Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was supported by grants from the National Institutes of Health (NIH) to Dr. Narayan (HL83359, HL103800, and HL149134) and to Dr. Rappel (HL122384 and HL145500). Dr. Zaman is funded by a Fulbright award and by a fellowship from the British Heart Foundation.
A major unresolved question in atrial fibrillation (AF) is whether mapping and targeting patient-specific mechanisms can improve the current 60% to 70% therapeutic ceiling of success from ablation. Ablation based on pulmonary vein isolation (PVI) is more effective than medications, yet in some patients AF may recur despite isolated pulmonary veins, whereas in others AF can be eliminated despite reconnected pulmonary veins. Anatomic strategies to extend beyond PVI have yet to improve AF outcomes, with no improvement and a trend for lower success when adding linear lines, complex fractionated electrograms, and even posterior wall isolation in one randomized trial to PVI compared with PVI alone.
AF mapping encompasses approaches to identify potential patient-specific mechanisms, with a view to personalized therapy. Potential mechanisms include localized electrical rotational and focal sources (or drivers of AF), localized regions of scar or fibrosis, or disorganized waves with no spatial predilection. This chapter is motivated by a growing body of bench-to-bedside data supporting drivers for AF , ; clinical experience that localized ablation may empirically eliminate persistent AF; data that AF exhibits consistent gradients in rate, organization, and spatial vector, suggesting a spatial hierarchy in organization; and data that extensive empirical ablation may not improve outcomes.
We examine the results of mapping AF in patients and ex vivo human atria. Our goal is to critically examine the localized source hypothesis, which is supported by optical mapping of AF in human atria , and animal models but why this hypothesis may be central to some patients and not others. , We conclude with future directions that we believe must be studied to advance the field.
Arrhythmias involve dynamic and static mechanisms. In AF, triggers such as ectopic beats, bursts of atrial flutter, or fluctuating autonomic balance , may initiate dynamic AF mechanisms that overlay on static mechanisms such as abnormal atrial architecture, surface curvature, and tissue composition (such as fibrosis).
Dynamic atrial repolarization and conduction may explain how triggers initiate AF. In Fig. 43.1A, a single premature atrial complex (PAC) produces dramatic oscillations of human left atrial action potential duration (APD). Specifically, APD of the PAC shortens compared with steady state, which then lengthens diastolic interval (DI) and APD of the next beat, which shortens DI and APD of the next but one beat and so on (see Fig. 43.1B ). If this so-called restitution relationship of APD to DI has slope greater than 1, a PAC can produce APD oscillations (alternans), which can in turn vary spatially (discordant alternans) and precipitate wave break.
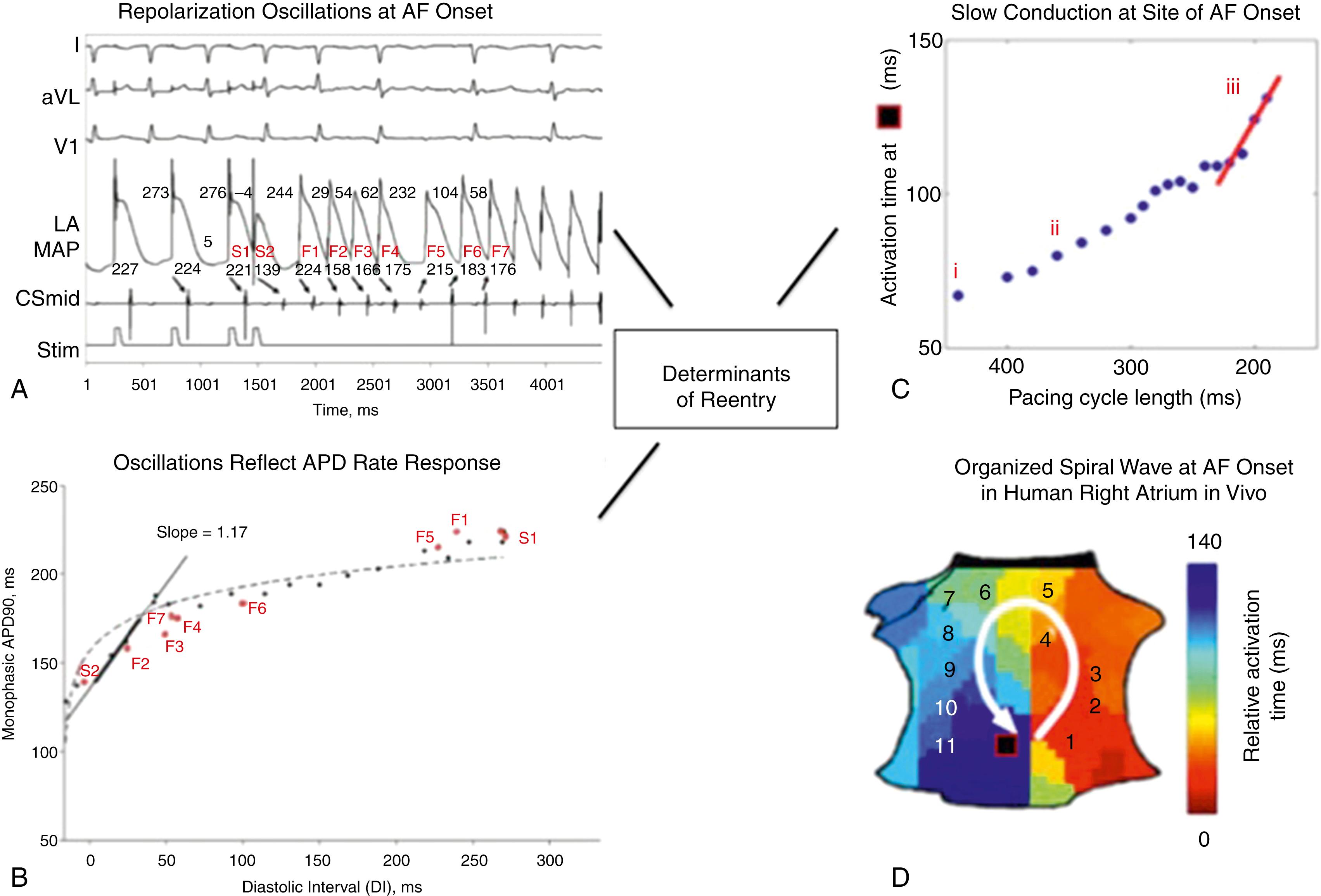
This dynamic milieu can be further exaggerated by rate-related slowing of atrial conduction velocity. In Fig. 43.1C a trigger dramatically slows human atrial conduction, which in this case enables reentry observed just prior to AF onset (see Fig. 43.1D ). Panoramic mapping studies of single PACs or tachycardia have shown that human AF may initiate by reentry when conduction restitution is engaged, or by repetitive focal firing.
Other mechanisms that may explain AF initiation include autonomic innervation, which may both trigger and sustain AF in animal models and can be targeted by ablation of epicardial ganglionated plexi or renal denervation. Interaction with fixed atrial anatomy or fibrosis may explain why separate AF initiations may occur at spatially conserved sites in a given patient even for diverse and varying triggers.
These mechanisms are consistent with theoretical studies of wave propagation in excitable systems, in which wave break results in one or more spiral waves, which drive AF and which may be either localized or meander.
Once initiated by triggers from pulmonary veins or other sites, disorganized wavefronts must be replenished for AF to sustain. Two mechanisms are proposed. In one model, disorganized activity self-sustains because new wavelets are generated across atrial tissue over time. Computer simulations reveal that such wavelets can be generated by unstable spiral waves, wave break, and new spiral waves. , Collision from repetitive foci can also destabilize wave propagation, again producing new wavelets. Notably, this hypothesis enables AF to occur in homogeneous tissue and does not require preferred regions. In a second model, preferred atrial regions of interest perpetuate AF. Specifically, such regions of interest may represent localized rotational or focal sources (also termed drivers); regional electrical disorder, which can drive surrounding fibrillation; or coincide with regions of fibrosis, microfibrosis, or tissue anisotropy. The process by which preferred regions cause surrounding disordered activation is termed fibrillatory conduction.
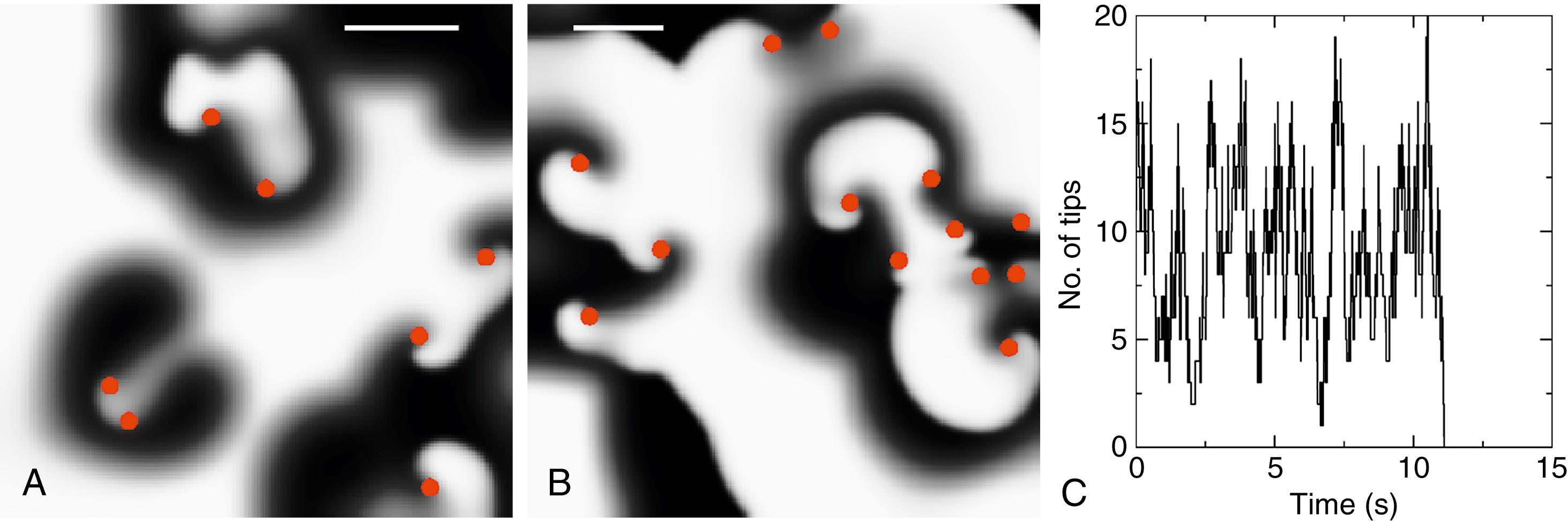
The quantitative balance between wavelet generation and termination determines AF duration and is critical to both models. Fig. 43.2 shows computer simulations of unstable spiral waves and multiwavelet reentry in homogeneous two-dimensional (2D) domains with nonconducting boundaries. In Fig. 43.2A , the number of spiral tips fluctuates and numbers approximately five for this 4.5 × 4.5 cm domain and parameter set. Because the number of tips is stochastic, there is a finite probability that the number of tips will spontaneously reach 0, typically by reaching a nonconducting boundary and annihilating, in which case AF will terminate. In 100 simulations with different random initial conditions, we found that the average spontaneous termination time was ≈3.3 seconds. Fig. 43.2B shows a simulation of identical parameters in a larger domain (5.625 × 5.625 cm) with eight spiral wave tips, in which termination time was ≈15 seconds. The result of a typical simulation, presenting the number of spiral tips as a function of time, is shown in Fig. 43.2C . Multiwavelet reentry thus lasts longer if spatial domains are larger, as seen clinically, but will ultimately terminate unless waves are regenerated. It is possible to derive expressions for the mean duration of AF once the rates for creation and extinction of wavelets are known.
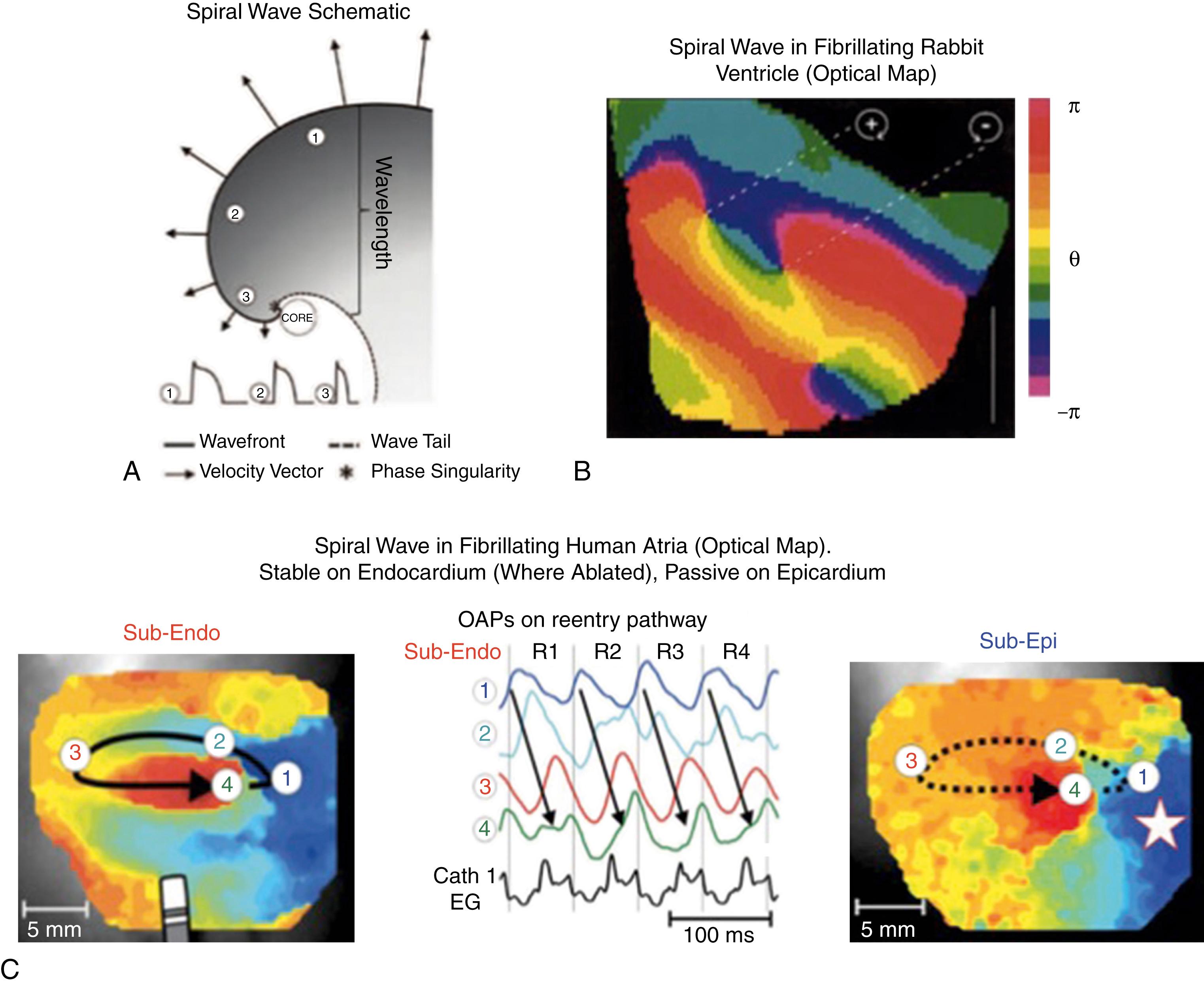
Localized rotational or focal sources provide one mechanism to replenish disordered wavelets. Rotors have been described in animal models of ventricular and atrial fibrillation since seminal work by Jalife and others in the 1990s. Classically, a rotor is defined as reentry around an inexcited yet excitable core, activating too rapidly for surrounding tissue to keep up and resulting in disordered waves (so-called fibrillatory conduction) ( Fig. 43.3A ). This has now been shown in multiple animal studies (see Fig. 43.3B ) and more recently in human atria during AF at sites in which ablation terminates AF (see Fig. 43.3C ). , The localized source hypothesis may explain how AF sustains in small volumes such as mouse hearts and how limited ablation may terminate persistent AF in patients. , There has been debate on specific definitions of a rotor. Given the resolution of clinical mapping, in this chapter all forms of rapid rotational activity with surrounding fibrillatory conduction are considered. Rapid focal activity can also produce fibrillatory activity, and it is considered a source or driver mechanism for AF. AF will continue as long as a source(s) exists, and source elimination should enable fibrillatory wavelets in AF to terminate stochastically.
AF exhibits spatially varying wavelets, which propagate rapidly at the limits of refractoriness. In designing AF mapping, one would thus ideally map activation and recovery at high temporal resolution and at multiple sites without cross talk (contamination) from adjacent tissue. Because AF is spatially nonuniform, mapping should be global. No mapping approach is perfect for this purpose in patients. However, several aspects can be accomplished by mapping AF signals in broad areas at moderate resolution (panoramic mapping), or by mapping AF in narrow focused areas successively at high resolution and correcting for variations over time.
Optical mapping of voltage-sensitive dyes is a gold standard for identifying electrophysiology. Optical mapping is well suited to AF because it identifies action potentials and thus simultaneously maps activation and recovery at high temporal and spatial resolution, with minimal cross talk from adjacent tissue activating at different rates (see Fig. 43.3 ). Phase analysis developed by Gray and associates (see Fig. 43.3B ) depicts progression of activation from depolarization through repolarization. Areas in fibrillation where phase exhibits a full rotation (i.e., −π to +π) are termed singularities or rotors. Rotational and focal activation can also be detected by activation mapping.
In human atria, ex vivo optical mapping of AF shows stable reentrant circuits on the endocardium, detectable without or with phase mapping, and disordered waves on the epicardium , (see Fig. 43.3C ). Endocardial sources may be single or multiple, in right or left atria, and were stable within 1 cm 2 areas of microfibrosis and anatomic complexity on the endocardium. Localized ablation at endocardial circuits terminated AF in these hearts. This demonstration of localized sources for human AF correlates well with at least one clinical mapping tool. These human optical studies provide powerful evidence for AF sources and provide a unique platform to validate results of various mapping tools in AF.
There are several limitations to this approach. First, optical mapping is not yet clinically feasible because of dye toxicity, the need to register images without immobilizing the heart, and difficulties of imaging through blood. It has been argued that optical studies may be contaminated by the use of pharmacologic agents such as pinacidil to induce AF, although those interventions shorten refractoriness and should facilitate disordered waves rather than stable features. Second, studies have questioned the use of phase mapping, although AF sources have been shown without phase mapping such as in optical studies of human AF. Although phase mapping may overemphasize rotational activity, activation mapping may overemphasize focal activity. Indeed, some criticisms of phase mapping are more accurately leveled at definitions of rotational or focal features rather than phase mapping per se. For instance, Vijayakumar reported phase mapping of sinus rhythm and found false rotations. Inspection of the raw data, however, shows rotational activity sites in the raw activation data, likely eddy currents from structural nonuniformities. Similarly, random waves propagating in opposite directions past a line of block may create transient rotational activity. However, this should not repeat if waves in AF are random; criteria requiring multiple rotations in the same location should identify organized sites from false positives. Thus the spatial and temporal criteria used to define repetitive rotational or focal sites is a central issue.
Attempts have been made in patients to map AF , using monophasic action potential (MAP) catheters, which accurately record activation and recovery as validated against intracellular recordings. However, it is difficult to record MAPs at multiple sites, and such efforts have been limited to a few centers. , Accordingly, the use of MAP has not yet resulted in a mapping approach to circumvent the lack of in vivo optical mapping in patients.
A series of elegant studies used intraoperative recordings to map AF in subjects with long-standing persistent AF at concomitant surgery or AF induced in control subjects. , Such studies used plaques typically placed on the right atrium and more recently the left atrium. AF electrograms were analyzed to assign one “best” activation time for each electrogram, which is the traditional approach, used to create contour maps of activation (isochronal maps) of AF. These epicardial maps typically show disordered waves with no rotational activity, transient rotations, or transient focal activity. ,
Although these data appear to contradict the results of optical mapping of the endocardium in human AF, they are in fact concordant with optical mapping of the epicardium. , Both epicardial approaches show transient focal beats, which may reflect breakthrough events, and few sustained organizational rotational elements. Conversely, endocardial optical and clinical mapping shows localized drivers.
There are other potential differences between surgical electrical and optical maps of AF. First, surgical AF mapping uses 1 to 2 cm 2 area plaques, which cover less than 10% to 20% of the more than 100 cm 2 atrial areas of long-standing AF patients at predetermined sites. Such recordings could potentially miss patient-specific regions of interest. Second, surgical patients with long-standing persistent AF caused by valve lesions or pathology may differ mechanistically from patients typically taken to the electrophysiology laboratory for ablation. Finally, interpreting AF electrograms by assigning an activation time can be difficult.
Electrogram interpretation in AF is a particular issue, as rules are somewhat arbitrary and results may not correlate with timings from action potentials mapped optically. , Atrial electrograms rarely record tissue repolarization, unlike action potentials, so in AF it is challenging to define which electrogram components reflect local activation, recovery, and far-field contamination. Bipolar electrodes, used in many historical AF mapping studies, compare extracellular potentials at two closely spaced poles but are highly directional. If a local wavefront reaches both poles simultaneously, no electrogram will be inscribed and activation will not be mapped. Conversely, wavefront collision or noise with a vector parallel to the bipole will be included in maps. Bipolar maps of AF may thus be misleading. Fig. 43.4 shows these effects in patients and simulations. Fig. 43.4A shows a simulation of a stable spiral surrounded by fibrillatory breakdown. The activation front arriving at the bipolar electrode (white dots in the coherent domain) is missed at time 2 because slight precession of the tip alters the direction of the activation wave relative to the recording bipole. Fig. 43.4B shows that clinical bipolar signals in AF may miss local activations captured by an adjacent MAP catheter.
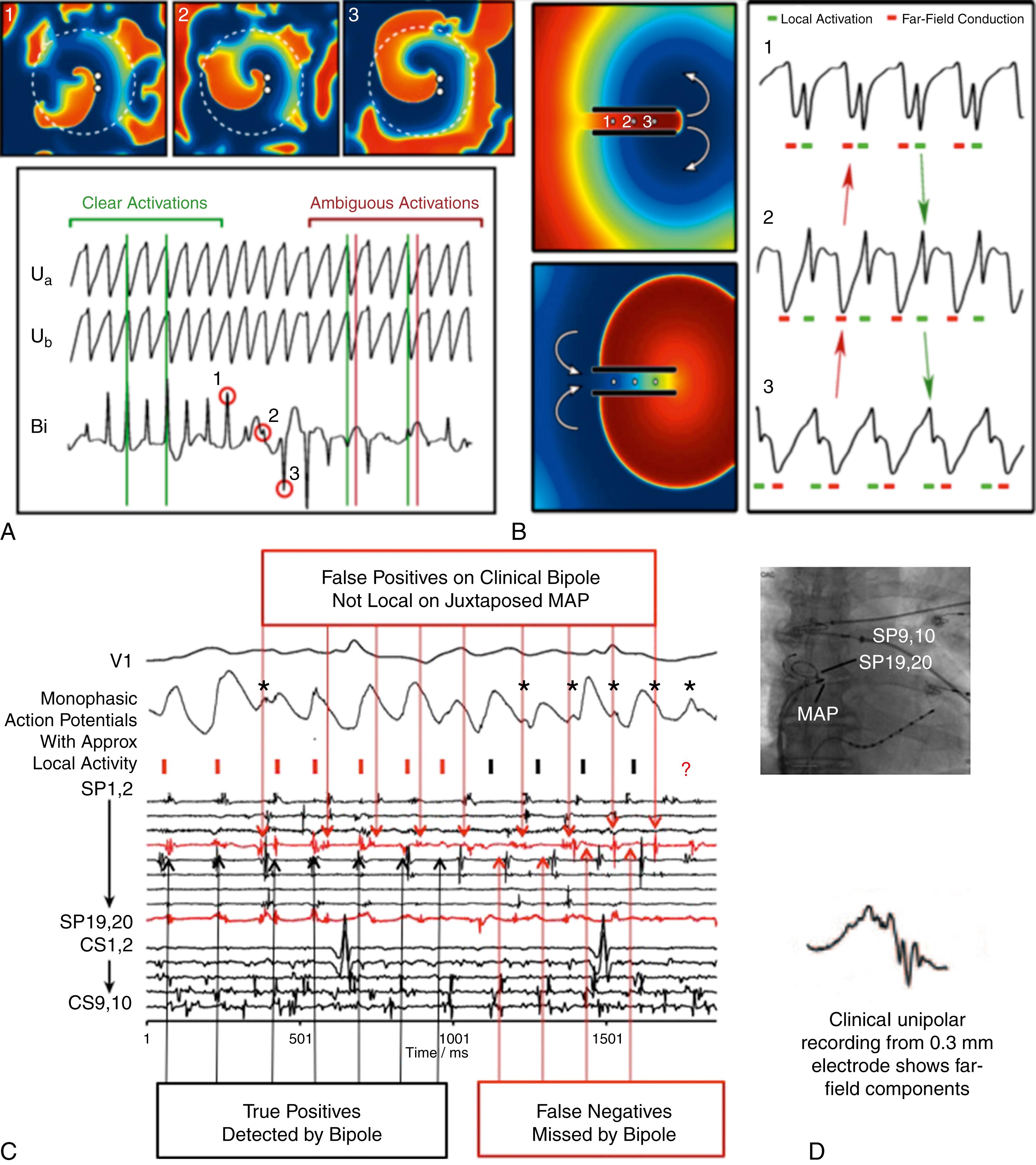
Unipolar electrode recordings avoid the directionality in bipolar signals but add greater far-field effects. This is true even for unipoles recorded from small 0.3 mm electrodes as seen in reference (see Fig. 43.4C ) and in simulations. Accordingly, unipolar signals can be challenging to interpret even in noncomplex arrhythmias. Fig. 43.4D shows simulated macroreentry through a narrow isthmus, in which unipolar electrograms at isthmus locations 1, 2, and 3 display multiple deflections representing local (green) and far-field (red) areas. This makes local activation time difficult to assign and can result in incorrect maps (red arrows). Electrograms may be even more complex in a fibrotic milieu, further influencing electrogram annotation and isochronal analysis.
It is unclear how to identify local activity in AF electrograms from far-field activity and noise. Although these components are readily separable in action potentials, action potentials are difficult to obtain in patients. These difficulties translate to ambiguities in mapping.
One advance in electrogram analysis has been to move beyond assigning a single activation time fiducial to individual electrograms; instead, use metrics that capture entire signal shapes or the relationships between signals. This analysis tells a different story to classical marking of activation times. Features such as dominant frequency, spatial vectors, or morphology often show AF organization consistent with sources. In AF patients, such studies have reported repetitive gradients in frequency distribution, , electrogram shapes, , and propagation vectors over time. , Moreover, these analyses suggest that AF is more stable than when analyzed by single marked electrogram onsets by these same groups. ,
In summary, the results of AF mapping are exquisitely sensitive to technique, introducing several challenges. First, it is not established whether mapping of the endocardium or epicardium is more relevant to AF therapy. Second, electrogram analysis methodology matters. At sites where newer mapping tools show organized rotational and focal regions, traditional electrogram analyses often show only disorder. Third, electrograms analyzed using organizational indices of rate, dominant frequency, or repetitiveness also reveal more organization than traditional mapping based on activation times. Accordingly, a common approach to define AF mechanisms has been to assess the effect of ablation at features identified by each mapping tool.
We set out in 2001 to probe mechanisms for human AF by attempting to recreate optical mapping in patients using computational analysis of signals acquired using contact electrodes in the electrophysiology laboratory. We did this by mapping both atria widely with baskets, , using algorithms to filter AF electrograms based on measured repolarization and conduction dynamics , from our studies of MAP dynamics in human atria. We further set out to test the relevance of identified features by targeted ablation. Our initial hypothesis was that localized sources do not exist in human AF.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here