Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
More than 3000 new cases of bone sarcoma are diagnosed annually in the United States.
No specific etiologic agents are identified in the majority of cases.
Secondary neoplasms are related to known oncogenic factors (e.g., ionizing radiation, alkylating chemotherapy agents, combinations of both).
Hereditary cancer syndromes (tumor suppressor genes) are responsible for some cases.
Plain radiographs are recommended for initial diagnosis.
Magnetic resonance imaging (MRI) of the primary tumor is the best radiographic study to perform for surgical planning.
Chest computed tomography (CT) is indicated to evaluate for pulmonary metastases.
Whole-body technetium-99m ( 99m Tc) bone scan is indicated to evaluate for metastases to other bones.
Positron emission tomography (PET) scanning is preferred for some sarcomas of bone, particularly Ewing sarcoma.
Needle or open biopsy is necessary for a tissue-specific diagnosis and to determine histologic grade.
In the pathology review, immunohistochemistry and cytogenetics are important.
Molecular genetic studies can be helpful for some sarcomas of bone.
Metastasis at presentation is an adverse prognostic finding.
Histologic grade is the next most significant prognostic indicator.
Size is less significant, but lesions larger than 10 cm in diameter have a poor prognosis.
Tumor response to neoadjuvant chemotherapy is prognostic in osteosarcoma and Ewing sarcoma.
Surgical margins of resection (minimum of a “wide” margin) have a significant effect on local control and, to some extent, overall survival.
The American Joint Committee on Cancer now monitors grade (high and low) and size (8 cm); designates “skip” lesions (T 3 ); and separates metastasis to bone from other sites (Mla, Mlb).
The Musculoskeletal Tumor Society monitors location (intracompartmental and extracompartmental), grade (high and low), and metastasis (skip lesions, nodal, bone, and lung are all lumped together).
A wide surgical margin is recommended.
Limb-sparing procedures are appropriate for 70% to 90% of patients.
Adjuvant irradiation is not routinely used for bone sarcomas.
Local recurrence rates for limb-sparing procedures approach 5% or less.
Reconstruction methods can be tailored to patients’ needs.
New and improved biocompatible implant materials and improved designs are available.
The search continues for new drugs, drug schedules, potentiating agents, and improved dose intensity.
Identification of risk factors (e.g., cytogenetic, molecular genetic, and signal transduction abnormalities) will improve to identify new methods of potential treatment.
It is estimated that 3260 new malignant tumors of bone (excluding multiple myeloma) will be diagnosed in the coming year in the United States, representing 0.2% of all new cancer cases. The femur is the most common site, but primary sarcoma can occur in any bone. Osteosarcoma, Ewing sarcoma, and chondrosarcoma account for approximately 90% of all primary sarcomas of bone. The management of osteosarcoma and Ewing sarcoma includes chemotherapy and surgery, whereas chondrosarcoma is treated by surgery alone. The management of these patients, from initial evaluation and biopsy through surgical therapy and long-term follow-up, is labor-intensive and technically demanding. Patients with a bone sarcoma should be treated in a center that has expertise in the management of these tumors.
Currently, the staging system adopted by the Musculoskeletal Tumor Society (MSTS) in 1980 and modified in 1986 is accepted by most musculoskeletal surgical oncologists. Malignant tumors are divided into only two histologic grades: low-grade malignant (G1) and high-grade malignant (G2). Low-grade malignant lesions (G1), comprising Broder I and II lesions, have a low probability of metastasis (25% or less). The majority of these tumors can be managed with relatively conservative surgical procedures and do not require chemotherapy. High-grade lesions (G2), Broder III and IV tumors, have a significantly higher incidence of metastases and require more radical surgical procedures and possibly neoadjuvant and/or adjuvant chemotherapy. Table 89.1 is a representative grouping of both low- and high-grade malignant tumors of bone and soft tissue origin.
| Low (G1) | High (G2) |
|---|---|
| Parosteal osteosarcoma | Classic intramedullary osteosarcoma |
| Periosteal osteosarcoma (typically intermediate grade) | High-grade surface osteosarcoma |
| Low-grade central osteosarcoma | Secondary osteosarcoma (Paget sarcoma of bone, radiation-induced sarcoma of bone |
| Secondary chondrosarcoma | Primary chondrosarcoma |
| Clear cell chondrosarcoma | Dedifferentiated chondrosarcoma |
| Mesenchymal chondrosarcoma | |
| Fibrosarcoma | Fibrosarcoma |
| Undifferentiated high-grade pleomorphic sarcoma of bone (malignant fibrous histiocytoma [MFH] of bone) | |
| Adamantinoma | |
| Leiomyosarcoma of bone | |
| Malignancy in giant cell tumor of bone | |
| Epithelioid hemangioma | Epithelioid hemangioendothelioma Angiosarcoma |
| Chordoma | Ewing sarcoma family of tumors |
Bone sarcomas that are totally intraosseous are intracompartmental, designated A. Those that penetrate the cortex are considered extracompartmental, designated B.
Patients without evidence of metastatic disease after radiographic staging are designated M0. In general, metastatic disease that is evident in the lung, in the lymph nodes, in other bones, or as an intramedullary “skip” lesion indicates a poor prognosis and is designated M1.
Surgical procedures are defined by the relationship of the circumferential surgical plane of dissection and the pseudocapsule. Surgical margins are defined as intralesional, marginal, wide, and radical. Examples of intralesional margins include curettage of a presumably benign tumor and cytoreductive debulking procedures. Marginal margins, achieved when the plane of dissection passes through the reactive zone of the pseudocapsule, are suitable for management of the majority of benign tumors. Such margins are accomplished when the surgeon “shells out” a neoplasm, cleaving the tissue between the reactive zone and the zone of compression. This technique leaves behind viable tumor satellites at the periphery of the lesion; thus marginal margins are not sufficient for local control of malignant or benign “aggressive” lesions.
A wide margin is obtained when the plane of dissection passes through absolutely normal nonreactive tissue that is distant from the pseudocapsule. Wide margins are sufficient for virtually all bone sarcomas, although the exact amount of tissue necessary to achieve a safe, wide margin has not been established and likely depends on the type of tissue that forms the margin. Fascia, for instance, is considered to be a better margin than fat, and thus a thinner fascial margin can be accepted compared with a fatty margin. It is presumed that pretreatment with chemotherapy and/or radiotherapy allows the surgeon to resect less normal tissue with the tumor than if no pretreatment is given.
Radical margins of a bone sarcoma are achieved when the entire involved bone and soft tissue compartments are removed. This process usually requires an amputation. A radical margin is rarely necessary and seldom performed.
The American Joint Committee on Cancer (seventh edition) has adapted the TNM (tumor-node-metastasis) staging system to bone. The topography (T) of the primary tumor now includes size based on relevant published reviews, in which the greatest dimension (8 cm) has replaced the compartment concept. Also, T3 has now been assigned to patients with skip metastases ( Table 89.2 ).
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor ≤8 cm in greatest dimension |
| T2 | Tumor >8 cm in greatest dimension |
| T3 | Discontinuous tumors in the primary bone site |
The problem of defining histopathologic grade (G) has been addressed, and now essentially all bone sarcomas are categorized as low- or high-grade lesions ( Table 89.3 ). G1 and G2 have been combined into low-grade and G3 and G4 into high-grade histopathologic types. Currently, all Ewing tumors are classified as G4 or high grade. This grouping is now identical to the G1 and G2 categories of the MSTS staging system.
| GX | Grade cannot be assessed |
| G1 | Well differentiated—low grade |
| G2 | Moderately differentiated—low grade |
| G3 | Poorly differentiated—high grade |
| G4 a | Undifferentiated—high grade |
Table 89.4 shows the stage groupings. Here, the committee has appropriately addressed the difference in prognosis of patients who have sustained metastases to lung (Mla) versus other sites, including bone (Mlb).
| Stage 1A | T1 | N0 | M0 | G1, 2 low grade, GX |
| Stage 1B | T2 | N0 | M0 | G1, 2 low grade, GX |
| T3 | N0 | M0 | G1, 2 low grade, GX | |
| Stage IIA | T1 | N0 | M0 | G3, 4 high grade |
| Stage IIB | T2 | N0 | M0 | G3, 4 high grade |
| Stage III | T3 | N0 | M0 | G3, 4 high grade |
| Stage IVA | Any T | N0 | M1a | Any G |
| Stage IVB | Any T | N1 | Any M | Any G |
| Any T | Any N | Mlb | Any G |
Conventional bone radiography remains the single most useful initial study for bone tumor evaluation. The study should include anteroposterior and lateral projections of the lesion and the whole bone in which the lesion is present. Malignant neoplasms usually result in ill-defined or “poorly marginated” radiographic margins with little or no reactive bone, loss of medullary trabeculation, and endosteal cortical erosion at the tumor–host bone interface, suggesting an active and destructive process. The pathologic process biologically overwhelms the normal time-dependent reactive processes of bone formation. Therefore the radiographic presence or absence of a reactive rim of bone is often useful in predicting the biologic aggressiveness of the pathologic process ( Table 89.5 ). Neoplastic bone formation is often seen in osteosarcoma, and calcification is often seen in chondrosarcoma.
|
The majority of bone sarcomas are best evaluated for extent and surgical planning with a single well-planned magnetic resonance imaging (MRI) examination with use of specific predetermined planes and images with intravenous contrast. T1-weighted images produce superior anatomic detail, whereas T2-weighted images best characterize the structure and composition of the lesion (solid, homogeneous, heterogeneous, cystic, or combinations of these characteristics). A T1-weighted coronal sequence of the entire bone is essential for judging the extent of the tumor in the bone itself and for planning surgical resection margins. The use of intravenous contrast material is valuable in assessing the tumor's vascularity and to reveal the tumor's relationship to the neurovascular bundle.
Overall, MRI provides optimal sagittal, coronal, and multiaxial anatomic detail in both soft tissues and bone. MRI data are indispensable in assessing the intramedullary extent of the lesion, intraarticular extension of tumor, and skip metastases either within the contiguous medullary canal or across adjacent joint surfaces.
Currently, MRI of the primary tumor appears somewhat predictive of tumor response to neoadjuvant therapy. Changes in the T2-weighted image signal intensity correlate with an obvious reduction in tumor volume (especially in Ewing sarcoma) and appear predictive of tumor necrosis. The addition of contrast enhancement does not appear to provide more viable tumor or necrotic tumor contrast than in T2-weighted images; however, the absence of contrast enhancement appears to be an indicator of tumor necrosis.
Computed tomography (CT) is superior to MRI only for evaluating a small lesion in the cortex, subtle bone formation, or calcification; otherwise, MRI is the study of choice for the primary site of a suspected sarcoma of bone. CT remains the standard for evaluation of the chest for occult metastases. The cross-sectional display usually provides sufficient resolution (<0.5 cm) to demonstrate subpleural metastases long before they become evident on plain chest films. Before definitive therapy or local management of a potentially malignant lesion, a staging CT evaluation of the chest and mediastinum should be performed.
The use of technetium-99m ( 99m Tc) bone scintigraphy remains the standard for surveying the skeleton for multiple osseous lesions. The test can be administered as a single, delayed, static study or can be displayed in multiple timed phases to evaluate the vascularity of the lesion. It is important to obtain a whole-body bone scan.
Positron emission tomography (PET) has been used to predict response to chemotherapy for Ewing sarcoma and osteosarcoma. PET scan might also be valuable in the initial screening for some sarcomas and has become more accepted as the standard initial staging modality for metastatic disease in Ewing sarcoma.
The staging biopsy might well be the most important and difficult procedure that is performed in the patient's management ( Box 89.1 ). The old adage that the surgeon who is going to perform the resection should do the biopsy may no longer apply now that core needle biopsies are more commonly performed than open biopsies. However, the principle still pertains in that it is critical that the radiologist performing the biopsy and the surgeon who will do the resection communicate and agree on the biopsy placement and trajectory. The placement, length, and orientation of the biopsy scar and the anatomic compartments that are contaminated during the biopsy procedure dictate which tissues and how many surgical compartments will require removal for local tumor management and limb-sparing surgery. A thorough knowledge of the soft tissue anatomic planes and muscle compartments is mandatory before proceeding with bone biopsy. Consideration should be given to the location and type of biopsy to be used, whether fine-needle aspiration biopsy, core needle biopsy, or open biopsy. In addition, it is critical that adequate diagnostic tissue be obtained so that an accurate diagnosis can be made. Usually, a core needle biopsy is sufficient.
Planning: biopsy tract must be in line of potential resection
Traverse only one compartment and only one muscle if possible
Avoid contamination of joint
Microbiologic culture
Prolonged gentle pressure for hemostasis
Planning: plan most appropriate biopsy tract, avoid transverse incisions
Use pneumatic tourniquet after gravity exsanguination
Avoid contamination of joint
Avoid exposure of neurovascular structures
Monitor biopsy with frozen sections
Microbiologic culture if there is question
Maintain integrity of deep tumor-host margin; could extend necessary surgical margin
Absolute hemostasis; thrombogenic agents (e.g., thrombin, Oxycel, Avitene)
Hemostatic closure of pseudocapsule
Subcuticular closure
Osteosarcoma, the most common primary sarcoma of bone, is a complex and heterogeneous group of neoplasms. It is defined as a sarcoma that directly produces tumor osteoid or bone. The classic osteosarcoma is a high-grade lesion, but there are many variants that vary in grade and behavior. Classic osteosarcoma is characterized by a bimodal age distribution, with the first peak in the second decade of life and the second later in life, in the sixth and seventh decades, although it can occur at any age. Osteosarcoma in adults is often associated with other underlying disease processes such as Paget disease of bone, bone infarct, and prior radiation.
High-grade osteosarcoma used to be a fatal neoplasm leading to metastases and death of 90% of patients despite aggressive local control, including radical amputations and/or radiotherapy. Previous versions of this chapter outlined the evolution of the use of adjuvant chemotherapy and the dramatic improvement in survival and event-free survival (EFS) that followed. Initially, the benefits of adjuvant chemotherapy were questioned, leading to the need for a randomized study comparing surgical management alone with surgery followed by multiagent chemotherapy. The active drugs were shown to be doxorubicin, high-dose methotrexate, and cisplatin. A randomized study, including both the randomly assigned patients and those who chose whether or not to have adjuvant chemotherapy, clearly demonstrated the survival benefits of adjuvant chemotherapy.
Surgical advances paralleled the advances in medical management of osteosarcoma patients. Originally, disarticulation or resection of the entire involved bone was recommended for surgical management of osteosarcoma. This recommendation was partly a result of the intramedullary origin of the tumor with proximal intramedullary growth and the reported 25% incidence of intramedullary skip metastases. Later studies reviewing the local recurrence rates for patients whose primary management was transmedullary amputation alone revealed local recurrences in approximately 5% to 10%, suggesting that the incidence of skip or intraosseous metastasis was probably lower than originally believed. The general standard of surgical management of patients with extremity osteosarcoma in 1980 included transmedullary amputation approximately 5 to 7 cm proximal to the intramedullary extent of the tumor.
Limb salvage therapy became more widespread in the 1980s with chemotherapeutic advances. Surgeons learned from examining amputation specimens that the nerves and vessels could often be separated from the tumor with a plane of normal tissue between the tumor and the neurovascular bundle, and imaging improved, so this could be determined accurately before the operation. MRI revolutionized the surgeon's ability to determine the extent of the tumor within the medullary cavity and soft tissues preoperatively.
Advances in design of custom prostheses subsequently made it possible to preserve mobile joints, borrowing from advances in arthroplasty for arthritis. Bone allografts were also used to reconstruct limbs after tumor resection and included osteoarticular grafts, allograft-arthrodeses, intercalary reconstructions, and allograft-prosthetic composites. Finally, the advent of modular prostheses, rather than custom ones, allowed the surgeon to custom design the implant for the specific defect in the operating room, and these modular prostheses are in current use and constantly undergoing design improvements.
One event that led to the popularization of limb salvage was the use of preoperative chemotherapy. Initially implemented during the time it would take to manufacture a custom prosthesis, it became apparent that the tumor showed clinical and radiographic “response” to the preoperative (neoadjuvant) chemotherapy. This seemed to make the subsequent surgery easier, if not safer, and gave the surgeon 10 to 12 weeks to work with the patient to decide on the best surgical option. There was initial concern that the delay in the resection might worsen the prognosis, but this issue was addressed in a randomized trial that showed no apparent advantage to having the surgery initially compared with the neoadjuvant mode of administration. In addition, it was learned that the histologic response to the preoperative chemotherapy was a predictor of outcome and, second only to the presence of metastases at diagnosis, was the best predictor of survival.
Conventional, or classic, osteosarcoma makes up the majority of all osteosarcomas. It occurs primarily in the metaphyses of adolescents with open physes or in young adults. Most patients with classic osteosarcoma are younger than 30 years, and many have no apparent predisposing factors. The lesion most often arises in the larger, more active metaphyses (e.g., distal femur, proximal tibia, proximal humerus), but it also can arise in the flat bones of the pelvis, skull, scapula, and ribs and in the spine. Overall, the majority of the lesions develop in the extremities and pelvis.
An estimated 3000 malignant bone tumors are diagnosed in the United States each year. Approximately 40% to 50% of these patients have classic or conventional osteosarcomas. Males are affected slightly more often than females. Classic osteosarcoma develops in females slightly earlier than in males, and there appears to be no race predilection. Although the common histologic presentation of malignant cells producing osteoid would suggest a homogenous group of tumors, the morphologic appearance can vary considerably, ranging from classic osteoblastic osteosarcoma (45% of cases) through fibroblastic (9%), chondroblastic (27%), anaplastic (17%), telangiectatic, low-grade central, and other osteosarcomas (2%).
An epidemiologic study conducted in Sweden between 1971 and 1984 investigated whether there were changes in the typical epidemiologic features of 227 conventional osteosarcomas. The mean annual incidence was 2.1 per million. The male-to-female ratio of 1.6:1 remained unchanged over the study period, as did the location and distribution of the tumors. The only clear change over the study period was an increase in the age of patients beyond the classic peak age range of 10 to 29 years.
In a study in the United States, the incidence rates of osteosarcoma for all races and both sexes were 4.0 (95% confidence interval [CI], 3.5 to 4.6) for the range 0 to 14 years and 5.0 (95% CI, 4.6 to 5.6) for the range 0 to 19 years per year per 1 million persons. Among childhood cancers, osteosarcoma occurs eighth in general incidence. Osteosarcoma has a bimodal age distribution, having the first peak during adolescence and the second peak in older adulthood. The first peak is in the 10- to 14-year-old age group, coinciding with the pubertal growth spurt. This suggests a close relationship between the adolescent growth spurt and osteosarcoma.
About 10% of patients with osteosarcoma are older than 60 years. This group composes the second peak of the bimodal age distribution curve. In these older patients, the anatomic region of presentation differs substantially from the sites of classic osteosarcoma. Whereas lesions develop in the region of the knee (the largest and most active physes) in more than 50% of patients with classic osteosarcoma in adolescence, osteosarcoma at that site develops in only 15% of the older patients. Moreover, osteosarcomas in the older population characteristically occur in regions that have had previous radiotherapy, underlying Paget disease of bone, fibrous dysplasia, or some other pathologic abnormality. In many ways, the older group can be thought of as having “secondary” osteosarcoma.
A separate group of osteosarcoma variants, including high-grade surface, extraskeletal, pagetoid, intracortical, low-grade central osteosarcoma, parosteal and periosteal osteosarcomas, small cell osteosarcoma, secondary tumors, and therapy-related tumors, will be discussed in the section Osteosarcoma Variants .
Osteosarcoma is considered a sporadic complex genotype sarcoma, as distinguished from the balanced translocation-associated sarcomas (e.g., Ewing sarcoma). Many cell cycle regulatory factors have been implicated, including p53, Rb, and others. Certain populations may, in fact, be at risk for developing osteosarcoma. Herein we provide a brief overview of the major regulatory pathways of the cell cycle with implications for sarcomagenesis in osteosarcoma.
In conjunction with the CDK4/6 inhibitor p16INK4A, mutations of the TP53 gene are the most recurrent genetic alterations associated with cancer. The TP53 gene encodes the well-known tumor suppressor p53, which plays an important role in various regulatory processes involved with cell cycle progression. Most important, p53 acts as an important negative regulator, facilitating growth arrest, senescence, and/or apoptosis when cells are exposed to genotoxic, cytotoxic, and/or physiologic stresses. A transcription factor, p53 possesses two transcriptional activation domains and a DNA-binding domain that recognizes specific sequences. After exposure to cellular stress, p53 activates expression of CDKN1A, which encodes the cyclin-dependent inhibitor p21CIP1. It is through the expression of p21 that p53 negatively regulates the cell cycle.
The expression level and activity of p53 is regulated by two different proteins: MDM2 and p14ARF. MDM2 is an E3 ubiquitin ligase, which facilitates the degradation of p53 in a ubiquitin- and proteasome-dependent manner. Interesting to note, MDM2 is a transcriptional target of p53, whose expression is increased in concert with increased levels of p53. In addition to promoting degradation, MDM2 can also inhibit p53 function through a direct protein-protein interaction, suppressing its transcriptional activity, in addition to translation of the messenger RNA transcript itself. To combat these negative regulatory effects, p14ARF is able to positively regulate p53, in part through the negative regulation of MDM2. p14ARF can actually be found at the same gene locus as CDKN2A, which encodes the CKI p16. The two gene sequences do overlap but possess alternate reading frames and are independently regulated. ARF binds MDM2, preventing it from interacting with p53. Consequently, p53 becomes stabilized and its overall activity increases within the cell.
The CIP/KIP family of proteins is the second group of cyclin-dependent kinase inhibitors (CKIs). There are three different members: p21CIP1, p27KIP1, and p57KIP2. Similar to the INK4 family of CKIs, CIP/KIP proteins inhibit the kinase activity of cyclin-dependent kinases (CDKs) by preventing their association with cyclin subunits and adenosine triphosphate molecules, both of which are required for the phosphorylation of target substrates. Unlike the INK4 family, however, CIP/KIP proteins are able to functionally inhibit multiple CDKs. For instance, both p27 and p57 can inhibit the kinase activity of CDK4/6 and CDK2, whereas p21 acts to control the function of both CDK2 and CDK1. Of the three family members, p21 is the most diverse, performing a variety of functions in addition to controlling CDK activity. For example, it was previously mentioned that p21 is a transcriptional target of p53. After exposure to various stresses, p53 activates the expression of p21, thus inducing a DNA damage response. In addition, p21 has been shown to localize to the cytoplasm, where it acts to inhibit the induction of apoptosis by interacting with proteins involved in mediating this process.
The last two cell cycle regulators that are discussed are C-MYC and Ki-67. Both of these proteins play crucial roles during cell proliferation but do so in very different ways. C-MYC influences several processes involved in cell cycle regulation via its function as a transcription factor. For example, C-MYC, when bound to its partner MAX, has been shown to induce the expression of cyclins D1 and D2 and of CDK4, subsequently promoting G 1 phase progression. The MYC-MAX heterodimer can also support continued cell cycle progression through the repression of multiple CKIs, including p15, p18, p21, and p27. Furthermore, C-MYC can increase the expression of E2F2 and cyclin A2, both of which affect the S phase of cell cycles and contribute to overall proliferation. Similar to C-MYC, Ki-67 has been shown to be vital for cell proliferation. Ki-67 is a cell proliferation–associated nuclear antigen that is thought to contribute to cell cycle progression via its involvement in ribosomal RNA and ribosome synthesis. Interesting to note, Ki-67 is expressed in all of the phases of cell cycle (excluding G 0 ), but whether or not it participates in other such related processes is currently unknown.
A small subset of osteosarcomas are hereditary. Osteosarcoma in siblings occurs in fewer than 1 in 1000 to 3000 osteosarcoma patients. Observation of two or more affected siblings in a family indicates an underlying genetic predisposition. When siblings in multiple generations are affected, an autosomal dominant disorder is most likely responsible. One example would be the hereditary form of retinoblastoma (RB). Individuals with hereditary RB (germline RB gene mutation) have a 2000-fold risk for osteosarcoma in the second decade of life when compared with the general population.
The gene for RB has been localized to the long arm of chromosome 13 (13q14). The RB gene is recognized as the prototype of a tumor suppressor gene and has been implicated in the pathogenesis of a number of human neoplasms. A tumor suppressor gene normally functions by restraining cell (tumor) growth, so loss of function or inactivation of a tumor suppressor gene results in tumor growth. Loss of 13q14 (the RB gene) is thought to be responsible for the development of RB. A two-hit kinetic model for this class of genes was proposed by Knudson. For hereditary RB, the primary mutation in one RB locus occurs in germinal cells; for sporadically occurring RB, the primary mutation exists in somatic cells. The second step, responsible for malignant transformation, is the loss of function of the remaining normal homologue in somatic cells by some chromosomal rearrangement or mutation identified as loss of heterozygosity for markers in or around the RB gene. Molecular analyses of both sporadic osteosarcomas and osteosarcomas from patients with RB have revealed homozygous loss of RB gene function in a high percentage of cases. Assessment of loss of heterozygosity at the RB gene in a study by Feugeas and colleagues revealed that RB gene locus loss of heterozygosity could be an early predictive feature for osteosarcomas with a potentially unfavorable outcome. Osteosarcoma develops in 12% of patients with bilateral RB, yet as many as 70% of osteosarcomas have a dysfunctional RB gene product. Thus other oncogenes are likely implicated in the oncogenesis of osteosarcoma.
Several investigators have demonstrated that the mutational profiles of the RB gene in osteosarcoma are basically the same as those for RB and that mutation of the RB gene plays an essential role in the development of osteosarcoma. Besides loss of gene function at the locus on chromosome 13, however, loss of heterozygosity for other chromosomal loci, such as 3q, 17p, and 18q, has been implicated.
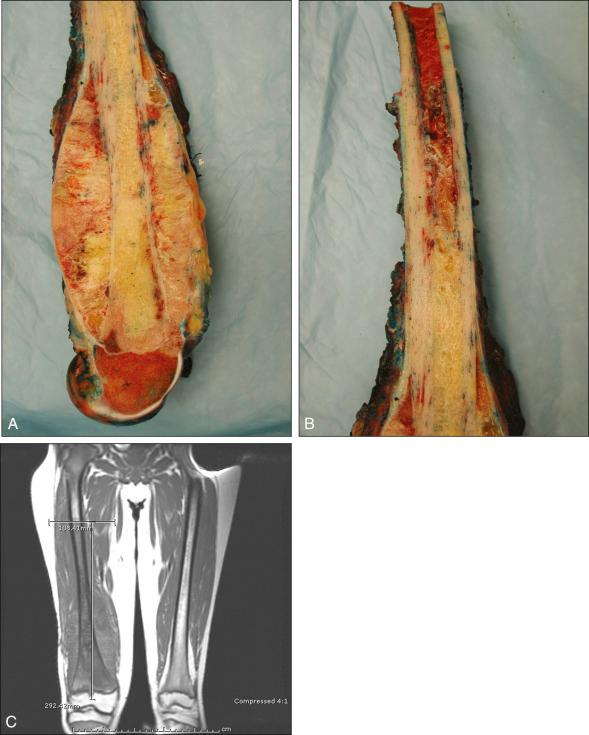
Osteosarcoma is a high-grade sarcoma consisting of malignant osteoblasts that vary in size and shape and have bizarre mitoses. Proposed histologic grading systems for osteosarcoma appear to be of little value. Attempting to grade an osteosarcoma presents many difficulties that limit the usefulness of any grading system. For example, many tumors are heterogeneous, and tissues sampled from separate areas of the same tumor may give different impressions. The number of mitoses, the degree of cellularity, and cellular anaplasia or pleomorphism can differ from site to site within the same tumor. Tumors of identical histologic appearance often differ in their clinical behavior. All classic or conventional osteosarcomas are considered high grade. Osteosarcoma is a vascular tumor, and the tumor osteoblasts produce tumor osteoid or woven bone ( Fig. 89.1A–B ). These tumors are poorly differentiated and may take on a fibroblastic or chondroblastic appearance on light microscopy ( Fig. 89.1C–D ), but if there are areas of bone formation, they are considered osteosarcomas. In fact, a high-grade chondroblastic sarcoma in a child or adolescent at biopsy is considered to be an osteosarcoma (and treated as such) until proved otherwise from examination of the entire specimen (chondrosarcomas are extremely unusual in children). The tumor usually originates in the metaphysis of the bone and percolates between the preexisting trabeculae of bone, incompletely destroying the existing bone, presumably because of its rapid growth (shown well in Fig. 89.1A ). The tumor eventually follows the vascular haversian and Volkmann canals in the cortex, partially resorbing the normal cortex and replacing it with tumor bone as it spreads to the adjacent soft tissue. The periosteum is lifted and tries to respond, but the response is incomplete, leading to the appearance of the Codman triangle on a radiograph. It may also cause perpendicular striations of bone, the so-called starburst appearance of osteosarcoma. Proximally, the tumor ends fairly sharply in the medullary cavity, but skip metastases may be detectable in the marrow surrounding the tumor in a small proportion of patients. The physis or growth plate is a relative barrier to tumor spread, but because the open physis has vascular channels, it is well documented that the tumor will cross the growth plate and enter the epiphysis. The articular cartilage is a more definitive barrier, and osteosarcomas seldom cross the articular cartilage unless there has been a fracture. It may spread into the joint at the periphery of the cartilage or enter the joint along ligaments such as the cruciate ligaments of the knee, but this is a relatively rare event ( Fig. 89.2 ).
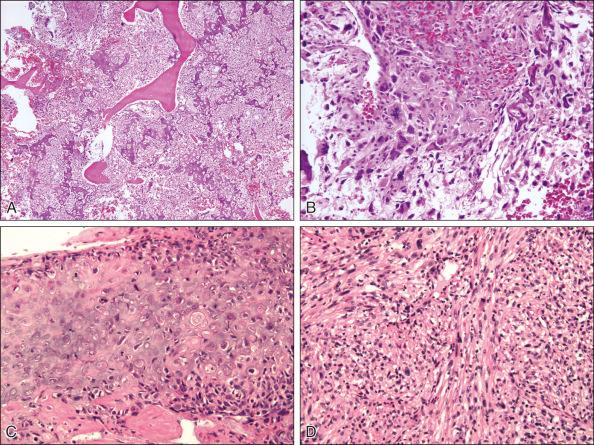
Telangiectatic osteosarcoma is a predominantly radiolucent, destructive osteosarcoma variant. Histologically, it is composed of single or multiple dilated spaces containing blood or degenerated tumor cells and lined with anaplastic, mitotically active sarcoma cells. Telangiectatic osteosarcoma must be differentiated from aneurysmal bone cyst, to which it can be similar in appearance both radiographically and histologically.
A review of 124 patients with telangiectatic osteosarcoma spanning the years 1921 to 1979 suggested no differences in survival compared with patients with conventional osteosarcoma. Further analysis demonstrated that the favorable outcome in 17 of the patients with telangiectatic osteosarcoma was related to their being treated with multiagent chemotherapy. Twenty-five patients had received this therapy, and 17 were free of disease at 5.5 years, demonstrating the response to chemotherapy in this highly vascular tumor. A more recent evaluation of 323 patients with telangiectatic osteosarcoma revealed that although these tumors may be at higher risk for pathologic fracture, with multimodal therapy, outcomes were similar to those of other osteosarcomas even with the negative prognostic factor of the fracture itself.
There are no specific clinical findings at physical examination for osteosarcoma. Bone sarcomas usually cause pain around a joint and a mass. A traumatic event is often in the history, but it is unlikely that the trauma “caused” the tumor; rather, the trauma is the event that calls the tumor to the patient's attention. The symptoms are often missed initially because other causes of joint pain are much more common. It is not unusual for symptoms to date back to 6 months before the documentation of the tumor. This is perhaps changing with the recent abundance of MRI in the evaluation of even minor joint pain.
The plain radiograph is the best diagnostic tool. Osteosarcomas may either completely destroy the bone (radiolucent lesion) or replace the bone with a blastic response (radiodense), but they most often do both. The radiograph shows areas of destruction of the host bone and blotchy densities of new (tumor) bone production. The lesion is most frequently in the metaphysis of a long bone in the adolescent or child and in the flat bones in the adult, but any bone can be involved at any age ( Fig. 89.3A–B ). The tumor is usually large, destroys the cortex, and is associated with a soft tissue mass. The mineralization of the matrix is often apparent, but because these tumors can contain areas of chondroblastic and fibroblastic in addition to osteoblastic differentiation, the pattern on mineralization might not be that of bone.
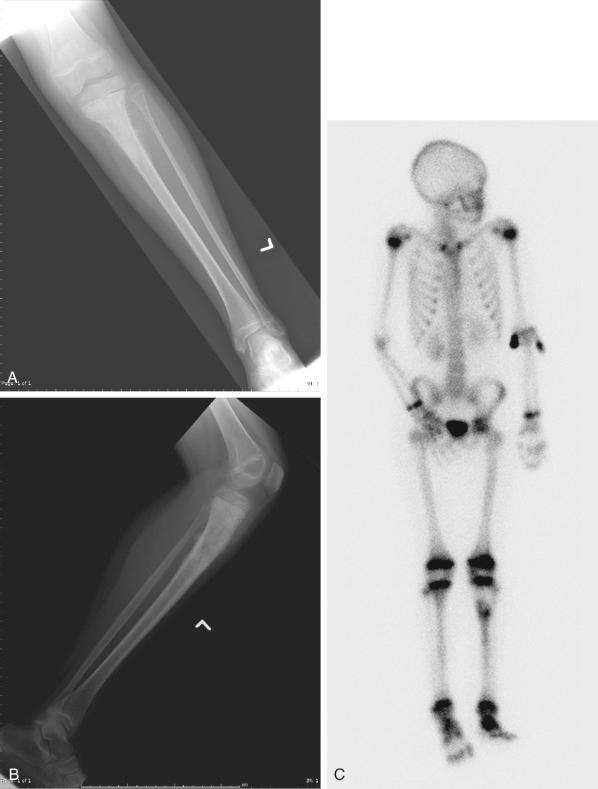
There are no blood laboratory studies that aid in the diagnosis of osteosarcoma, but it has been shown in several large series of patients that an elevated level of alkaline phosphatase and/or lactic dehydrogenase is associated with a worse prognosis.
MRI is the single most useful study in evaluating the intraosseous and extraosseous extent of the primary tumor and in detecting intramedullary or transarticular skip metastases. It has been well demonstrated that MRI better identifies the edema (high water content) in and around the reactive zone of the pseudocapsule, illuminating the potential surgical margin. MRI has also been shown to be superior to CT in displaying the medullary canal extent of the tumor, suspected skip lesions, soft tissue extension, and overall anatomic location of an extremity tumor (see Fig. 89.3D–F ). MRI, with or without magnetic resonance angiography, is the single most valuable tool for planning limb-sparing surgical procedures. The use of MRI has significantly improved radiologic staging and presurgical planning. Serial MRI studies are less reliable, however, in evaluating tumor response to primary chemotherapy, and are more predictive of a poor rather than a good response. By demonstrating an increase in size of the tumor, more bone destruction, and soft tissue invasion on serial studies, MRI is more accurate as a measure of a poor chemotherapy response.
Metastases to bone and/or lung are usually assessed with a whole-body 99m Tc bone scan and a complete CT of the chest and mediastinum. The 99m Tc bone scan has been considered superior to other imaging studies for surveying the skeleton for metastatic or multiple lesions and for later detecting the development of skeletal metastases (see Fig. 89.3C ).
Regional lymph node involvement is unusual unless the tumor directly involves the skin or regional lymphatic structures.
PET scans have been used in an attempt to stage and separate high-grade from low-grade tumors. Brenner and associates summarized the current usefulness of fluorine-18 fluorodeoxyglucose–positron emission tomography ( 18 F-FDG–PET) in patients with osteosarcoma. High-resolution CT has been shown to be superior to 18 F-FDG–PET for detecting pulmonary metastases and is not recommended to detect bone metastases except when a suspected skip lesion has been identified at MRI. PET does not make it possible to differentiate between high- and low-grade osteosarcoma. It could be useful, however, in determining the appropriate area in which to perform biopsy to identify viable representative tumor tissue and in distinguishing benign aggressive lesions from other lesions in which local recurrence is likely.
18 F-FDG–PET may be most useful to determine the response to neoadjuvant chemotherapy and to demonstrate a region of viable tumor. The timing between the initiation of preoperative chemotherapy and the point at which 18 F-FDG–PET becomes predictive remains uncertain. The response to soft tissue postchemotherapy inflammation and healing surrounding the tumor must be characterized before this technique becomes predictive of preoperative response to chemotherapy. PET also has potential usefulness in patient follow-up, differentiating postoperative tissue changes and possible tumor recurrence, especially in patients with metallic reconstructions. Despite improved techniques to mitigate metal artifact, orthopedic implants often hamper assessment with CT or MRI, and sequential 18 F-FDG–PET scans could be used to differentiate between recurrent tumors and the normal healing process. Also, because 18 F-FDG–PET provides scanning of the entire patient, it may be useful when combined with CT of the chest to detect first evidence of pulmonary metastasis. A study has shown that PET correlates only moderately with histologic response but may be predictive of progression-free survival.
Once the staging workup is complete, a biopsy is performed. The procedure can be either an open biopsy or a needle biopsy (core needle or fine-needle aspiration) depending on the experience of the surgeon, the interventional radiologist, and the pathologist. If an open biopsy is performed, it should be done by the surgeon who will be responsible for local control, with a view to placement in a site that can be resected with the definitive specimen. If a core needle biopsy is performed by an interventional radiologist (which is becoming more frequent), the radiologist and the surgeon should agree on the placement of the needle track. Communication between the surgeon and interventional radiologist is essential, as is communication with the entire multidisciplinary team to determine whether tissue should be sent for cytogenetic evaluation or other special biologic studies. Table 89.6 provides a diagnostic workup algorithm.
|
Once the biopsy results are available, the sarcoma can be staged as discussed earlier.
The diagnosis of a bone sarcoma is usually obvious, although differentiation between an osteosarcoma and a Ewing sarcoma in a child or adolescent might depend on biopsy results; radiographically the two may appear similar. In general, osteosarcomas are metaphyseal lesions, and Ewing tumors are diaphyseal or in the flat bones in young patients, but there are frequent exceptions to this rule, and both types of tumor can occur in any bone. Matrix production may be apparent in a Ewing tumor because of the host response and absent in an osteosarcoma because the osteoid is incompletely mineralized or absent (as in telangiectatic osteosarcoma). In the adult, it can be difficult to differentiate osteosarcoma from malignant fibrous histiocytoma (MFH) of bone and chondrosarcoma clinically, but a biopsy usually settles the issue.
Osteomyelitis and Langerhans cell histiocytosis may mimic an osteosarcoma and should always be considered, especially in obtaining tissue for a biopsy. A fatigue fracture (stress fracture) may also appear with an aggressive periosteal response, and the MRI study shows extensive marrow and soft tissue edema. This can mimic an osteosarcoma, but a CT scan, especially with coronal reconstructions, is usually diagnostic.
In the adult, one must look for underlying causes of osteosarcoma, such as Paget disease and prior irradiation. Because the bone is abnormal to begin with in these two circumstances, the diagnosis may be difficult. Paget disease itself may be painful, but a change in the pain pattern and new destruction compared with old radiographs if available are helpful. Metastatic carcinoma and multiple myeloma are much more common causes of a destructive bone tumor in the adult, but it should always be remembered that, although rare, osteosarcoma can present as a purely radiolucent lesion.
Other bone tumors, such as giant cell tumor of bone, aneurysmal bone cyst, and chondroblastoma, may have an aggressive radiographic appearance and mimic an osteosarcoma, but the histologic type is usually diagnostic in these situations. An aneurysmal bone cyst, however, might have scant tissue from a biopsy, as does a telangiectatic osteosarcoma, so at times the distinction is difficult. An experienced bone pathologist is essential in these instances even if it necessitates an outside referral. Similarly, osteosarcomas can have giant cell–rich areas that can mimic a giant cell tumor. Again, the expertise of the bone pathologist and a team of clinicians and radiologists working in concert are essential to make these distinctions.
The use of adjuvant chemotherapy for osteosarcoma was introduced in the 1970s, and after initial concern relative to its efficacy, a randomized trial confirmed its benefit. It is now established as an essential part of the treatment of osteosarcoma. Initially used as an adjuvant after amputation or resection of a tumor, chemotherapy is now used in the neoadjuvant setting, although no clear survival benefit to that approach has been documented. The use of neoadjuvant chemotherapy does allow the assessment of histologic response to the chemotherapy, which has prognostic implications, allows time for planning of limb salvage operations, and makes those resections easier and probably safer.
A number of single-institution and multiinstitution studies have reported the results of treatment protocols, including multiagent neoadjuvant chemotherapy and limb-sparing surgery. The specific drugs and their methods of administration are important. Delépine and colleagues published a meta-analysis of the relationship of total dose and dose intensity of methotrexate from nine single-institution and nine multiinstitution randomized trials. They concluded that both methotrexate dose and dose intensity had major prognostic value. Intravenous administration of chemotherapy appears to be as effective as the intraarterial route of administration. A meta-analysis of 16 regimens published by a group from the National Cancer Institute found that dose intensity was the most important determinant of a favorable outcome, defined as a good histopathologic response to neoadjuvant chemotherapy. Other studies likewise have supported the importance of doxorubicin treatment in patients with osteosarcoma.
The use of preoperative chemotherapy was extended to selected patients in an attempt to contain growth of the primary tumor while awaiting construction of a custom prosthesis (usually 12 to 16 weeks). The sequence of several courses of primary chemotherapy and subsequent surgery afforded the opportunity to examine and histopathologically grade the tumor tissue response to multiple chemotherapy agents. Patients with greater than 90% tumor necrosis (good) were shown to have a better disease-free survival than those with a poor (<90%) response to chemotherapy. Goorin and associates completed a study of 106 patients admitted to Pediatric Oncology Group Study 8651 who were randomly assigned to immediate surgery or to preoperative chemotherapy with high-dose methotrexate, doxorubicin, and cisplatin for two cycles (10 weeks). Six patients were excluded from analysis. Of the remaining 100 patients, 45 were randomly assigned to immediate chemotherapy, and 55 were randomly assigned to immediate surgery. At 5-years follow-up, 67 patients remained disease free. At 5 years, the projected EFS rate was 65% for immediate surgery and 61% for presurgical chemotherapy. There was no apparent advantage for the group that received preoperative chemotherapy. This was a difficult study to complete because at the time there was a surgical bias toward neoadjuvant chemotherapy for limb salvage patients, making accrual slow. The number of limb-preserving procedures was nearly equal in both groups (55% with immediate surgery and 50% with presurgical chemotherapy). Although the percentage of patients in the two groups was similar, the overall percentage of patients receiving limb-sparing procedures was much lower than the usual 60% to 90% limb-preserving procedures reported. Only one local recurrence was reported among all 100 patients.
Surgical resection of the primary tumor is usually planned for 10 to 12 weeks after induction chemotherapy. Limb preservation is elected if the tumor responds favorably—that is, there is evidence of a clinically favorable response, including diminished local pain; radiographic evidence of mineralization of the previously unmineralized soft tissue portion of the tumor on radiographs; and MRI evidence of retention of a normal fatty tissue plane between vascular and neural structures and the tumor. However, if the lesion enlarges and the vessels become involved secondarily (poor response), ablative surgery is recommended. Once the operative wound heals (usually within 2 to 3 weeks), adjuvant chemotherapy is initiated and is maintained for approximately 40 weeks, depending on the particular study. Wilkins and colleagues described their results at two institutions with a dose-intensified neoadjuvant protocol using intravenous doxorubicin and intraarterial cisplatin administered until a maximum angiographic response was observed (usually four courses). Despite showing results similar to others previously reported, it requires extraordinary resources, making it unlikely that a similar study could be carried out in an extensive cooperative manner.
Long-term outcomes were studied by Bacci and coauthors, who reported the results of treatment of 164 patients with nonmetastatic extremity osteosarcoma who were followed for a minimum of 10 years. Preoperative chemotherapy consisted of high-dose methotrexate, cisplatin, and doxorubicin. Postoperatively, good responders (≥90% tumor necrosis) received the same three drugs, whereas poor responders (<90% tumor necrosis) received ifosfamide and etoposide in addition to the three-drug chemotherapy regimen. Follow-up showed that 101 patients (62%) remained continuously free of disease, 61 had experienced relapse, and two had died of doxorubicin-related cardiotoxicity. There were no differences in outcome between good and poor responders.
Limb-preserving surgery was performed in 136 of the patients (83%) in the Bacci study, and 117 (71%) had a good histologic response. Despite the large percentage of patients with limb-sparing procedures, only four local recurrences developed (2.4%). The complications of chemotherapy included doxorubicin-induced cardiotoxicity (six patients) and secondary malignancies (seven patients) at a median follow-up of 11.5 years.
In 1997, Bramwell conducted a review to provide answers to important questions about the role of chemotherapy in the management of patients with nonmetastatic osteosarcoma of the extremities. Many studies were analyzed, including a study by Link and coworkers that clearly demonstrated the role of adjuvant chemotherapy in the treatment of patients with conventional osteosarcoma. The role of adjuvant chemotherapy was also confirmed by another study that provided additional objective evidence for the efficacy of multiagent chemotherapy in preventing and/or delaying relapse. The five studies that were reviewed by Bramwell approached the results of Rosen and associates ; three were multicenter studies and two were reports from a single institution. Bramwell concluded that although the Rosen T10 regimen is complex and toxic, it can be given in a multicenter setting without apparent major compromise in efficacy.
Bramwell concluded that outcomes with multiagent chemotherapy were similar to those of regimens containing the most active drugs (doxorubicin and cisplatin) that were used in two consecutive European Osteosarcoma Intergroup protocols when compared with results from multicenter studies using the T10 regimen.
It is apparent that histopathologic response to neoadjuvant chemotherapy correlates with improved survival. The reports by Picci and colleagues (Bologna, 355 patients), Kempf-Bielack and coworkers (Cooperative Osteosarcoma Study Group, 504 patients), and Delépine and associates (Paris, 112 patients) all demonstrated that a good response to neoadjuvant chemotherapy was an independent prognostic factor. Meyers and colleagues reported the relationship between duration of preoperative chemotherapy and histopathologic response. In univariate analysis, the duration of preoperative chemotherapy did not correlate with relapse-free survival. With longer preoperative treatment, a greater proportion of patients had a favorable histopathologic response to therapy, but the correlation of the response with outcome decreased. Bramwell postulated that with prolonged preoperative chemotherapy, a good response to chemotherapy might lose its prognostic significance. The CCG-782 study published by Provisor and coworkers involving 268 patients with nonmetastatic osteosarcoma of the extremity used the resected tumor histologic response to neoadjuvant chemotherapy to determine postoperative chemotherapy. In 206 patients, the tumor was morphometrically assessed for residual viable tumor; 28% displayed a good (<5% viable tumor) histologic response, whereas the remaining patients were judged to have a poor histologic response (>5% residual viable tumor). The patients who had a good response had an 8-year postoperative EFS rate of 81% and a survival rate of 81%. Patients with a poor histologic response had an 8-year postoperative EFS rate of 46% and an overall survival rate of 52%. The researchers concluded that EFS and overall survival appeared to be related directly to histologic response to neoadjuvant chemotherapy. If that is true, then increasing the percentage of necrosis by intensifying chemotherapy should improve outcome even further. A study compared standard chemotherapy with an intensified arm to assess this contention. Conventional treatment consisted of six 3-week cycles of cisplatin (100 mg/m 2 by 24-hour infusion) and doxorubicin (25 mg/m 2 per day by 4-hour infusion for 3 days). Intensified therapy was treatment with identical total doses of cisplatin and doxorubicin, planned as six 2-week cycles supported by granulocyte colony-stimulating factor. In this study, 497 eligible patients were evaluated, and good histologic response (>90% tumor necrosis) was observed in 36% of standard arm and 50% of the intensified arm. However, there was no evidence of a difference in overall survival between the two treatment regimens. The study found that intensification of chemotherapy could increase the percentage of necrosis but not progression-free survival or overall survival. Another study looked at increasing the dose of chemotherapy with the same drugs in 196 osteosarcoma patients. The authors failed to find a difference in percentage of necrosis, 5-year EFS, or overall survival, suggesting that response to chemotherapy is related to the specific drugs used but that increasing the intensity is of little benefit. The tumors either respond or do not, suggesting biologic differences in the tumors.
Currently, there are no strong data showing that changing drugs in the poor histologic responders improves the survival of those patients. A retrospective study reported by Benjamin and associates compared outcomes from three consecutive cohorts of patients receiving intraarterial cisplatin and intravenous doxorubicin between 1980 and 1992. In cohort 1 (37 patients), the postoperative chemotherapy was the same. In cohort 2 (59 patients), the postoperative chemotherapy for poor responders consisted of high-dose methotrexate, bleomycin, cyclophosphamide, and dactinomycin alternating between doxorubicin and dacarbazine. In cohort 3 (28 patients between 1988 and 1992), poor responders were managed with three alternating regimens of high-dose methotrexate, ifosfamide, and alternating doxorubicin and dacarbazine. The significant 5-year relapse-free survival rate for poor responders for the three cohorts was 13%, 34%, and 67%. Bramwell believes that although the results appear significant, they could also be explained on the basis of small sample size, increasing dose intensity, total dose, and increased duration of preoperative chemotherapy.
A meta-analysis of North American and European studies of nonmetastatic osteosarcoma showed that three-drug regimens had better EFS and overall survival rates than two-drug regimens (EFS, 48% and 58%; and overall survival, 62% and 70%, respectively). Adding a fourth drug (e.g., ifosfamide) did not improve outcome further, but dose intensification improved histologic response to chemotherapy. There was no benefit to intraarterial chemotherapy.
A randomized multiinstitutional study of patients from North America and Europe, EURAMOS-1, is attempting to address this question more definitively by randomly assigning poor responders, defined as showing at least 10% viable cells after preoperative methotrexate, doxorubicin, and cisplatin (MAP) chemotherapy, to continued MAP therapy or an intensified arm in which ifosfamide and etoposide were added to the MAP: MAPIE. A total of 618 patients were randomized, and at a median follow-up of 5 years, the EFS did not differ between the treatment groups, although the MAPIE regimen was associated with more grade 4 toxicity. The researchers concluded that intensified therapy with this regimen did not improve the outcome of patients with a poor response to neoadjuvant therapy. This study also looked at the good responders, randomly assigning them to continued MAP or MAP with the addition of interferon-α. There was no observed benefit of adding interferon-α in this study, although because a considerable portion of patients never received interferon-α, longer-term follow-up continues.
The issue of whether other agents incorporated into intensive multiagent regimens will improve survival further is less clear. Preliminary trials incorporating ifosfamide into multiagent chemotherapy appeared promising, but a recent study of the Children's Oncology Group patients who were randomly assigned to multiagent regimens that either contained or did not contain ifosfamide shed some doubt on this. In this study, 677 nonmetastatic osteosarcoma patients were treated with one of four prospectively randomized treatments. All patients received identical cumulative doses of cisplatin, doxorubicin, and high-dose methotrexate and underwent definitive surgical resection of the primary tumor. They were randomly assigned to receive or not to receive ifosfamide and/or muramyl tripeptide (MTP), a pulmonary macrophage stimulant. The addition of ifosfamide in this dose schedule to standard chemotherapy did not enhance EFS. The addition of MTP to the ifosfamide arm appeared to add further benefit, but the study was not designed to test this, and this question remains uncertain but intriguing. Whether MTP should be included in standard treatment protocols remains a topic of debate at this time. There was a suggestion in a small cohort of patients with metastatic osteosarcoma that addition of liposomal muramyl tripeptide phosphatidylethanolamine (L-MTP-PE) may enhance EFS and overall survival, but this study was not of sufficient size to be definitive.
To test whether intensified ifosfamide therapy might be of benefit, a study of 182 patients treated with two cycles of high-dose ifosfamide (15 g/m 2 ), methotrexate (12 g/m 2 ), cisplatin (120 mg/m 2 ), and doxorubicin (75 mg/m 2 ) was conducted. Postoperatively, patients received two cycles of doxorubicin (90 mg/m 2 ) and three cycles each of high-dose ifosfamide, methotrexate, and cisplatin (120–150 mg/m 2 ). Granulocyte colony-stimulating factor support was mandatory after the high-dose ifosfamide-cisplatin-doxorubicin combination. No disease progression was recorded during primary chemotherapy. With a median follow-up of 55 months, the 5-year probability of EFS was 64%, and the overall survival rate was 77%. The addition of high-dose ifosfamide to methotrexate, cisplatin, and doxorubicin in the neoadjuvant setting was found to be feasible but associated with major renal and hematologic toxicities. Survival rates were similar to those obtained with four-drug regimens including just standard-dose ifosfamide. Growth factors do appear to offer a benefit in increasing dose intensity. In a pilot study, the European Osteosarcoma Intergroup demonstrated that the use of granulocyte colony-stimulating factor supported increased dose intensity, making chemotherapy every 2 weeks feasible. Thrombocytopenia remained dose limiting, and whether attainable dose intensification improves survival remains uncertain.
The histologic subtypes of osteosarcoma also appear to influence response rates. Bacci and colleagues at the Rizzoli Institute in Bologna correlated the histopathologic response to preoperative chemotherapy in 1058 patients with conventional osteosarcoma of the extremity. They classified the tumors as osteoblastic (70%), chondroblastic (13%), fibroblastic (9%), and telangiectatic (6%). At diagnosis, 911 patients had localized disease and 147 had resectable pulmonary metastases. The response to preoperative chemotherapy was good (≥90% tumor necrosis) in 59% of patients and poor (<90% tumor necrosis) in 41%. Notably, the rate of good responders was significantly higher ( P = .0001) in patients with fibroblastic (83%) and telangiectatic tumors (80%) than in those with osteoblastic (62%) and chondroblastic (60%) tumors. In all subtypes (excepting the chondroblastic), the 5-year overall survival rate was significantly higher ( P = .0001) in good responders (68%) than in poor responders (52%).
Essential to the improvement in survival in osteosarcoma is the combination of effective surgical resection along with the use of chemotherapy. Jaffe and colleagues in the Department of Pediatrics at the University of Texas MD Anderson Cancer Center attempted the cure of 31 patients with nonmetastatic osteosarcoma. Their protocol for selection included initial treatment with chemotherapy consisting of high-dose methotrexate and leucovorin rescue (MTX-LF) in three patients and intraarterial cisplatin in 28 patients. After response at 3 months, entry into the study was permitted, and chemotherapy treatment was maintained for a total of 18 to 21 months with a combination of MTX-LF, intraarterial cisplatin, and doxorubicin. Only 3 of 31 patients (10%) were cured exclusively with chemotherapy. Four additional patients requested surgical extirpation of the tumor after the cessation of chemotherapy. Histopathologic examination revealed no evidence of viable tumor. Adding these patients to the three mentioned previously yielded a total of seven patients (23%) who had a cure from chemotherapy alone. Because the expected cure rate with conventional strategies is 50% to 65%, the authors concluded that their results do not justify the option of current forms of chemotherapy as exclusive treatments for osteosarcoma.
In summary, although great improvements have been made since the 1970s in the outcome of patients with nonmetastatic osteosarcoma, it seems that a plateau has been reached with little new improvement in outcome in recent years. It is likely that future progress will result from novel treatments capitalizing on knowledge of the molecular biology of osteosarcoma and the development of new, targeted agents to specific molecular targets. Many have been proposed, but none are in routine clinical use, nor have they been successfully studied in clinical trials. This remains an area of intense interest and research.
Morphometric analysis of pathologic specimens was instituted after it was recognized that chemotherapy-induced necrosis correlated with clinical outcome. Picci and coauthors described their methodology in 50 patients. Necrosis was divided into three categories: good (100% to 80% necrosis), fair (80% to 50% necrosis), and poor (<50% necrosis). Other authors have used different classifications. Depending on the system that is used, tumor necrosis ranging from 60% to 95% is common. The information gained has prognostic significance. Winkler and associates were early to report that patients with unfavorable pathologic responses to preoperative chemotherapy experienced a poorer (49%) disease-free survival than did patients with a favorable pathologic response (87%; P = .005). Glasser and coworkers later reviewed 279 consecutive patients with stage II osteosarcoma of the appendicular skeleton who were treated between 1976 and 1986. Continuous disease-free survival for the overall group was 70% at 5 years and 69% at 10 years. The only independent predictor of a favorable outcome was found to be the histopathologic response to chemotherapy as defined by pathologic review of the surgical specimen.
A literature review by Davis and colleagues attempted to identify prognostic factors that could influence survival in patients with nonmetastatic high-grade osteosarcoma of the extremities. Eight previously reported large series of patients included sufficient data to evaluate the numerous identified variables. Only two variables proved significant to univariate analysis: tumor size and chemotherapy-induced tumor necrosis after primary chemotherapy. Only tumor necrosis remained significant after multivariate analysis, however. Other large series have demonstrated similar prognostic responses to neoadjuvant chemotherapy.
E-Table 89.1 summarizes recent data regarding treatments for nonmetastatic osteosarcoma reported by 10 internationally recognized institutions. The table suggests that results have improved owing to management by multiagent neoadjuvant chemotherapy combined with adequate local surgery performed by experienced musculoskeletal surgeons. Several conclusions can be drawn from these studies:
Patients who request limb-sparing operations do not appear to be at greater risk for development of local or distant relapse than patients who have transmedullary amputations.
The administration of sequential multiagent adjuvant chemotherapy has significantly improved disease-free survival for patients without metastasis.
The risk of local recurrence appears to be no greater in patients who complete limb-preserving procedures than for those who have an amputation, although this never has and likely never will be proven in a randomized study for obvious reasons.
| Institution | No. of Nonmetastatic Extremity Total* | Resection (No. of Patients) | Rotationplasty (No. of Patients) | Amputation (No. of Patients) |
Local Control (No. of Patients) |
Disease-Free Survival |
|---|---|---|---|---|---|---|
| MD Anderson | 60 | 31 | — | 28 | 4 (7%) | 74% (TIOS-III) |
| Dana Farber | 74 | 36 | — | 38 | 1 (1%) | 87% |
| Memorial Sloan-Kettering | 271 | 159 | 9 | 103 | 18 (7%) | 77% |
| Vienna University Clinic | 73 | 41 | 22 | 10 | 2 (3%) | 76.7% |
| Birmingham Service | 99 | 74 | — | 15 | 4 (4%) | 48% |
| French Study | 100 | 79 | 3 | 18 | 1 (1%) | 82% |
| Brazil Group | 92 | 34 | — | 58 | 6 (7%) | 41.1% |
| Mie Japan | 52 | 25 | — | 27 | 2 (4%) | 58.9% |
| COSS 86 | 159 | 65 | 39 | 44 | 3 (2%) | 84% |
| Rizzoli Institute | 125 | 106 | 9 | 10 | 1 (0.08%) | 87% |
The role of neoadjuvant chemotherapy in facilitating limb preservation appears to be well established. The relationship among margins, chemotherapy, and local recurrence is not as straightforward. Although, in general, local recurrence is higher in patients with inadequate margins, some patients who are so treated do not have recurrence, whereas in some patients with wide or radical margins, disease does recur or persist locally. The effect of neoadjuvant chemotherapy in this regard is suspected to be beneficial, but only retrospective data are available. Four of the institutions reporting data shown in E-Table 89.1 also provided data on the pathologic margins achieved at surgery and correlated the surgical margins with locally recurrent disease. Of the 271 margins reported by Memorial Sloan-Kettering, 266 were adequate (261 wide and 5 radical), and five were inadequate (three marginal and two intralesional). Of the 18 locally persistent tumors, however, all developed in patients who were thought to have wide margins. There were no locally recurrent tumors from the five known inadequate surgical margins. The University of Vienna group reported 61 margins as wide, with 12 inadequate (nine marginal and three intralesional) margins. Only two local recurrences developed, one in a patient having an intralesional amputation and the other with a marginal resection and reconstruction.
A similar review from Birmingham, England, revealed 23 marginal and 15 intralesional margins in 99 patients, with a 4.5% recurrence rate. Investigators in a French study reported 100 patients with nonmetastatic osteosarcoma managed by neoadjuvant chemotherapy and surgery with “numerous” marginal margins but no intralesional margins. These researchers reported one local recurrence. A Rizzoli Institute study, examining 125 patients retrospectively, identified 15 patients with inadequate margins. Only one patient had a local recurrence.
Investigators from the Rizzoli Institute in Bologna have shown by multivariate analysis that the incidence of local recurrence in 355 patients was related closely to surgical margins ( P < .0001) and response to preoperative chemotherapy ( P < .0001). There were 28 patients who experienced local recurrence (7%), three of whom survived (11%). Six of 10 patients who did not receive preoperative chemotherapy had local recurrence. In the other limb salvage procedures, 110 patients had wide margins, 12 were marginal, seven had intralesional margins, and seven had wide contaminated margins. Although 27 patients had inadequate margins, only three developed local recurrence, all within the first 2 years after diagnosis.
To better illustrate the relationship between surgical margins and local recurrence, Picci and colleagues reported on a single-institution study retrospectively reviewing 355 patients with nonmetastatic high-grade osteosarcoma of the pelvis and extremities. The average length of follow-up was 65 months for surviving patients.
Pathologic review demonstrated less-than-wide margins in 65 of the 355 patients. The most common anatomic site for inadequate margins was the popliteal region near the vascular and neural structures, where 20 of 140 patients were found to have inadequate margins (either marginal or intralesional). Only 7 of the 20 patients had local recurrence, however. Locally persistent disease developed in only 3 of 15 patients with inadequate margins associated with lesions around major joints locally persistent disease. The intramedullary canal was the site of inadequate margins in 20 of 237 patients, and local recurrence developed in 6 patients. It is of interest that 7 of the 11 patients with intralesional surgical margins and 16 of the 21 with contaminated margins did not have local recurrence. These findings could not be explained on the basis of poor survival or early death. The observation is believed to be related to the effectiveness of preoperative chemotherapy at producing tumor necrosis and the development of a “mature” capsule surrounding the tumor where satellite tumor nodule formation had been suppressed.
An analysis of a more recent group, 164 patients with nonmetastatic osteosarcoma of the extremities, also came from the Rizzoli Institute. Limb-sparing procedures were performed in 136 patients (83%), 18 patients (11%) had amputation, and 10 (6%) had rotationplasty. The surgical margins were reviewed. In amputations, 18 margins were wide or greater; in rotationplasty, nine were wide and one was intralesional. In the limb salvage procedures, 110 patients had wide margins, 12 were marginal, seven had intralesional margins, and seven had wide contaminated margins. Although 27 patients had inadequate margins, only three experienced local recurrence, all within the first 2 years after diagnosis.
The unanswered question is whether the poor correlation with margin and recurrence relates to neoadjuvant chemotherapy or the underlying tumor biology or some combination of the two. It may also relate to the ability of the surgeon in conjunction with the pathologist to accurately determine whether margins are truly wide because the soft tissues around the tumor move about during the operation and after the specimen has been removed, making it difficult to accurately determine whether a margin is wide, marginal, or intralesional even when the specimen is inked. Adequate surgical resection is certainly a worthwhile goal, but the causes of local recurrences are probably more complex than adequacy of margin.
The goal of surgical management is complete resection of the tumor with a cuff of normal tissue surrounding the tumor (wide resection). This can be accomplished with either amputation or local resection as long as this goal is achieved. Radical resections are seldom performed today. The most important question regarding limb salvage surgery is, “Is it safe?” Suffice it to say that no randomized studies have ever compared amputation with local resection, nor are they likely ever to be performed. What is known comes from retrospective analyses of tumor specimens and from outcomes of patients treated in clinical trials.
To identify the potential risks of delaying resection to prepare for limb-sparing surgery versus immediate amputation, the results of data on 279 patients treated at Memorial Sloan-Kettering between 1975 and 1984 were reviewed retrospectively. Sixty-three patients who completed primary local control surgery (amputation primarily, although this is not clear in the report) and adjuvant chemotherapy were compared with patients who had primary preoperative chemotherapy followed by local control surgery and adjuvant chemotherapy. Univariate analysis showed no difference in outcome between these groups ( P = .34).
A multiinstitutional retrospective questionnaire study of members of the MSTS evaluating patients with nonmetastatic osteosarcoma of the distal femur reported treatment results for 227 patients who were managed with limb-sparing procedures, above-knee amputation, and hip disarticulation between July 1975 and June 1980. The Kaplan-Meier estimates for the three surgical groups revealed no significant difference in continuous disease-free and ultimate survival rates for each group (Mantel-Cox test, P = .8) after a median follow-up of 5.5 years. The continuous disease-free survival rate for the entire group was 42%, with an overall survival rate of 55% at 5 years. The researchers concluded that limb-sparing procedures for osteosarcoma of the distal end of the femur did not compromise either disease-free survival or overall survival. Outcome results showed that one-third of patients with limb-sparing procedures required at least one additional surgical procedure, and one-fourth eventually required amputation.
A follow-up of the aforementioned study 8 years later reviewed the original 227 patients. In this study, 213 patients had been classified as having stage IIB osteosarcomas. Seventy-three patients had limb-preserving procedures, 115 had above-knee amputations, and 39 had hip disarticulations. Eighty-four percent of patients were followed for a minimum of 10 years. The Kaplan-Meier estimate of the disease-free survival rate for all patients at 10 years was 41%. Fourteen of the original 17 patients experiencing a local recurrence in the first study did so within the first 2 years after the index procedure, and only one of the original 17 patients survived. There were nine local recurrences after above-knee amputations and eight after limb-sparing procedures. No patient who had a hip disarticulation (radical margin) experienced a local recurrence. Although the function of patients with limb-sparing procedures was superior to that of both the amputation and disarticulation groups, no differences could be identified regarding patient acceptance or psychosocial outcome (quality of life [QoL]) among the three operative groups.
Limb-preserving procedures are currently performed in approximately 80% to 90% of osteosarcoma patients with nonmetastatic extremity tumors, in contrast to the previously reported high amputation rates. Advances in chemotherapy, imaging technology, implant design and materials, and subspecialization in orthopedic oncology have reversed the trends of previous decades. Limb preservation, however, has not altered disease-free survival rates when compared with ablative procedures. Local recurrence rates after neoadjuvant chemotherapy and resection or amputation appear to be similar; these are low-frequency but still serious events (0.8% to 7%). Although limb-sparing surgery in appropriately selected patients appears to be safe from an oncologic viewpoint, it is not clear that functional outcome and QoL are superior in limb salvage patients compared with those who have undergone amputation. The long-term outcomes and reoperation rate (including eventual amputation) of the various limb salvage constructs are unknown. Most patients prefer to keep their limbs, however, and limb-sparing procedures are now routinely offered.
Problems associated with limb sparing include an increased early complication rate of 25% to 35%. Many reconstruction alternatives are available, and the method that is chosen depends on such variables as patient age and employability, tumor location and size, and the potential of the elected procedure to provide curative margins. Of paramount importance are recognition of the patient's desires and discussion of realistic expectations. In addition, the surgeon's experience and expertise play a role in the choice of procedure.
Lindner and associates, from the University of Muenster, reported the results of their study with 133 patients who had high-grade osteosarcoma of the extremities treated with intravenous neoadjuvant chemotherapy and surgery between 1978 and 1994. Seventy-nine patients had limb-preserving procedures, including 32 with endoprosthesis, 39 with allograft replacement, six with autograft reconstruction, and two with shortening procedures. Twenty-one patients had rotationplasty, and 33 patients elected amputation. With the MSTS (1993) functional evaluation scale, major complications were experienced after all procedures; 20 of 32 patients with endoprosthetic procedures experienced a major complication, and 6 of the 20 required removal of the prosthesis. Twenty of the 39 patients with allografts experienced a major complication, and 6 of the 20 patients required removal as well. Ten of the 21 patients with rotationplasty also experienced major complications, but none required revision to amputation. Eight of the 33 patients who were treated with transmedullary amputation experienced a major complication, and three of the eight required a more proximal reamputation. Lindner and associates concluded that the extent of preoperative primary tumor necrosis, surgical margins, and tumor volume were the most important oncologic prognostic factors and that functional outcome after rotationplasty was superior to that of amputation and other limb-preserving techniques.
A minimum of a “wide” margin should be achieved for adequate local control of high-grade primary sarcomas of bone. If there is invasion of a major peripheral nerve from lesions arising around the knee, then the equivalent of a wide amputation should be performed, but this is a rare occurrence. If the nerve is spared, then consideration may be given to limb-sparing resection. One issue is the definition of “wide.” As defined, it is a cuff of normal tissue completely surrounding the tumor and reactive zone around the tumor. In the early days of limb salvage, most surgeons desired 1 cm or more of soft tissue and 7 to 10 cm of uninvolved bone marrow when performing resections or amputations. As more experience has been gained and neoadjuvant chemotherapy has come into common use, the “acceptable” thickness of the margins has decreased for most surgeons, but it is unclear how close is too close to the tumor before risk of local recurrence increases. Most surgeons now are accepting 1- to 3-cm bone marrow margins and soft tissue margins in the millimeter range (avoiding “ink on tumor”), especially if the histologic response is good. One problem is that the response is not known predictably until the resection has been carried out. Suffice it to say that this issue has not been resolved, and the margins that are accepted vary depending on the experience and judgment of the surgeon and treatment team on any given case. It is clear that intralesional margins are likely to lead to local recurrence, especially if there has been a poor response, but some osteosarcomas recur despite wide margins and good histologic response, suggesting that the aggressiveness of the tumor may play an important but at this point undefined role. Once the decision whether to perform amputation or limb salvage has been made, it is necessary to decide on the type of reconstruction. This requires lengthy discussion with the patient and, in the case of pediatric patients, the parents.
The type of reconstruction varies with the location and extent of the tumor, the age of the patient, the experience of the surgeon, and the desires of the patient. In general, patients who desire unrestricted athletic activities are encouraged to have amputations or rotationplasty in the lower extremity because they are more durable options and not subject to mechanical failure, loosening, or fracture. Patients who are more concerned with preserving the limb must first undergo careful imaging to ensure resectability and must understand the various reconstruction options. After neoadjuvant chemotherapy, the plain radiograph and MRI are repeated. The surgeon and radiologist must critically assess these studies to determine whether the major neurovascular structures are free of the tumor and the extent of tumor in the medullary cavity and in the soft tissues. If sufficient muscle cannot be preserved, soft tissue healing may be problematic, and the muscle power of the limb will be compromised. The presence or absence of tumor extending into the adjacent joint will determine whether an intraarticular resection or extraarticular resection can be carried out, and this will affect the type of reconstruction.
Intraarticular resections that preserve the joint muscles and adjacent bone are reconstructed with tumor prostheses, osteoarticular allografts, or allograft-prosthetic composites. Early function is excellent; however, the implants have a finite life span and will likely require subsequent revision surgery depending on the patient's age and activity level. Extraarticular resections result in difficult reconstructive challenges. Resection arthrodesis was used exclusively by some surgeons in the past, but is less commonly performed today for lesions around the knee or shoulder. Endoprostheses or allograft-prosthetic composites are more commonly used today. Extraarticular resections around the knee that include the patella and quadriceps mechanism can be treated with allograft-prosthetic composites that use a tibial graft with a patella and quadriceps that can be repaired to the host quadriceps tendon as an alternative to arthrodesis, amputation, or rotationplasty.
The age of the patient has a major influence of the type of reconstruction that is chosen. In young patients (younger than 10 years in girls and 12 years in boys) with lower extremity tumors, limb length inequality becomes a major issue. It is less of an issue in upper extremity lesions. In young patients with tumors around the knee, an amputation might be the optimal treatment option. Young amputees with modern prostheses do quite well, and these prostheses allow the child to return to unrestricted activities with a single operation. The complication rates and type of complications are much less than those with limb-sparing prosthesis. Alternatives to amputation include rotationplasty, expandable endoprostheses, and osteoarticular allografts. Some centers have tried novel techniques, such as distraction osteogenesis, transepiphyseal reconstructions, and combinations of allografts with vascularized fibulae that have growth centers.
Rotationplasty for tumors around the knee and hip is a very functional alternative to amputation ( Fig. 89.4A–E ), but carries a cosmetic disadvantage. For skeletally immature patients, patients with a pathologic fracture, or patients with large tumors, especially of the distal femur, a rotationplasty should be considered. A similar procedure of hip rotationplasty has been described and may be used at times for proximal femoral tumors.
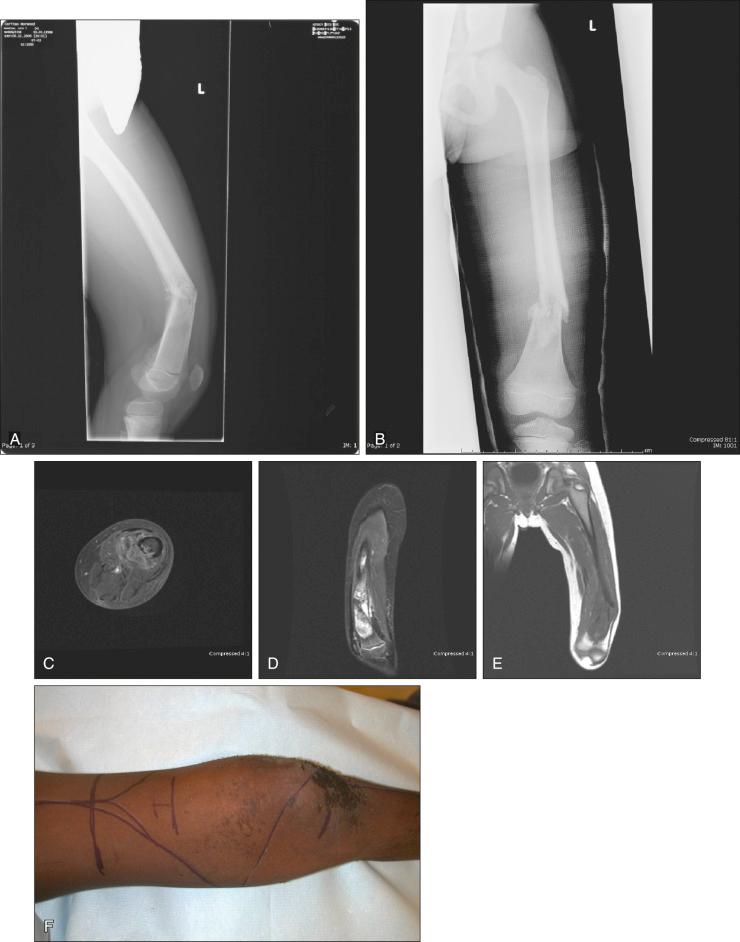
Tibial rotationplasty was first described by Borggreve in 1930 for management of a limb that was markedly shortened by tuberculous involvement of the knee. In 1950, van Nes and others extended the use of the procedure to treat congenital femoral focal limb deficiencies. The first tibial rotationplasty used for reconstruction after a radical resection of the distal femur for osteosarcoma was performed by Dr. Martin Salzer and coworkers in Vienna in 1974 and reported in 1981.
The largest collective experience, reported in 1991, involved 70 patients with sarcoma of bone who were managed with rotationplasty. Forty-seven patients had stage IIB osteosarcoma; other conditions included MFH of bone, parosteal osteosarcoma, chondrosarcoma, Ewing sarcoma, giant cell tumor of bone, periosteal osteosarcoma, and undifferentiated sarcoma of bone. Sixty-two of the lesions originated in the distal femur, six involved the diaphysis, and two originated in the tibia. At a mean follow-up of 4.3 years, Kaplan-Meier analysis revealed a 70% probability of survival and a 58% probability of disease-free survival.
The surgical oncology principles of tibial rotationplasty yield surgical margins that are comparable to those of a transmedullary amputation. In patient selection, the only absolute requirement is the absence of tumor involvement of the sciatic nerve and a previously untraumatized, essentially normally functioning foot and ankle (see Fig. 89.4F–N ). The procedure is applicable for lesions that involve the distal half of the femur and the proximal third of the tibia that spare both the peroneal and tibial divisions of the nerve. Large, locally invasive lesions of the distal femur, even with knee joint involvement, are often amenable to tibial rotationplasty.
The procedure is ideally used in young, skeletally immature patients in whom the anticipated adult limb length inequality (associated with the loss of the distal femoral and proximal tibial epiphyses) would be substantial if other reconstructions were used. The desired final effect is to have the axis of rotation of the rotated ankle joint slightly proximal to the axis of rotation of the normal knee at skeletal maturity. A recent study from Italy showed that the QoL in survivors as assessed with the 36-item Short Form (SF-36) was very similar to, and sometimes better than, that in the general Italian population, although the study stressed the importance of support from family and others.
A recent study looked at 25 patients who survived osteosarcoma and underwent rotationplasty at 15 years from treatment. They noted generally good MSTS functional scores, but that when the rotationplasty “knees” were distal to the normal knee, there were gait and cosmetic issues. They also noted early osteoarthritis of the foot and ankle joints.
Borrowing from techniques of the pediatric orthopedic surgeon, distraction osteogenesis has been used by some for limb salvage in children. This technique involves slowly transporting a segment of vascularized bone with an external fixation device to fill the gap that tumor resection creates. It requires the ability to preserve the articular cartilage, and the potential risks include close margins and infection from the percutaneous pins in patients receiving chemotherapy. The results of treating patients who have undergone a local resection for tumor of bone and treatment with bone transport (10 patients), shortening-distraction (three patients), and distraction osteogenesis (six patients) have been reported, but distraction osteogenesis has not had widespread acceptance in the United States.
Canadell and colleagues described an innovative physeal-sparing procedure in skeletally immature patients in whom the primary tumor was limited to the metaphysis. During the neoadjuvant chemotherapy phase, distraction forces were applied to the epiphysis, “pulling” the tumor away from the epiphysis and providing new, widened uninvolved metaphyseal bone for a margin of resection without sacrificing the adjacent joint. The procedure was used in 20 patients with a mean follow-up of 54 months without evidence of local recurrence ( E-Fig. 89.1 ).

Limb length is an issue to be considered in a skeletally immature patient with a tumor of the lower extremity. In general, patients younger than 8 years are probably best treated with amputation or, when possible, rotationplasty. Girls aged 8 to 10 years and boys aged 12 years have significant growth remaining, such that resection of a physis about the joint will lead to significant limb length discrepancy. One alternative for managing this issue in children is to use standard allografts or endoprostheses and rely on techniques of epiphysiodesis or subsequent limb lengthening to equalize the extremities. Another alternative is to use expandable prostheses to deal with limb length. There are several on the market, some of which require operations to expand the prostheses and others of which can be lengthened with use of electromagnetic devices in a radiology suite ( E-Fig. 89.2 ). One prosthesis allows noninvasive extension of the limb, which can be performed in the clinic as the child grows. The data on the use of this prosthesis are early but encouraging. One report of 34 children with femoral and tibial sarcomas, 27 of whom remained alive at a mean follow-up of 44 months, showed mean MSTS scores of 85% and a mean extension of 32 mm (range, 4 to 80 mm). Deep infection developed in six patients, and five patients eventually underwent amputation. The early reports of these prostheses are encouraging, especially to parents, but the long-term outcomes are unknown. They are very expensive and complex prostheses, and few data are available with respect to two key issues: whether a very young child can reach skeletal maturity with equal limb lengths and good function, and whether the results of the required revision to an adult prosthesis at skeletal maturity will be successful. Early reports show that lengthening can be achieved and that the complication rates are likely higher than in skeletally mature patients treated with nonexpandable endoprostheses. A higher rate of flexion contractures had been noted, because young children are not as cooperative with physical therapy, and loosening and failure of the implant also can occur in these complex prostheses. The optimal method of fixation of the stems to the host bone remains controversial. It is also unclear whether sufficient bone stock will remain when the patient needs to be converted to an adult prosthesis, because of stress shielding of the stem in these immature bones. A study has shown that children treated with an expandable prosthesis have high emotional acceptance as assessed with the Pediatric Outcomes Data Collection Instrument, although only 12 of 26 potential families completed the assessment.
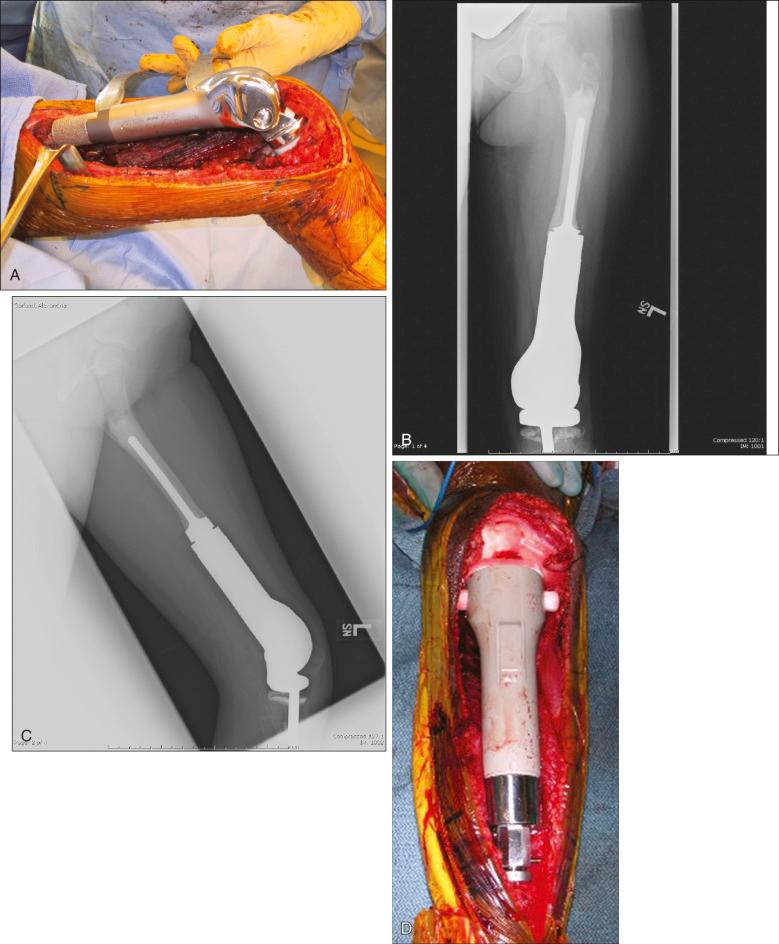
Ablation or primary amputation may be considered for patients with pathologic fracture and for patients who are younger than 9 years in whom lower extremity limb length inequality would be considerable. Also, stage IIB lesions that arise below the knee and below the elbow may be best treated with primary amputation. It should be recognized, however, that selected patients with favorable lesions that arise below the knee and elbow might also be candidates for limb-sparing procedures. Also, some pathologic fractures have been shown to heal during intensive neoadjuvant chemotherapy. We have become so accustomed to salvaging limbs that amputations are probably not being used enough. It is wise to remember that with modern prosthetics for the lower extremity, function and QoL can be quite good, and perhaps equivalent to or better than that after limb salvage for tumors of the distal femur and below. Improvements such as bony ingrowth mechanisms for attachment of the prosthesis directly to bone are being evaluated clinically. Amputation should not be viewed as a failure or sign of defeat until we develop better methods for limb reconstruction.
Early experience with metallic reconstructions for limb salvage of tumors required custom-made implants, which took time to manufacture and had design features that limited longevity. Now there are modular prostheses for most anatomic sites of the upper and lower extremities that allow the surgeon to reconstruct individual defects at the time of the resection without custom implants ( Fig. 89.5 ). Design improvements in rotating knee hinges and cementless stem options will likely lead to increased longevity of these implants. The advantages of endoprostheses include their relative ease of use, a lower reported infection rate, and a more rapid return to function because there is no waiting for union as in bone allografts. The main drawbacks include the concern for longevity of the implants and their fixation to the host bone. The complications and functional outcomes of these implants have been included in several articles. A multicenter study classified a large group of endoprosthetic complications into five categories: soft tissue complications, aseptic loosening, structural failures, infection, and tumor failure, with infection being the most common complication.
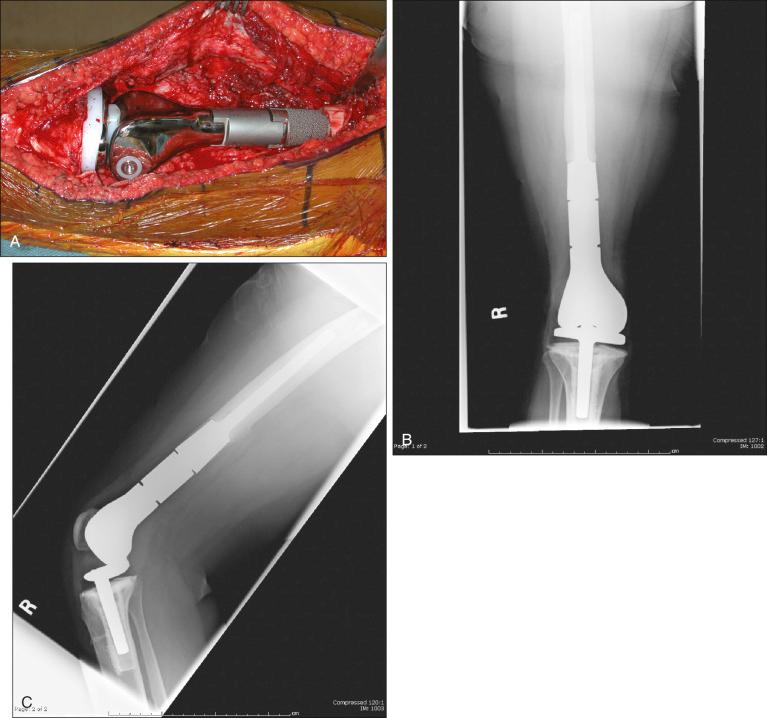
A stem design that uses constant compression forces and a cementless fixation system that is purported to induce bone hypertrophy and lessen stress shielding has been reported, but only in preliminary form. Long-term reports with sound comparisons to other types of stems are lacking in the literature. A study of 22 patients treated with an osseointegration compressive device for stem fixation reported outcomes in 18 survivors at 5 years. Aseptic mechanical implant survivorship was 16 of 18 and overall survivorship was 12 of 18. Most of the failures occurred early—that is, within the first 30 months—which indicated to the authors that there is a transient period of implant stabilization. In young children, it is unreasonable to expect a complex implant to last the patient's lifetime if the patient survives the disease, and multiple revisions are to be expected. Advances in fixation of implants to the host and design improvements in the joints will likely improve the longevity and usefulness of these implants.
Bone allografts are another alternative to limb salvage for sarcomas. They offer the potential advantage of incorporation by the host and greater longevity but are technically more demanding than prostheses. Availability of a bone bank with a variety of shapes and sizes of allografts is a prerequisite, although several large commercial bone banks that are accredited by the American Association of Tissue Banks are now available. Younger patients are more suitable because the recovery period is longer because of the need to protect the osteosynthesis site until it heals. Allografts can be used as osteoarticular grafts ( Fig. 89.6 ), replacing one side of a joint, or can be combined with a standard prosthesis to create allograft-prosthetic composites ( Fig. 89.7 ). Intercalary defects are particularly suited to allografts, because the joint on either end of the diaphyseal defect is intact ( Fig. 89.8 ). An arthrodesis of a shoulder, knee, or hip can also be created with allografts. The main drawbacks are the potential for disease transmission and the relatively high reported complication rates compared with endoprostheses.
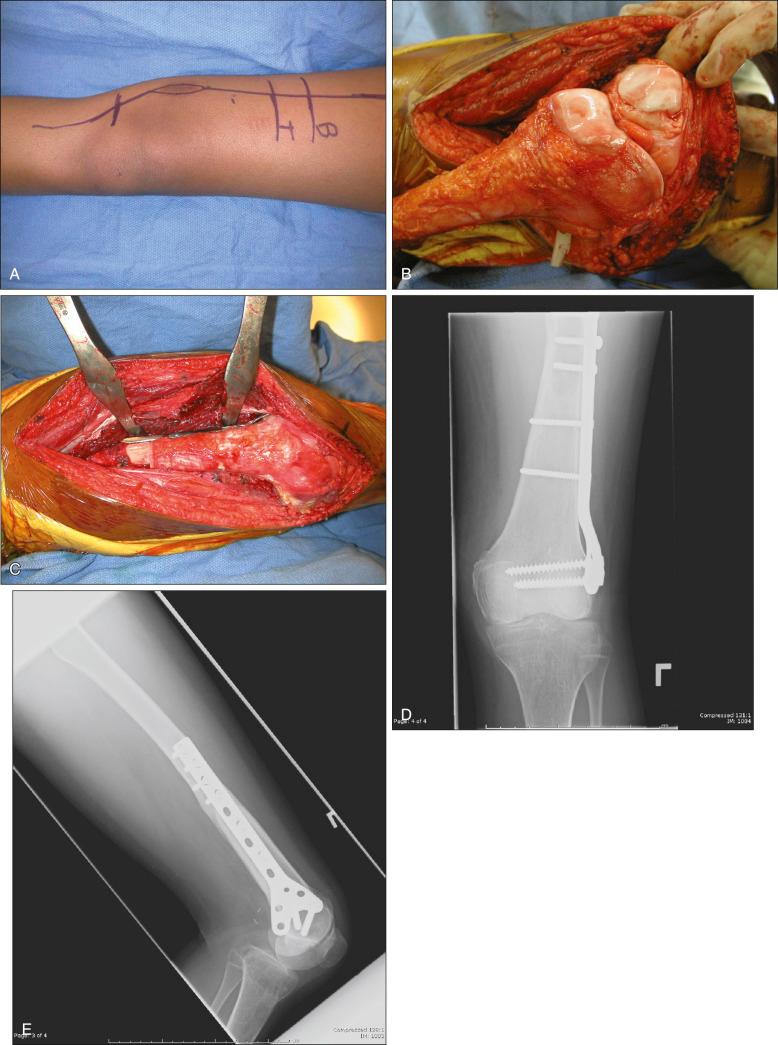
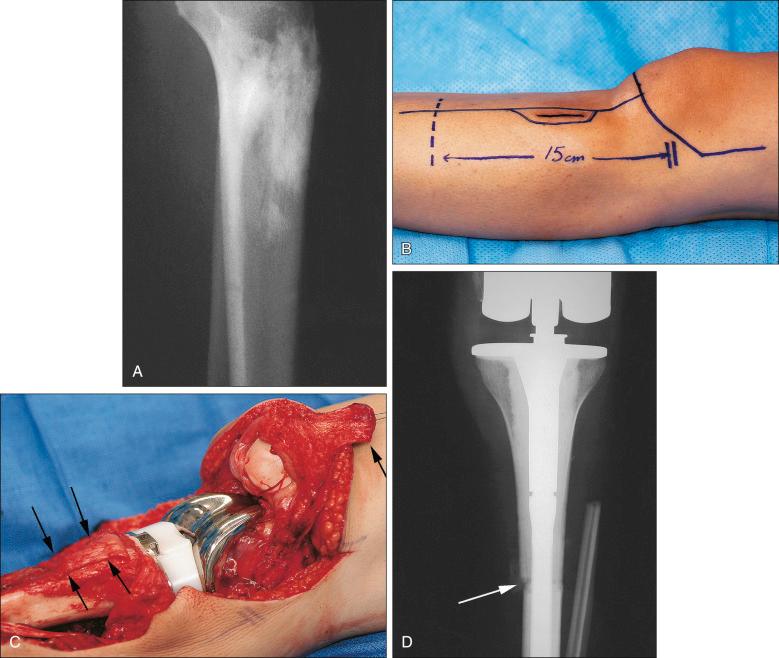
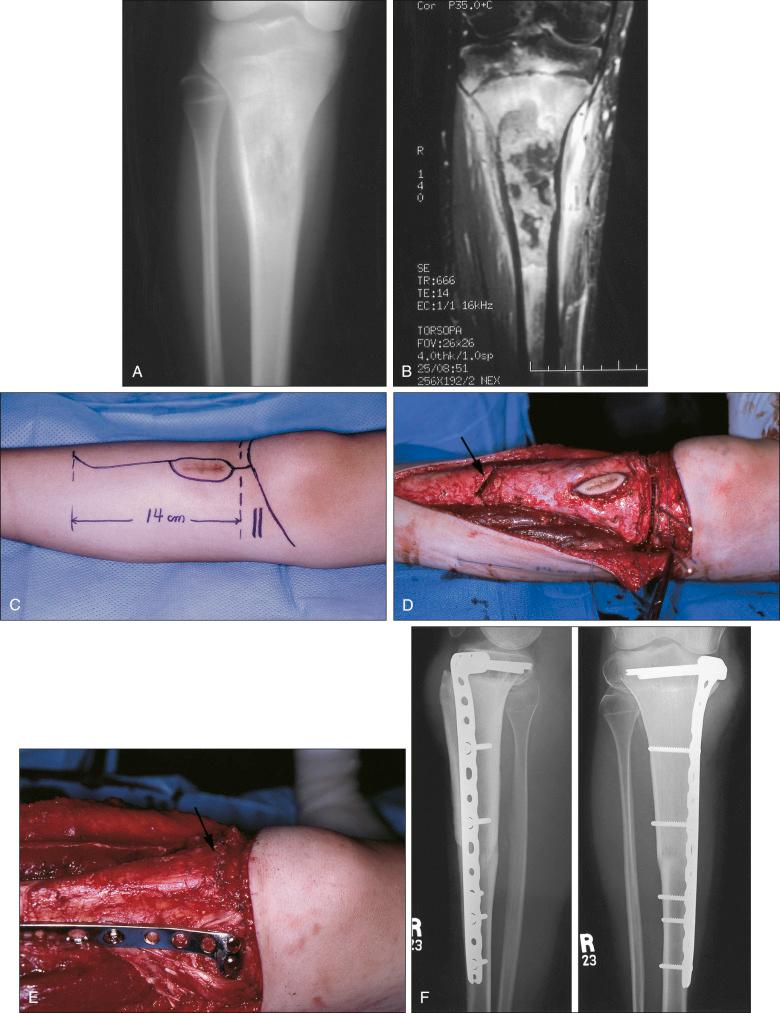
The experience with large-bone allografts for reconstruction in high-grade osteosarcoma at the Rizzoli Institute in Bologna has been reviewed by Donati and colleagues. Between 1986 and 1994, 112 large-bone allograft reconstructions were performed. Forty-one were used for arthrodesis of the knee, and three were used in the ankle. Thirty-nine were used as intercalary grafts (see Fig. 89.8 ), and 22 were used as osteoarticular allografts ( E-Fig. 89.3 ) (3 humeral, 6 in the distal femur, and 13 in the proximal tibia). Seven composite constructs were reported: two were in the proximal and distal humerus, one in the proximal femur (as in Fig. 89.9 ), and four in the proximal tibia. Complications included delayed union (>1 year without radiographic union) in 49% of patients and fracture in 30%. There were no deep infections. With the modification of the Mankin scale by Gebhardt and coworkers, good to excellent functional results were reported in 74% of the 92 patients who were available for review. Details of the options and functional results of these reconstructions at various sites are beyond the scope of this chapter. Articles that address these reconstructions include Gilbert and colleagues, Aponte-Tinao and colleagues, Abdeen and colleagues, Muscolo and colleagues, and Bianchi and colleagues.
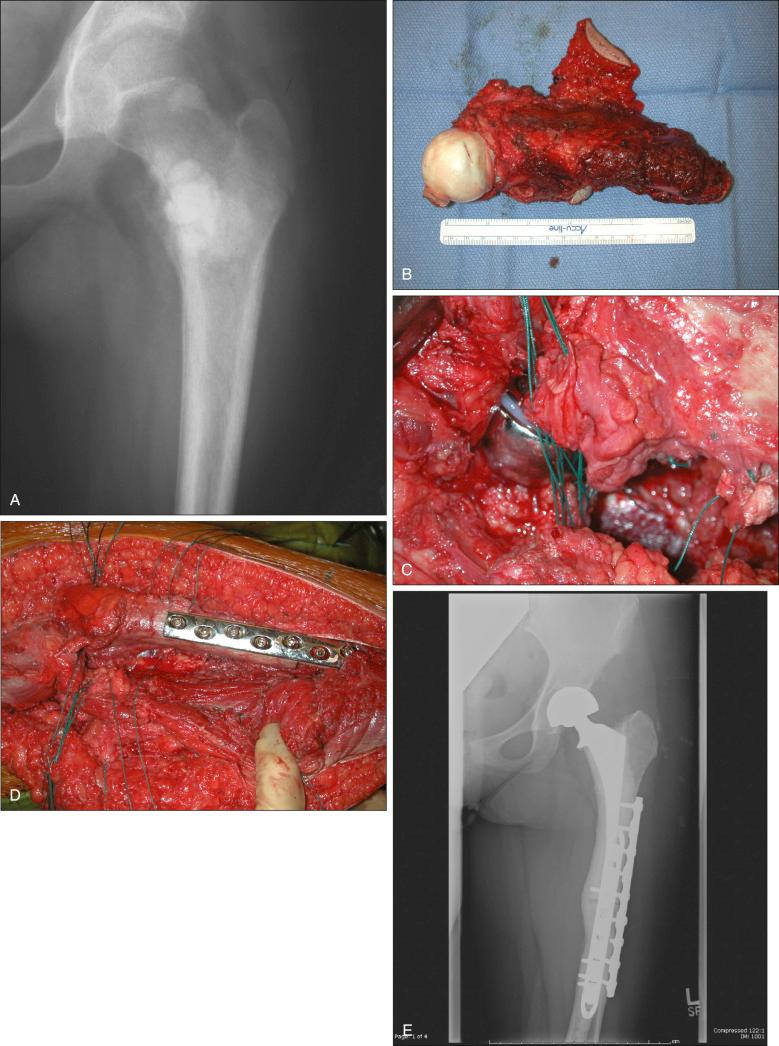
Two studies have indicated that local recurrence within 18 to 24 months of surgery has a substantial negative influence on long-term survival. Ferrari and colleagues, from three Italian institutions, reviewed the data on patients who were treated for nonmetastatic osteosarcoma of the extremities between October 1986 and June 1995; they found 162 patients with recurrence or relapse. The main prognostic factors for postrelapse survival were the relapse-free interval, site of metastasis, and number of pulmonary nodules. Complete surgical resection of the recurrence was found to be pivotal in the strategy of treatment. When the risk factors were combined, patients with a relapse-free interval longer than 24 months and with only one or two pulmonary nodules had a postrelapse survival rate of 72%. Patients with a short relapse-free interval and three or more lung pulmonary nodules had a poor postrelapse survival rate of 5%. Patients with unresectable recurrence did receive benefit from second-line chemotherapy, but the authors’ data did not support the generalized use of chemotherapy after complete surgical resection of the first recurrence.
Weeden and colleagues reported the effect of local recurrence on survival of 559 patients entered into two randomized controlled trials of the European Osteosarcoma Intergroup in which preoperative chemotherapy was used, but there was no survival benefit between chemotherapy arms. A landmark analysis allowed the assessment of 440 patients at 18 months, and 22 patients (5%) had experienced a local recurrence. Patients with a local recurrence had a fourfold greater risk of death during the 18 months after surgery. Similar analyses were conducted with landmark time points at 1 and 2 years. With a multivariate proportional hazards model, 368 patients were assessed to investigate the relative prognostic importance of histologic response and local recurrence. It appears clear that histologic response to chemotherapy and local recurrence had a significant effect on survival. A study from St. Jude's Research Hospital analyzed prognostic factors for survival in 26 patients with a local recurrence of osteosarcoma who were treated between 1970 and 2000. The initial surgical procedure was amputation in 20 patients (76.9%) and limb salvage in six patients (23.1%). Eleven patients (42.3%) developed an isolated local recurrence, and 15 patients (57.7%) developed local and distant recurrence. The 5-year estimate of postrecurrence survival (PRS) for the 26 patients was 19.2%. The factors that affected survival after recurrence were as follows:
Recurrence 2 or more years from the time of diagnosis (5-year PRS of 50.0% ± 20.4% compared with earlier recurrence (10.0% ± 5.5%) ( P = .037).
Patients with negative margins after initial surgery were found to have improved survival (5-year PRS of 33.3% ± 13.6%) compared with patients with positive margins (7.1% ± 4.9%) ( P = .015).
Patients who underwent complete surgical resection at the time of recurrence were found to have a better PRS (5-year PRS of 41.7% ± 14.2%) compared with patients who did not undergo surgery (0% ± 0%) ( P < .001).
This demonstrated that the prognosis for patients after local recurrence of osteosarcoma is poor, but attempts at salvage should be made. Complete surgical resection at the time of recurrence is essential for survival.
A study from the European Osteosarcoma Intergroup of 202 patients assessed the primary surgical treatment alternatives. Three centers differed in the rates of limb salvage, and all local recurrences arose in limbs with attempted limb salvage. Local recurrence was closely related to the adequacy of the margins of excision and to the chemotherapeutic response. Interesting to note, patients who had undergone limb salvage surgery and who experienced local recurrence still had a better survival rate than did patients who underwent primary amputation (37% versus 31% survival at 5 years), suggesting that they had a less-aggressive tumor. Of patients who experienced relapse, 31% of those with local recurrence alone were cured with further treatment, compared with only 10% of those with metastases.
A retrospective analysis of the management and outcome of 44 patients who experienced local recurrence from 1983 to 1999 after neoadjuvant chemotherapy of osteosarcoma of the extremities was performed in a single institution. In 24 patients (54.5%), local recurrence was the first sign of recurrence; in eight patients (18.2%), local recurrence followed systemic recurrence, and in 12 patients (27.3%) the two events were concurrent. The only prognostic factor that was identified for disease-free survival of patients after local recurrence was the presence of systemic recurrence at the time of diagnosis of the local recurrence or before. The 5-year postrecurrence EFS rate was 29.1% for patients without metastases at the time of local recurrence versus 0% for those with metastases ( P = .02). The analysis confirmed that patients with osteosarcoma of the extremities who experience local recurrence are at a significantly high risk for metastatic disease and dying of the tumor despite the use of effective neoadjuvant chemotherapy. Another study looked at 407 patients and found 23 patients with resectable local recurrence. Median time to local recurrence was 13 months. All patients were treated with chemotherapy after recurrence. The 5-year and 10-year survival rates in the recurrent cases were 29% and 10%, respectively. Increased risk of local recurrence ( P < .0001) was strongly correlated with positive margins of resection. The strongest correlates with poor survival were local recurrence within the first year after primary resection ( P = .001) and, as in the Bacci study, metastasis at the time of first local recurrence ( P = .04). Failure to achieve clinical remission after disease recurrence ( P = .04) was also a poor prognostic factor.
To determine whether inappropriate surgical procedures based on an initial misdiagnosis affected recurrence and survival rates, one group retrospectively reviewed the surgical treatment and results of 117 patients with high-grade osteosarcomas. Nine patients had intralesional curettage performed at other institutions based on an erroneous diagnosis of a benign lesion. Two of the nine patients underwent amputations, and seven patients underwent limb salvage procedures. Of the 108 patients who were not misdiagnosed, six patients underwent amputations and 102 patients underwent limb salvage procedures. All patients received neoadjuvant therapy. Fifteen of the 117 patients had local recurrences. Patients who had inappropriate surgical procedures at diagnosis had an increased risk of local recurrence and lower 10-year survival rates.
A study assessed the prognostic factors for survival in 449 patients with extremity osteosarcoma without metastatic disease at initial diagnosis and treatment (38 with local recurrence, 411 without local recurrence). They compared the survival difference between patients with local recurrence ( n = 38) and without local recurrence (control; n = 76) matched for age, location, initial tumor volume, and tumor volume change after chemotherapy, and assessed prognostic factors in this subgroup. In a cohort study, multivariate analysis revealed that initial tumor volume, tumor enlargement, inadequate margin, and local recurrence predicted poor survival. In the case-control study, the 10-year metastasis-free survival rates of two groups were 13.1% ± 10.7% and 19.3% ± 9%, respectively. Tumor enlargement and initial tumor volume showed multivariate significance. The investigators concluded that local recurrence has a small impact on survival in patients with high-risk osteosarcoma.
The incidence of the development of pathologic fractures of the extremity in patients with high-grade osteosarcoma varies from 10% to 13%. The occurrence of a pathologic fracture is associated with a poorer prognosis and in the past was felt to demand an immediate amputation. More recently, limb-sparing procedures have been used if there is a good response to preoperative chemotherapy.
The cause of a pathologic fracture is multifactorial and probably related to the type of osteosarcoma, the location of the lesion, the activity of the patient, and the type of trauma. The more destructive lesions, such as telangiectatic osteosarcoma, result in a thinned expanded cortex and are more subject to fracture than are other types. Lesions involving a weight-bearing bone, such as the femur, are also more likely to fracture.
Before the advent of neoadjuvant chemotherapy, the majority of patients who experienced pathologic fractures were treated with amputation. Jaffe and colleagues, however, observed that some pathologic fractures would heal while under the influence of systemic chemotherapy. They observed “reduction of the associated soft tissue mass, repair of periosteum, and deposition of new mineral in the tumor and about the fracture.” These observations made the closed management of some selected pathologic fractures feasible during treatment with neoadjuvant chemotherapy and in preparation for a limb-preserving procedure.
The subsequent healing of the fracture is also a function of the response of the tumor to neoadjuvant chemotherapy. Patients with healing fractures in general would be expected to have better overall outcomes than patients without a favorable histopathologic healing response to primary chemotherapy.
Current recommendations for treatment of patients who experience pathologic fractures from tumors that are potentially sensitive to chemotherapy include the following:
The patient must have the ability to tolerate conventional intensive chemotherapy.
The fracture should be treatable by closed means—slings (usually upper extremity fractures) or casts, external fixators that are well removed from the tumor margins, fracture braces, or traction for lower extremity fracture management. In some femoral lesions, open reduction and provisional fixation might be appropriate to stabilize the bone during neoadjuvant chemotherapy if it is determined that the procedure will not contaminate the tumor bed more extensively than the fracture itself. This is a complex decision requiring an experienced surgeon and an informed patient.
The fracture should be monitored with physical examination (e.g., decreased fracture motion and pain) and the demonstration of a radiographically favorable response (e.g., increased maturation of the tumor, increased mineral deposition, subperiosteal new bone). If no evidence of improvement or favorable tumor response can be demonstrated after chemotherapy, then consideration must be given to immediate limb-preserving surgery or amputation.
Current small retrospective reviews have suggested that patients with pathologic fracture who can tolerate both the proposed method of fracture treatment and intensive multiagent chemotherapy do not appear to have a greater risk of an unfavorable outcome compared with fracture patients who are treated with immediate amputation, unless the fracture demonstrates poor healing and unfavorable clinical response to treatment. The proximal femur may be a particularly difficult site for a pathologic fracture, as shown by the dismal 5-year survival rate of 14% in one study.
Metastatic disease is principally the result of hematogenous spread to the lungs and bone, followed by secondary involvement of the kidney, liver, and brain. Regional lymph nodes are involved in less than 10% of cases. Metastatic disease at diagnosis does not preclude long-term disease-free survival when the disease is sensitive to chemotherapy and all sites of disease can be surgically resected.
Aggressive surgical treatment of pulmonary metastases (often requiring more than one procedure) can yield disease-free survival rates of 17% to as high as 40%. In general, favorable prognostic indicators include metastasis later than 1 year from original diagnosis, unilateral disease, presence of fewer than five nodules, and completeness of resection. Early pulmonary metastases, unresectable bilateral disease, and hilar, nodal, or pleural-based lesions have a poor prognosis.
Bacci and coauthors, from the Rizzoli Institute, reported their results of treatment of 44 patients with osteosarcoma of the extremity with detectable pulmonary metastases between January 1993 and June 1995. Twenty-three patients were evaluable, having completed primary chemotherapy consisting of methotrexate, cisplatin, doxorubicin, and ifosfamide as defined by their protocol. After primary chemotherapy, lung metastases disappeared in three of the 23 evaluable patients, whereas in four patients, the pulmonary disease was assessed as unresectable. All seven patients received surgical therapy for the primary tumor only. In the remaining 16 patients, simultaneous resection of the primary tumor and of the pulmonary metastases was performed. Remission was complete in 15 of the 16 patients and incomplete in one patient. Ten patients (45.5%) remained continuously free of disease at a mean follow-up of 30 months.
Kaste and coworkers reported the experience of St. Jude Children's Research Hospital in evaluating 215 patients with extremity osteosarcoma, of whom 32 (15%) had evidence of metastatic disease. Thirty-one patients demonstrated evidence of pulmonary metastases, and only one demonstrated metastases to bone. The histologic subtype that was most commonly associated with metastases was the osteoblastic subtype ( n = 17). Both the number of nodules and the number of lobes that were involved were found to be significant predictors of survival ( P = .0009 and P = .04, respectively); multiple nodes were bilateral in 61% of patients. In the nonmetastatic cohort, the 5-year survival rate plus or minus standard error was 69% ± 4%, and the 5-year EFS rate plus or minus standard error was 52% ± 4%. This is in contrast to the results of the metastatic group ( n = 32), in which the 5-year survival plus or minus standard error was 29% ± 8%, and the 5-year event-free rate was 14% ± 7%. Notably, the risk of death increased 1.4-fold for each additional lobe that was involved with pulmonary metastases (95% CI, 1.018). The authors found a 2-year EFS rate similar to those previously reported, in spite of the inclusion of cases accrued over a 20-year span. Bacci and colleagues have reported an estimated 2-year survival rate of 45% for patients with resected pulmonary metastases. The 2-year survival rate estimated for their cohort was 52%. Meyer and colleagues reported an estimated 5-year survival rate of 11% compared with the reported cohort estimate of 14% of patients with metastases. They concluded that not only the number of pulmonary metastases but also their distribution among pulmonary lobes affected survival.
The Cooperative German-Austrian-Swiss Osteosarcoma Study Group reported their findings with patients with proven high-grade osteosarcoma of the extremities or trunk who were registered between 1979 and 1998. Of the total 1765 patients, 202 (11.4%) had clinically detected metastatic osteosarcoma. In univariate analysis, survival of the patients with metastases was correlated significantly with patient age, site of primary tumor, number and location of metastases, number of involved organ systems, histologic response of the primary tumor to preoperative chemotherapy, and completeness and time point of surgical resection of all tumor sites. After Cox multivariate regression analysis, however, only multiple metastases at diagnosis and macroscopically incomplete surgical resection remained significantly associated with poor outcomes. At a median follow-up of 1.9 years, 60 patients were alive, and 37 of the original 202 patients were in complete and continuous surgical remission. The overall actuarial survival rate at 5 years was 29%, and at 10 years it was 24%. The researchers concluded that the number of metastases and the completeness of surgical resection of all clinically detected tumor sites are of independent prognostic value in patients with proven primary metastatic osteosarcoma. A more recent study of 21 patients with metastatic osteosarcoma showed a cumulative 5-year survival rate after complete resection of 34.2%. Complete resection was found to be a significant prognostic factor for survival following metastasectomy ( P = .04). The authors concluded that every attempt should be made to completely resect all clinically detected metastases even if it means performing repeat thoracotomy in recurrent disease.
Attempts have been made to substitute newer drugs in an attempt to improve survival of patients with metastatic osteosarcoma. To date, many of these have been disappointing. Substitution of cisplatin with carboplatin does not appear to be effective. The use of topotecan is similarly disappointing. One recent ray of hope was the use of L-MTP-PE in newly diagnosed osteosarcoma patients treated in the Children's Oncology Group Intergroup-0133 (INT-0133) study. These researchers compared three-drug chemotherapy with cisplatin, doxorubicin, and high-dose methotrexate (regimen A) with the same three drugs with the addition of ifosfamide (regimen B). The 5-year EFS for patients who received L-MTP-PE ( n = 46) was 42% versus 26% for those who did not ( n = 45; P = .23), and the 5-year overall survival rates for patients who received MTP-PE versus no MTP-PE were 53% and 40%.
In addition to the psychosocial outcomes of therapy discussed previously, late effects of chemotherapy, including cardiomyopathy from doxorubicin, ototoxicity from cisplatin, and thyroid disorders, remain problematic in survivors. The addition of dexrazoxane has been shown to reduce the toxic effects of doxorubicin on the myocardium. Infertility is another concern for patients who receive chemotherapy, especially with alkylating agents. A study has shown that although patients who undergo chemotherapy should be informed of the possibility of infertility, many survivors of pediatric sarcoma treatment can be expected to have successful childbirths. Teenage males might want to consider sperm banking.
Aung and colleagues described a single-institution assessment of patients who experienced metachronous skeletal osteosarcoma from 1973 to May 2000. A retrospective review of records from 426 patients with nonmetastatic high-grade and primary osteosarcoma revealed 23 patients, with a median age of 18.7 years, who experienced metachronous osteosarcoma. Initial therapy included combination chemotherapy and surgery. Treatment of the metachronous relapse consisted of chemotherapy or radiation alone or surgery with or without additional individualized chemotherapy.
The median time to the diagnosis of a metachronous lesion was 1.4 years, with a range of 0.2 to 11.3 years. Patients who experienced late metachronous osteosarcoma experienced significantly longer postmetachronous osteosarcoma survival compared with those whose metachronous disease developed early. For the former group, the 2- and 5-year postmetachronous osteosarcoma survival rates were 72.7% and 61%, respectively. In contrast, all five patients who experienced metachronous disease between 12 and 24 months of their original presentation died within 1.5 years of follow-up. Among patients who experienced late metachronous disease, further analysis based on type of therapy suggested that those who were treated with a combined modality (including surgery and aggressive chemotherapy) for their metachronous disease fared better than did those who received monotherapy. The authors also concluded that local therapy with surgery directed at the bulky primary tumor is the only effective method for local control.
Rodriguez and associates described the treatment of five patients in whom primary osteosarcoma had been treated effectively with chemotherapy and resection of the primary tumor and in whom another osteosarcoma developed at another site but without pulmonary metastases. The interval between management of the primary tumor and the subsequent development of another, apparently unrelated metachronous osteosarcoma ranged from 99 to 150 months. All patients remained otherwise free of disease 24 to 96 months after treatment of the metachronous lesion, except for one patient who experienced acute myelogenous leukemia, most likely as a late effect from chemotherapy. Fitzgerald and colleagues reported that among 800 patients, 12 experienced metachronous osteosarcoma, and none experienced pulmonary metastases, a quite different response from that of patients who were reported with primary metastatic osteosarcoma. Others have observed that aggressive treatment of metachronous osteosarcoma is indicated and suggested that the etiology in some might be related to hereditary RB or Li-Fraumeni syndrome.
Aung and associates retrospectively reviewed the experience of the Memorial Sloan-Kettering Cancer Center with long-term survivors of osteosarcoma and the subsequent development of secondary malignancies. The patients were treated between February 1973 and March 2000, and all had received chemotherapy and/or surgery. Chemotherapy consisted of a combination of agents that included high-dose methotrexate, doxorubicin, bleomycin, cyclophosphamide, dactinomycin, vincristine, cisplatin, and ifosfamide. Of 509 patients, 14 were identified with secondary malignant neoplasms. The time interval from diagnosis of the primary osteosarcoma to development of the secondary malignant neoplasm ranged from 1.3 years to 13.1 years (median, 5.5 years). The second neoplasms occurred in the central nervous system in four patients. There were two cases of acute myeloid leukemia and one case each of myelodysplastic syndrome, non-Hodgkin lymphoma, high-grade pleomorphic sarcoma, leiomyosarcoma, fibrosarcoma, carcinoma of the breast, and mucoepidermoid carcinoma. The standard incidence was 4.6% when patients with a history of RB or Rothmund-Thompson syndrome were excluded.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here