure threshold by metabolic derangements, fever, impaired sleep, or other factors. Antiseizure medications (ASMs) may have to be increased, and benzodiazepines frequently are given also to prevent seizures during the acute illness. These medication changes are usually temporary and do not need to be continued.
Second, seizures provoked in persons without pre-existing epilepsy by an acute metabolic derangement or brain insult are relatively common. Such seizures do not in themselves necessitate a diagnosis of epilepsy and are termed acute symptomatic seizures (previously termed provoked seizures or situation-related seizures). In these patients, recognizing and treating the underlying medical illness early is imperative. Acute symptomatic seizures may occur with disorders that are transient and reversible (e.g., alcohol intoxication) or in the acute phase of an irreversible brain injury (e.g., stroke) that may subsequently lead to the development of spontaneous recurrent seizures (epilepsy). Although persons experiencing acute symptomatic seizures from a brain insult may be at an increased risk for developing epilepsy in the future, this risk is clearly less than for unprovoked seizures and probably is explained by different neurophysiologic mechanisms. As a corollary, most persons with acute symptomatic seizures are started on ASMs during the acute illness, but do not require long-term ASM treatment.
Finally, acute symptomatic seizures in comatose or critically ill patients can be difficult to recognize, as seizures in these patients may have only subtle or no clinical manifestations. Electroencephalography (EEG) is required in such circumstances to evaluate for seizures and to guide therapies, as discussed in a later section.
Seizures are common in acute uremia, typically developing between 7 and 10 days after the onset of renal failure, while the patient is anuric or oliguric. By contrast, seizures are relatively unusual in chronic renal insufficiency, and usually only occur if a significant encephalopathy is present. Risk factors for acute symptomatic seizures in renal failure include metabolic disturbances, especially hypocalcemia and hypomagnesemia, which may increase the risk for convulsive status epilepticus, and impaired renal clearance of proconvulsant medications (e.g., penicillin).
Hypertensive encephalopathy is a classic disorder associated with renal failure and blood pressure fluctuations (with the level usually exceeding 180/120 mmHg) that presents with altered mental status and headache. Generalized convulsive seizures are seen in most patients and may be the presenting symptom. Treatment is with intravenous antihypertensive medications to reduce blood pressure by 25 percent within the first hour and then to less than 160/110 mmHg by 6 hours.
Hemodialysis also increases the risk for generalized convulsive seizures, usually toward the end of a hemodialysis session and for several hours thereafter, as part of the dialysis disequilibrium syndrome . Fluid shifts may be responsible, leading to cerebral edema from increased brain osmolality in the uremic state. Improved dialysis techniques have reduced the incidence of seizures. The dialysis encephalopathy (dementia) syndrome of myoclonus, asterixis, a distinctive speech disorder, psychiatric disturbances, and seizures due to increased aluminum levels in the brain has largely disappeared in clinical practice in response to removing aluminum from the dialysate.
Prominent myoclonus as well as asterixis (in which the hands flap due to momentary “negative” myoclonus) is often seen in renal failure. Usually, myoclonus occurs in the context of encephalopathy, and may or may not be epileptic. Cephalosporins, amlodipine, verapamil, diltiazem, and nifedipine may induce acute myoclonic jerking without convulsive seizures. Metformin in the presence of end-stage renal disease and pregabalin (frequently prescribed for diabetic neuropathic pain) may also induce myoclonus in patients receiving hemodialysis.
The treatment of seizures in renal failure requires both the correction of metabolic abnormalities and the use of ASMs. Several points regarding ASMs in renal impairment are important to remember. First, newer third- and fourth-generation ASMs tend to be cleared renally and should be used cautiously in patients with renal impairment or failure. If used, these newer ASMs usually require dose adjustments in the setting of reduced renal function. Conversely, earlier first- and second-generation ASMs do not usually require dose adjustments for renal function. However, uremic molecules may downregulate the expression of cytochrome P-450 enzymes, thereby decreasing hepatic metabolism and increasing the risks of drug toxicities for these earlier first- and second-generation ASMs that are hepatically metabolized ( Table 57-1 ).
| Volume of Distribution ( V d L/kg) (0.6= V d for H 2 O) | Renal Elimination | Hepatic Metabolism | Molecular Weight (Hemodialysis More Effective if Low) | ||
|---|---|---|---|---|---|
| First- and Second-Generation ASMs | |||||
| Minimal or no protein binding |
|
|
|
|
|
| Moderate protein binding | Phenobarbital | 0.5–1.0 | Minimal | High | 232 |
| High protein binding |
|
|
|
|
|
| Third- and Fourth-Generation ASMs | |||||
| Minimal or no protein binding |
|
|
|
|
|
| Moderate protein binding |
|
|
|
|
|
| High protein binding |
|
|
|
|
|
† The active metabolite 10-OH-carbamazepine is 40% bound (minimal-moderate).
§ Approved in Europe for SMEI (Dravet syndrome).
¶ The mean t(1/2) of perampanel is about 105 h, and steady state is achieved in 2–3 weeks; but t(1/2) increases to 305 h in mild hepatic impairment. Dosage adjustments are required in patients with mild or moderate hepatic impairment.
Second, many ASMs bind extensively to serum albumin ( Table 57-1 ). Patients with chronic renal disease are hypoalbuminemic, so protein binding is decreased; a larger amount of free drug is therefore available to exert a pharmacologic effect. Uremic molecules may also bind to plasma proteins, displacing drugs. For these reasons, the free serum level—where available—should guide therapy.
Third, some ASMs are cleared by dialysis. As molecular size decreases, solubility increases such that the fraction removed increases. Conversely, as protein binding increases and the volume of distribution decreases, the fraction removed declines. Highly lipophilic ASMs with large volume and tissue distribution are less available for removal by dialysis. This volume is independent of renal clearance, and usually is not modified in renal impairment ( Table 57-1 ). For this reason, ASMs that are cleared significantly by hemodialysis should be taken after the dialysis session or supplemental doses should be prescribed.
There are few data or systematic reviews on the use of third- and fourth-generation ASMs in renal failure, including patients on dialysis. Data for the pharmacokinetics of individual ASMs and recommended dosing adjustments for newer ASMs are summarized in Tables 57-1 and 57-2 , respectively.
| ASM Dose | |||||
|---|---|---|---|---|---|
| GFR 60–90 mL/min | GFR 30–60 mL/min | GFR 15–30 mL/min | GFR ≤15 mL/min | Hemodialysis | |
| Third- and Fourth-Generation ASMs | |||||
| Gabapentin | 300–1,200 mg t.i.d | 200–700 mg b.i.d | 200–700 mg/day | 100–300 mg/day | Plus 125–350 mg after HD |
| Levetiracetam | 500–1,000 mg b.i.d | 250–750 mg b.i.d | 250–500 mg b.i.d | 500–1,000 mg/day | Plus 250–500 mg after HD |
| Topiramate | 50% decrease for ≤70 mL/min | 50% decrease | 50% decrease | Plus 50–100 mg after HD | |
| Zonisamide | 100–400 mg/day | 100–400 mg/day | |||
| Oxcarbazepine | 300–600 mg b.i.d | 300–600 mg b.i.d | 300 mg/day (starting dose) | ||
| Felbamate | None | 50% decrease | |||
| Lamotrigine * | None | None | None | None | |
| Brivaracetam | None | None | None | None | Not recommended (not studied) |
| Vigabatrin | 25% decrease | 50% decrease | 75% decrease | ||
| Rufinamide | None | None | None | Plus 30% of dose after HD | |
| Lacosamide | None | None | 300 mg/day | Plus ≤50% replacement dose | |
| Perampanel | None | Close monitoring | Not recommended | Not recommended (not studied) | |
| Eslicarbazepine | None | 400–600 mg/day | 400–600 mg/day | ||
| Clobazam | None | None | None | None | |
| Pregabalin | None | 50% decrease | 25–150 mg/day | 25–75 mg/day | Plus 25–150 mg after HD |
* The product monograph for Lamictal XR recommends reduced maintenance doses in patients with significant renal impairment, and caution in patients with severe renal impairment. Lamotrigine pharmacokinetics were essentially unchanged in a study comparing 10 subjects with renal failure (estimated creatine clearance of 10 to 25 mL/min) to 11 healthy subjects (Wootton R, Soul-Lawton J, Rolan PE, et al: Comparison of the pharmacokinetics of lamotrigine in patients with chronic renal failure and healthy volunteers. Br J Clin Pharmacol 43:23, 1997).
Seizures are relatively uncommon in patients with acute hepatic encephalopathy, if seizures related to alcohol withdrawal are excluded. Either focal or generalized seizures may occur, usually in severe hepatic encephalopathy. Treatment involves management of the hepatic dysfunction and hepatic encephalopathy.
Chronic liver disease does not usually cause convulsions. The occurrence of seizures in alcoholics with hepatic cirrhosis is usually related to alcohol withdrawal. Seizures are common in Reye syndrome and rare in Wilson disease. Convulsions in patients with acute hepatic necrosis are frequently associated with severe hypoglycemia. In these patients, ASM therapy often is not required except when an underlying cause of epilepsy (e.g., prior trauma in an alcoholic) is present.
Gabapentin, vigabatrin, and levetiracetam are the only ASMs not hepatically metabolized. Certain ASMs, including selected newer agents, undergo extensive hepatic metabolism ( Table 57-1 ), but unless hepatic dysfunction is severe its effects on their pharmacokinetics are difficult to predict. Barbiturates, and benzodiazepines, as well as other central nervous system (CNS) depressants, may unmask hepatic encephalopathy in patients with compensated liver disease, and are relatively contraindicated. Valproic acid is potentially hepatotoxic and should be used with care in patients with pre-existing liver disease. The free fraction of highly protein-bound ASMs may increase due to hypoalbuminemia, and (as for renal impairment) the free serum level—where available—should guide therapy.
In persons without hepatic disease, ASMs may cause drug-induced hepatic injury. In most cases, these reactions are reversible by stopping the ASM but, occasionally, acute hepatic failure may necessitate transplantation. ASMs (as a class) were the third most-common cause (phenytoin, n =10; valproic acid, n =10) of acute drug-induced liver failure requiring liver transplantation in the United States between 1990 and 2002 ( n =270).
Overall, the incidence of clinically important hepatotoxicity seems less for third- and fourth-generation ASMs than for older ASMs, but cases of severe hepatotoxicity have been reported for newer ASMs, including lamotrigine, levetiracetam, gabapentin, pregabalin, and oxcarbazepine. Felbamate specifically has been associated with multiple cases of aplastic anemia and acute liver failure, and has a “black box warning” label that restricts its use.
Two types of hepatic injury by ASMs are described. First, ASMs may induce one of several systemic immune-mediated drug hypersensitivity syndromes that may cause hepatic injury and are potentially life-threatening. These syndromes are characterized by fever, rash, and increased serum levels of aminotransferases several weeks after starting an ASM. The risk for these hypersensitivity reactions is highest for first- and second-generation ASMs, and is estimated to be 1 to 10 per 10,000 for phenytoin, carbamazepine, and phenobarbital, but also for lamotrigine (third-generation). For carbamazepine specifically, the HLA-B*15:02 allele is strongly associated with Stevens–Johnson syndrome/toxic epidermal necrolysis in Southeast Asia. For this reason, the U.S. Food and Drug Administration (FDA)–approved drug label for carbamazepine now recommends testing for HLA-B*15:02 for all patients with ancestry in populations having an increased frequency of HLA-B*15:02 , prior to initiating carbamazepine therapy.
Second, first- and second-generation ASMs may cause hepatic injury as a direct effect of hepatic metabolism, and not as a systemic hypersensitivity syndrome. This is a concern specifically for felbamate and (especially in children) valproic acid. For valproic acid, the risk for hepatic injury in young children less than 2 years old and on multiple ASMs is as high as 1 in 800, and the drug is relatively contraindicated in this population. In adults and children, hyperammonemia may be seen with normal liver function tests in the majority of persons on valproic acid, usually with no associated clinical symptoms. Topiramate may increase this risk of hyperammonemic encephalopathy and hepatic injury from valproic acid.
Cannabidiols (CBD) derived from Cannabis sativa have become very popular in recent years for a variety of conditions, particularly pain. In June 2018, the first CBD-based drug, Epidiolex, was approved by the FDA for treatment of selected severe epilepsy syndromes (Dravet and Lennox–Gastaut syndromes). In the trials on which approval was based, CBD was associated with elevated serum levels of aminotransferases. Except for Epidiolex, CBD products are not regulated and the risks for hepatic injury are unclear.
Epidemiologic studies have consistently shown that people with epilepsy have a higher prevalence of structural cardiac disease than those without epilepsy, possibly reflecting shared genetic risk factors that affect the heart and brain. Cardiovascular disease also seems to be a significant contributor to the increased mortality of people with epilepsy, compared with the general population. As a group, patients with epilepsy have lower heart rate variability than normal, a finding that predicts arrhythmias and mortality in cardiac patients but is of uncertain significance in epilepsy.
Cardiac disease may also cause epilepsy indirectly due to stroke or from global cerebral ischemia after cardiac arrest. Stroke accounts for about one-third of newly diagnosed seizures in people over the age of 60 years.
The use of first- and second-generation ASMs may also increase cardiovascular disease risks in persons with epilepsy. The enzyme-inducing ASMs phenytoin or carbamazepine may lead to elevated serologic vascular risk markers (e.g., total cholesterol, low-density lipoproteins, homocysteine) and atherosclerosis. Some first- and second-generation ASMs (e.g., valproic acid, carbamazepine) cause weight gain and may increase the risk of developing nonalcoholic fatty liver disease and metabolic syndrome.
Cardiovascular disease and epilepsy may co-exist, especially in the elderly, persons with congenital cardiac disease, and persons with alcohol and polysubstance use. This complicates treatment of acute seizures and status epilepticus. Intravenous benzodiazepines may cause hypotension in medically ill or elderly patients. Rapid intravenous administration of phenytoin may cause sinus arrest and hypotension; fosphenytoin, a water-soluble prodrug of phenytoin that does not require propylene glycol as a diluent, is generally preferred for treating status epilepticus as its infusion rate is faster and there is a lesser risk of arrhythmia than with phenytoin. In these patient populations, valproic acid, levetiracetam, or lacosamide are preferred if fosphenytoin is not available.
Various cardiac arrhythmias have been described in persons with epilepsy, including ictal asystole (usually self-limiting), postictal asystole (not self-limiting), ventricular tachycardia/ventricular fibrillation, bradycardia, and atrioventricular block. In some epilepsies, these abnormalities may reflect genetic ion channel mutations expressed in the brain as well as in the heart, and may in part explain the increased risk for sudden unexplained death in epilepsy (SUDEP). The most recognized example of this is Dravet syndrome, a severe epilepsy syndrome with high premature mortality, caused by an SCN1A mutation. Biomarkers associated with the risk of sudden cardiac death (e.g., decreased heart rate variability) have been found in patients with this syndrome. In mutant SCN1A knock-out mice, postictal bradycardia and seizure-triggered ventricular fibrillation have been recorded before a death resembling SUDEP.
ASMs that affect sodium channels may influence cardiac excitability and conduction, but clinically relevant arrhythmias are usually related to ASM overdose. Long-term ASM use has been associated only rarely with significant cardiovascular complications. A prolonged PR interval is seen in patients on lacosamide and carbamazepine. Symptomatic arrhythmias have been reported in patients receiving lacosamide and carbamazepine in therapeutic dosages, but underlying cardiac abnormalities or the use of multiple sodium channel-blocking ASMs usually (but not always) have been present. Shortening of the QT interval is seen in patients on rufinamide. Routine electrocardiograms should be considered in all patients started on these ASMs and in all patients with pre-existing cardiac arrhythmias.
Anticonvulsant agents may also interact with certain cardiac medications. The concomitant use of phenytoin and quinidine may increase ectopy in patients with ventricular arrhythmias. The metabolism of quinidine, digoxin, lidocaine, and mexiletine may be increased by phenytoin and phenobarbital because of induction of hepatic microsomal enzymes. Amiodarone increases phenytoin levels, and calcium-channel blocking agents, such as verapamil, may increase serum carbamazepine concentrations. For these reasons, serum ASM levels and cardiovascular function should be followed closely when either antiseizure or cardiac medications are introduced or altered.
Acute symptomatic seizures in patients who are critically ill are detected typically on continuous EEG monitoring, may have subtle or no clinical signs, and are probably under-recognized. The published incidence of seizures in critically ill patients is highly variable but is nonetheless impressive, ranging from 8 percent to as high as 48 percent in various studies. The possibility of nonconvulsive seizures and status epilepticus should be suspected in any critically ill patient with altered mental status of unknown cause. Subtle motor activity, such as rhythmic twitching of the fingers or eye movements, should raise suspicion of seizure activity in this setting. Diagnosis depends on the EEG findings. Prompt recognition and treatment are essential because delay is associated with a poor outcome.
Continuous EEG monitoring of brain function in critically ill patients usually includes simultaneous video recording and quantitative analysis of EEG trends ( Fig. 57-1 ). The guideline recommendation of the American Clinical Neurophysiology Society for recording for at least 24 to 48 hours using continuous EEG enables the identification of 80 to 95 percent of patients with nonconvulsive seizures. Monitoring should be initiated as soon as possible when nonconvulsive seizures are suspected, and is required in newly admitted critically ill patients with altered mental status and observed seizures.
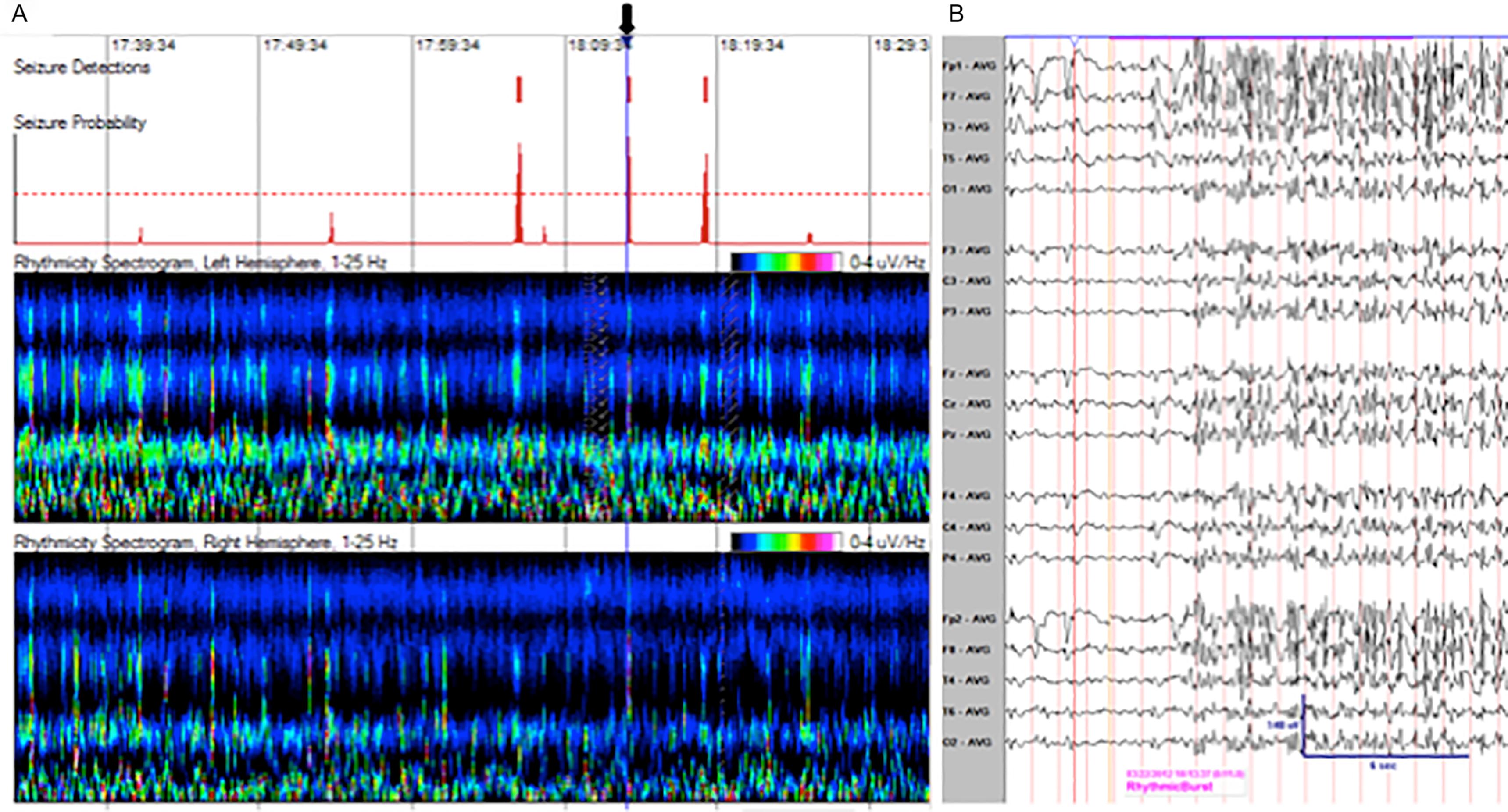
When evaluating the causes of seizures in critically ill patients, various metabolic derangements must be considered; if detected, therapy is directed at their correction. The electrolyte abnormalities contributing most frequently to seizures are reviewed in a later section. Reversible causes, such as medications (or acute withdrawal of them, including anticonvulsants, benzodiazepines, and barbiturates) and substance abuse are important ( Table 57-3 ).
| Class | Examples | Notes |
|---|---|---|
| Antimicrobials | ||
| β-Lactams | Penicillins, cephalosporins, carbapenems | β-Lactams interact with the GABA A receptors, interfering with inhibitory effects of GABA in a concentration-dependent manner. |
| Antituberculous agents | Isoniazid | Isoniazid inhibits pyridoxine phosphokinase, the enzyme that converts pyridoxine for the synthesis of GABA from glutamate, reducing GABA. Give pyridoxine and benzodiazepine. |
| Antimalarials | Mefloquine, chloroquine | |
| Fluoroquinolones | Ciprofloxacin | May inhibit the GABA A receptor; seizures are rare but have been reported. |
| Analgesics | ||
| Opioids | Morphine, tramadol, codeine, fentanyl | All opioids appear capable of lowering the seizure threshold. |
|
Theophylline, aminophylline | Interacts with adenosine A1-receptor; also interferes with activity of BZD. |
| Antipsychotics | Clozapine (and to a lesser extent, other antipsychotics including risperidone, olanzapine) | Seizures affect 3–6% of clozapine-treated patients. |
| Antidepressants | Buproprion | A recent systematic review found no evidence for an increase in seizure frequency with other classes of antidepressants. Seizures may be seen in toxicity with other antidepressants. |
| Antiseizure medications | All | Most can induce paradoxical seizures in overdose; carbamazepine and oxcarbamazepine can exacerbate generalized epilepsies. Stopping them without taper seems invariably to lower seizure threshold. |
When ASM treatment is necessary, the increased potential for complex drug interactions in patients receiving numerous other medications and the possibility of altered pharmacokinetics (e.g., due to renal or hepatic impairment) and of adverse systemic effects must be considered. For example, in hypoalbuminemic patients, it may be necessary to monitor free drug levels when using a first- or second-generation ASM (e.g., phenytoin) that exhibits significant serum protein binding. Many first- or second-generation ASMs that are inhibitors or inducers of certain metabolic enzymes may also affect the serum concentration of other medications taken concurrently.
Ideally, ASMs should initially be administered parenterally as loading doses to attain therapeutic serum concentrations, especially in patients with gastrointestinal dysfunction, so limiting the selection of ASMs to those with intravenous formulations.
The use of continuous renal replacement therapy (CRRT) in critically ill patients complicates the use of the third- and fourth-generation ASMs, as most of these are cleared renally. There are as yet no data available for ASM dosing in patients on CRRT. For this reason, ASMs with readily measured serum levels are preferred to guide dosing in patients on CRRT. In general, an ASM that is eliminated renally or affected by dialysis will be removed using CRRT, but the degree of removal will depend largely on the replacement parameters and the specific ASM.
The use of extracorporeal membrane oxygenation (ECMO) is also increasing in critically ill patients, but as for CRRT, no data are available for evaluating specific ASM dosing. The biggest impact of ECMO on drug dosing lies in the propensity for the ECMO circuit to sequester drugs, resulting in a larger than expected volume of distribution. This phenomenon may decrease over time with continued dosing due to saturation of binding sites. In general, medications with higher volumes of distribution/lipophilicity and higher protein binding tend to be sequestered more. Patients on ECMO frequently receive concomitant CRRT, further complicating dosing. For these reasons, ASMs that have readily available serum levels are strongly preferred.
A high proportion of patients who have been resuscitated after a cardiac arrest go on to develop seizures due to global anoxic-ischemic cerebral injury. Seizures typically commence within 24 hours of cardiopulmonary arrest and may consist of generalized tonic-clonic, tonic, myoclonic, or partial seizures, as well as tonic-clonic or myoclonic status epilepticus. Electrographic status epilepticus with restricted clinical manifestations (usually limited to extraocular or facial muscles) is also well described after cardiopulmonary arrest, occurring in 12 to 22 percent of patients, but may be difficult to recognize.
Published guidelines have emphasized the importance of neurocritical care for patients with brain injury after cardiac arrest, including EEG monitoring for the detection and treatment of seizures. Certain EEG patterns are associated with poor outcome. Thus, generalized background suppression to 20 µV, a burst-suppression pattern with generalized epileptiform activity, or generalized periodic complexes on a flat background are strongly but not invariably associated with poor outcome.
The occurrence of focal seizures or even of generalized tonic-clonic status epilepticus does not determine the eventual clinical outcome of patients in postanoxic coma. By contrast, myoclonic status epilepticus (defined as spontaneous, repetitive, unrelenting, generalized multifocal myoclonus in comatose patients) seems invariably associated with poor outcome and may well represent a marker of severe anoxic-ischemic brain injury. Although hypothermia has antiseizure effects, seizures in resuscitated patients after cardiac arrest may occur during therapeutic hypothermia and, in many patients with nonconvulsive status epilepticus, seizures first appear on EEG during therapeutic hypothermia.
The available evidence does not support the use of prophylactic ASMs in these patients. The same ASMs used for the treatment of status epilepticus from other causes may be considered for seizures and status epilepticus after cardiac arrest, but bearing in mind the potential cardiac effects of selected ASMs reviewed earlier. The neurologic complications of anoxic-ischemic encephalopathy are reviewed in detail in Chapter 9 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here