Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Rickettsial diseases are emerging infectious diseases. Because of better diagnostic tools, increased awareness, and changes in tick exposure, new rickettsial diseases continue to be described in recent years. Three families of diseases are grouped under rickettsioses: ehrlichioses and anaplasmoses and Q fever ( Table 302-1 ).
| GENUS | GROUP | SPECIES | SUBSPECIES | |
|---|---|---|---|---|
| Rickettsiae | Rickettsia | Typhus | R. prowazekii | |
| R. typhi | ||||
| Spotted fever | R. aeschlimannii | |||
| R. africae | ||||
| R. akari | ||||
| R. asembonensis | ||||
| R. australis | ||||
| R. conorii | Conorii | |||
| Israeli | ||||
| Caspia | ||||
| Indica | ||||
| R. felis | ||||
| R. fournieri | ||||
| R. helvetica | ||||
| R. heilongjiangensis | ||||
| R. honei | ||||
| R. japonica | ||||
| R. massiliae | ||||
| R. monacensis | ||||
| R. parkeri | ||||
| “ R. philipii ” | ||||
| R. raoultii | ||||
| R. rickettsii | ||||
| R. sibirica | Sibirica | |||
| Mongolitimonae | ||||
| R. slovaca | ||||
| R. tillamookensis | ||||
| Candidatus R. xinyangensis | ||||
| Orientia | Scrub typhus | O. tsutsugamushi | ||
| Ehrlichiae | Ehrlichia | E. chaffeensis | ||
| E. ewingii | ||||
| E. canis | ||||
| E. muris –like | ||||
| Candidatus Neoehrlichia mikurensis | ||||
| Anaplasma | A. phagocytophilum | |||
| Neorickettsia | N. sennetsu | |||
| Wolbachia | W. pipientis | |||
| Coxiellae | Coxiella | C. burnetii |
The agents of rickettsial diseases are small, gram-negative, intracellular bacteria that are associated with eukaryotic cells. Except for Coxiella burnetii , which is the agent of Q fever, they have not yet been grown in axenic media, but rather require living hosts (e.g., cell cultures, embryonated eggs, or susceptible animals) for growth. With the exception of Rickettsia prowazekii , which is the agent of epidemic typhus and which is specifically associated with the human body louse, these bacteria infect humans incidentally and are mainly animal pathogens. Polymerase chain reaction (PCR) amplification of DNA from blood samples, biopsy specimens, and cutaneous eschar swabs has helped identify new species.
Rickettsia spp are small gram-negative bacteria that multiply freely in the cytoplasm of their host cells. The genome of Rickettsia is small, between 1.1 and 1.6 Mb; some have plasmids and potential for conjugation. These bacteria have a family of outer membrane proteins of the surface cell antigen family, including rOmpA (lacking in the typhus group) and rOmpB. These proteins are major antigens that help identify the rickettsial species by serology, and their encoding genes are used for amplification and sequencing for diagnostic or taxonomic purposes. Complete genome sequencing of Rickettsia species demonstrates that these bacteria are undergoing a genomic reduction process as a result of various phenomena, including progressive gene degradation and genomic rearrangements and also a paradoxical expansion of small RNAs and short palindromic elements. Genome sequence–based taxonomic information can reliably classify rickettsial isolates at the genus and species levels.
The target cells in humans are vascular endothelial cells or monocytes, and vasculitis is the most prominent result. These bacteria invade cells by phagocytosis and escape the phagosome vacuole.
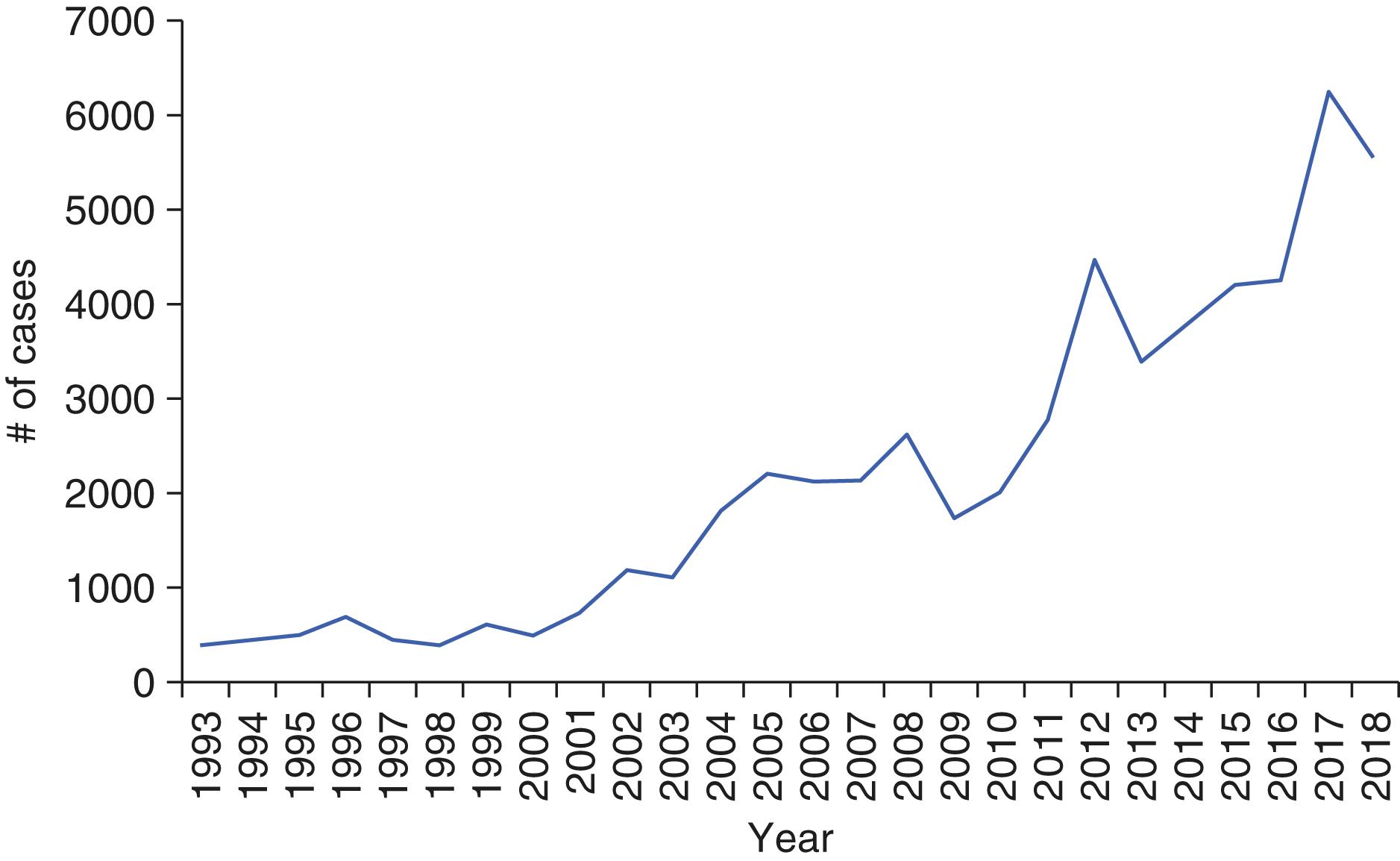
Rocky Mountain spotted fever (RMSF), which is the most severe of tick-borne rickettsioses, is caused by Rickettsia rickettsii ( Table 302-2 ). Rocky Mountain spotted fever is the major tick-borne rickettsiosis recognized in America and is prevalent in at least 44 states in the United States ( Fig. 302-1 ) as well as in Central and South America (Argentina, Brazil, Colombia, Costa Rica, Mexico, and Panama).
| DISEASE | ORGANISM | ARTHROPOD HOST | GEOGRAPHIC AREA | RASH | ESCHAR TACHE NOIRE | REGIONAL LYMPH NODE | HIGH FEVER | FATALITY RATE |
|---|---|---|---|---|---|---|---|---|
| TICK-TRANSMITTED SPOTTED FEVERS | ||||||||
| Rocky Mountain spotted fever | R. rickettsii | Dermacentor andersoni | America (North, Central, and South) | Yes, may be purpuric | Very rare | No | Yes | High |
| Dermacentor variabilis | ||||||||
| Rhipicephalus sanguineus | ||||||||
| Amblyomma cajennense | ||||||||
| Mediterranean spotted fever, Astrakhan fever, Indian tick typhus, Israeli spotted fever | R. conorii | Rhipicephalus sanguineus | Mediterranean, India, Caspian Sea, Africa | Yes, papular; may be purpuric | Yes | No | Yes | Moderate |
| African tick-bite fever | R. africae | Amblyomma hebraeum | Sub-Saharan Africa, West Indies | Yes, half of cases may be vesicular | Yes (frequently multiple) | Yes | No | Low |
| Amblyomma variegatum | ||||||||
| Queensland tick typhus | R. australis | Ixodes holocyclus | Eastern Australia | Yes, may be vesicular | Yes | ? | Yes | Moderate |
| Siberian tick typhus | R. sibirica | Dermacentor nuttallii | Siberia, China, Mongolia | Yes | Yes | No | Yes | Low |
| Scalp eschar, neck lymphadenopathy after tick bite (SENLAT) | R. slovaca or R. raoultii | Dermacentor marginatus | Europe, Pakistan | Very rare | Yes, may be erythematous | Yes (painful) | No | Low |
| Dermacentor reticulatus | ||||||||
| Lymphangitis-associated rickettsiosis (LAR) | R. sibirica mongolitimonae | Hyalomma asiaticum | Mongolia, Africa, Europe | Yes | Yes | Yes | Yes | Low |
| Flinders Island spotted fever | R. honei | Ixodes granulosus | Flinders Island, eastern Australia | Yes | Yes | Yes | Yes | Low |
| Japanese spotted fever | R. japonica | Ixodes ricinus | Japan, Korea (China?) | Yes | Yes | No | Yes | Low |
| Pacific coast tick fever | “ R. philipii ” | Dermacentor occidentalis | Northern California | Yes | Yes | No | Yes | Unknown |
| Unnamed | R. aeschlimannii | Hyalomma sp | Mediterranean, Africa | Yes | Yes | Yes | Yes | Unknown |
| R. helvetica | Ixodes ricinus | Europe, Asia | No | Yes | No | No | Unknown | |
| R. massiliae | Rhipicephalus sanguineus | Europe, United States | Yes | Yes | No | Yes | Unknown | |
| R. monacensis | Ixodes Ricinus | Europe | Yes | Yes | No | Yes | Unknown | |
| R. parkeri | Amblyomma maculatum | America | Yes | Yes | No | Yes | Unknown | |
| Candidatus R. xinyangensis | Haemaphysalis longicornis | China | No | Yes | Yes | Yes | Unknown | |
| FLEA-TRANSMITTED DISEASES | ||||||||
| Murine typhus | R. typhi | Xenopsylla cheopis | Worldwide | Yes | No | No | Yes | Low |
| Ctenocephalides felis (mosquitoes) | ||||||||
| Flea-borne spotted fever | R. felis | Ctenocephalides felis , Aedes mosquitoes | Worldwide | Sometimes | Sometimes | Unknown | Yes | Unknown |
| Unnamed | R. asembonensis | Ctenocephalides felis | Peru, Zambia | Unknown | Unknown | Unknown | Yes | Unknown |
| LOUSE-TRANSMITTED DISEASES | ||||||||
| Epidemic typhus | R. prowazekii | Pediculus humanus corporis | Worldwide | Yes | No | No | Yes | High |
| Amblyomma ticks (?) | ||||||||
| American sylvatic typhus | R. prowazekii | Flying squirrel ectoparasites | United States | Yes | No | No | Yes | Low |
| Brill-Zinsser disease (relapse of epidemic typhus) | R. prowazekii | Worldwide | Yes, could lack | No | No | No | Low | |
| MITE-TRANSMITTED DISEASES | ||||||||
| Rickettsialpox | R. akari | Liponyssoides sanguineus | Worldwide | Yes, vesicular | Yes | Yes | Yes | Low |
| Scrub typhus | Orientia tsutsugamushi | Leptotrombidium sp (chiggers) | Central and Eastern Asia, Australia | Yes | Yes | Yes | Yes | High, may relapse |
In ticks, R. rickettsii is transmitted transovarially from one generation to the next. The infecting ticks are mainly Dermacentor andersoni (a wood tick) in the Western United States; Dermacentor variabilis (the American dog tick) in the East, the Midwest, and the South; and Rhipicephalus sanguineus in Arizona. In Central and South America, Amblyomma cajennense is the major vector. Humans are infected through infected saliva after a tick bite. The duration of attachment is critical in any tick-borne rickettsiosis. Transmission is unlikely when the tick feeds for less than 20 hours. Since the tick bite is painless, it is often unnoticed. An eschar at the tick bite site is rarely observed. The epidemiology of Rocky Mountain spotted fever varies annually. This temporal repartition is determined by tick activity and human encounters. More than 500 cases occur each year, and more than 90% are reported from April to September. The disease is more common in children under 10 years of age.
Two days to two weeks after the tick bite, fever and headaches appear. The fever is high (temperature >102°F) and associated with nonspecific symptoms such as malaise, myalgias, nausea, vomiting, anorexia, and diarrhea. Due to the absence of specific symptoms, Rocky Mountain spotted fever is not frequently diagnosed at this stage, but patients with high fever who live in or have a history of travel to an endemic area during the “tick season” and, possibly, have a history of tick bite should be considered as possible cases of Rocky Mountain spotted fever.
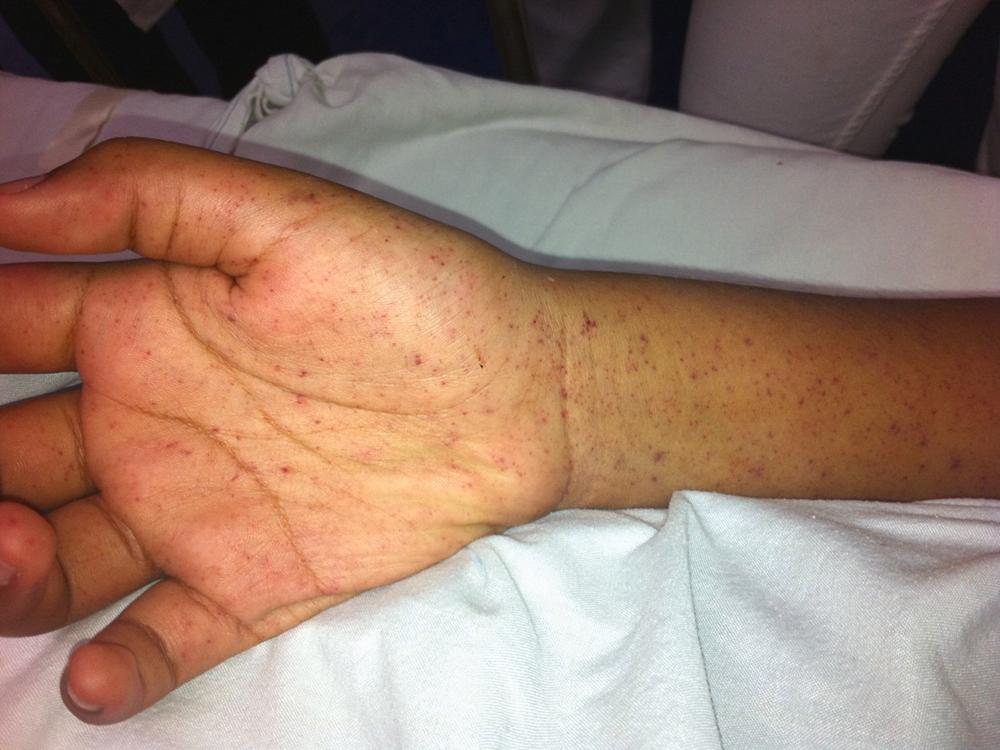
The most characteristic feature is a rash. However, the classic triad of fever, headache, and rash is present in only 44% of confirmed cases. A rash is found in 14% of cases on the first day of disease and in less than 50% in the first 3 days. The rash is macular; spots are 1 to 5 mm in diameter and can evolve from pink to purpuric ( Fig. 302-2 ). The rash appears first on the ankles and wrists and then generalizes, but it can appear later or even not at all. Involvement of the palms and soles theoretically differentiates the typhus diseases (in which it is absent) from spotted fever rickettsioses. “Spotless” Rocky Mountain spotted fever represents one-third of cases.
Untreated patients worsen progressively. The disease is associated in various degrees with general manifestations related to vascular inflammation and increased vascular permeability, which can lead to multiple organ dysfunction syndrome. In severe forms, patients suffer from edema, hypovolemia, hypoalbuminemia, and hypotension leading to shock. In very severe cases, necrosis and gangrene of the extremities occur. In some instances, noncardiogenic pulmonary edema develops; pulmonary involvement leading to respiratory distress can cause death. Renal failure can result either from hypovolemia and shock and be reversible or from acute tubular necrosis and require hemodialysis. The usual neurologic symptoms are confusion, lethargy, and stupor. In severe cases, delirium, coma, and seizures are observed. In some series, cerebrospinal fluid (CSF) sampling exhibits meningitis in one third of cases; in general, a few mononuclear cells (10 to 100) are observed, along with increased protein but normal glucose levels. Cardiac arrhythmias may occur. Intestinal tract involvement is manifested as abdominal pain, diarrhea, vomiting, and severe bleeding. Upper gastrointestinal hemorrhage can cause death. Ocular involvement may consist of conjunctivitis and retinal abnormalities, including hemorrhages, papilledema, and arterial occlusion.
The white blood cell count is usually normal, but immature myeloid cells are frequently present. Thrombocytopenia is observed in 30 to 50% of cases and may be marked in severe cases. Anemia develops in 30% of patients. Coagulopathy with decreased clotting factors (including fibrinogen) and prolonged coagulation times may contribute to bleeding. There may be hypoalbuminemia, and proteins of the acute phase response are increased (C-reactive protein, ferritin, fibrinogen). Hyponatremia and hypocalcemia may be noted and correlate with severity, as does an increase in creatinine. Increased concentrations of serum liver and muscle enzymes such as aminotransferases (aspartate [AST] and alanine [ALT] aminotransferase), lactate dehydrogenase (LDH), and creatine kinase usually reflect the severity of organ involvement, including the lung, heart, liver, and rhabdomyolysis.
The diagnosis of Rocky Mountain spotted fever should rely on clinical and epidemiologic findings. The most important clue is unexplained fever in a patient with a history of tick exposure in an endemic area. When a rash is present, Rocky Mountain spotted fever should be suspected and the patient should be treated accordingly unless another cause is demonstrated.
The differential diagnoses include other rickettsioses (such as those caused by R. parkeri in southern states), meningococcemia ( Chapter 274 ), enterovirus infections ( Chapter 349 ), typhoid ( Chapter 284 ), leptospirosis ( Chapter 298 ), ehrlichiosis, gonococcemia ( Chapter 275 ), toxic shock syndrome ( Chapters 267 and 269 ), syphilis ( Chapter 295 ), rubella ( Chapter 339 ), measles ( Chapter 338 ), and Kawasaki syndrome ( Chapters 254 and 406 ). Drug hypersensitivity ( Chapter 234 ), especially after antimicrobial use for febrile illness, is sometimes confused with Rocky Mountain spotted fever.
Direct detection of the bacterium by demonstration of specific antigens by immunodetection or genomic amplification by PCR provides a definite diagnosis of infection. A biopsy specimen or a swab of a skin lesion, preferably of an inoculation eschar, is the best sample for this purpose. Direct detection in removed ticks using PCR is also very useful. In contrast, blood contains inhibitors and only a few copies of rickettsial DNA.
The diagnosis can also be confirmed by serology, but treatment should never be delayed to obtain its results. Criteria for laboratory confirmation include a four-fold or greater change in antibody titer determined by serology (measured by immunofluorescence antibody assay). Two serum samples should be tested (early and convalescent). The early serum is usually negative because patients seroconvert between the 7th and 15th days. A cutoff value of 1:64 for total immunoglobulin and 1:32 for IgM antibodies is required for diagnosis. The latex agglutination cutoff is 1:64 or 1:128.
However, serology is nonspecific. Cross-reactive antibodies have been reported, mainly with infections caused by other rickettsioses, but also infections with Ehrlichia, Bartonella, Legionella , and Proteus spp. False-positive results, including IgM, may be observed when rheumatoid factor is present in serum and in patients who have viral infections that generate nonspecific B-lymphocyte proliferation (e.g., cytomegalovirus [ Chapter 347 ], Epstein-Barr virus [ Chapter 348 ]).
Culture of rickettsiae takes 3 to 7 days and is restricted to specialized laboratories. Immunodetection by immunofluorescence antibody assay or immunohistochemistry is sensitive and specific but requires specific antibodies. It can be performed with frozen or fixed and paraffin-embedded material and allows retrospective diagnosis.
Doxycycline is life-saving in patients with Rocky Mountain spotted fever, and the prognosis depends on the timing of antimicrobial treatment. The recommended dose is 100 mg two times a day, and treatment should be continued for at least 3 days after the fever resolves. Oral treatment is effective, but the intravenous route should be used in patients with gastric intolerance or coma. Fluoroquinolones, rifampin, and some macrolides (azithromycin and clarithromycin but not erythromycin) are also effective in vitro, but lack of clinical experience precludes their use for Rocky Mountain spotted fever. β-Lactam antimicrobials, aminoglycosides, and cotrimoxazole are not effective.
Severely ill patients should be treated in intensive care units. Fluid administration must be carefully monitored. Mechanical ventilation is used in case of respiratory distress ( Chapter 90 ), hemodialysis in patients with renal insufficiency ( Chapter 117 ), and antiseizure drugs in patients with seizures ( Chapter 372 ). Anemia and coagulation abnormalities may also be corrected. For patients with gangrene of the extremities, amputation may be necessary. Glucocorticoids have not proved useful.
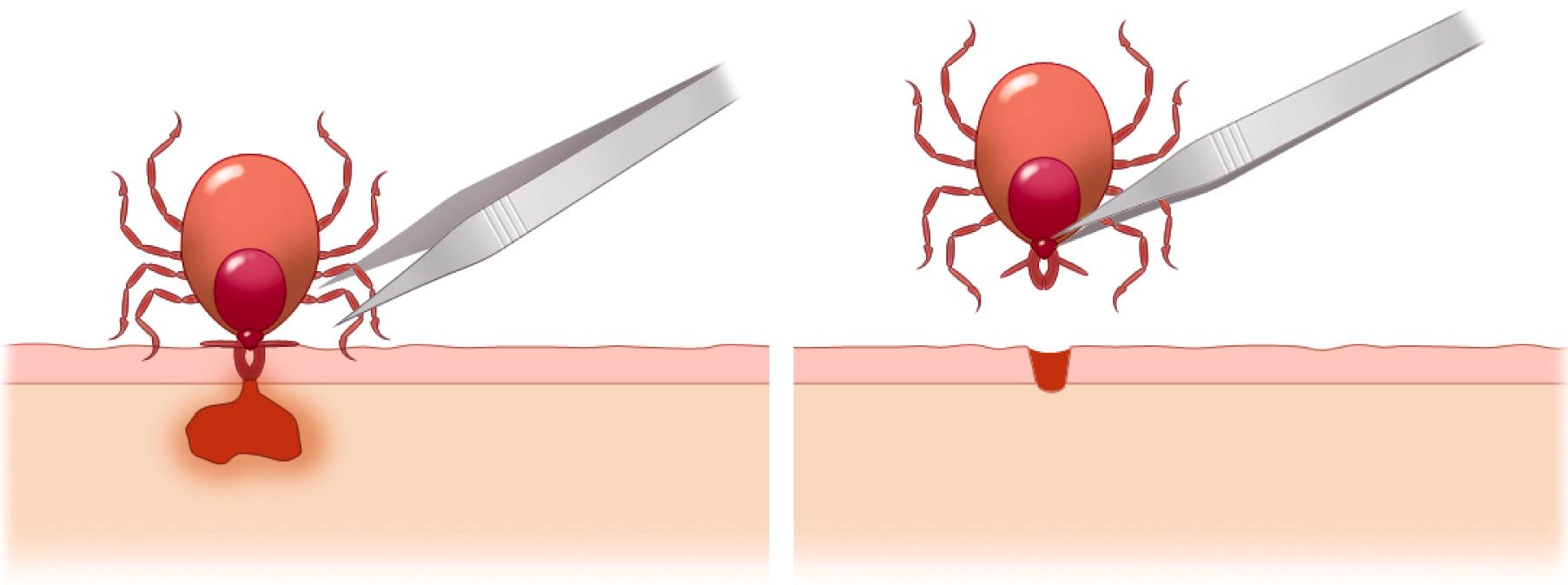
Prevention is based on the use of repellents, protective garments, or both to avoid tick bites. Repellents containing permethrin can be sprayed on boots and clothing and will persist for several days. Repellents containing DEET ( N,N -diethyl- m -toluamide) can be applied to the skin but will persist only a few hours before reapplication is necessary. After being outdoors in areas of potential tick exposure, careful examination of the scalp, groin, and axillae is also recommended. The tick can be removed by forceps, and the skin should be disinfected ( Fig. 302-3 ).
The prognosis of Rocky Mountain spotted fever depends strongly on the timing of diagnosis and antimicrobial treatment. The overall case-fatality rate is 2.5%, but it is higher in patients older than age 70 years (9%). No significant difference in outcome has been observed between Blacks and Whites. Patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency are more susceptible to severe infection. Recovery is usually complete, but neurologic sequelae can remain, and amputation of extremities may be necessary after gangrene.
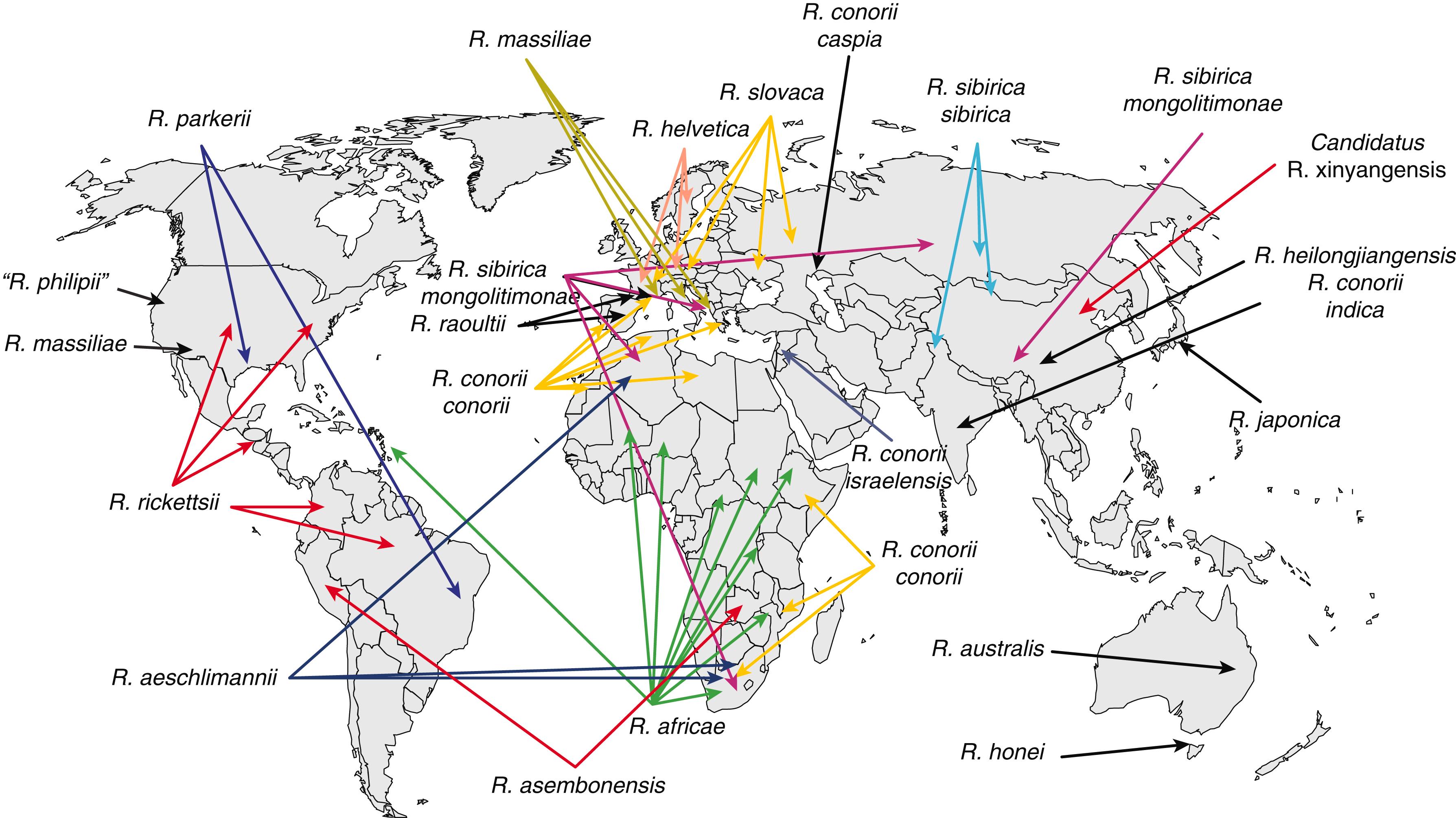
Tick-borne rickettsioses have a limited geographic distribution that is determined mainly by the ecology of the tick vector ( Fig. 302-4 ). R. parkeri has been identified in the United States and South America. R. philipii is seen in the United States. In Europe, R. conorii is distributed around the Mediterranean and Caspian seas ( caspia subspecies); R. slovaca, R. raoultii , and possibly R. helvetica are seen in Western and Central Europe; and R. sibirica mongolitimonae is detected in France and Greece. Elsewhere, a number of specific agents of rickettsial disease have been identified (see Table 302-2 ).
R. africae causes African tick-bite fever, which may be the most common rickettsiosis worldwide. It is extremely common in travelers visiting safari parks in Southern Africa. It is transmitted by Amblyomma hebraeum and Amblyomma africanum ticks. These ticks are often infected; as many as 60% can harbor R. africae . They usually feed on ungulates but also attack human beings in groups and cause a high prevalence of infection in rural Africa and in travelers. The tick attacks typically generate clusters of cases as well as multiple eschars in safari tourists.
R. conorii comprises different but closely related subspecies. Many names are given to the infection caused by R. conorii : mainly Mediterranean spotted fever, but also boutonneuse fever, Marseilles fever, and Kenya tick typhus for infections caused by the subspecies R. conorii conorii ; Astrakhan fever (caused by R. conorii caspia ); Israeli spotted fever (caused by R. conorii israelensis ); and Indian tick typhus (caused by R. conorii indica ). R. conorii is closely related to R. rickettsii , with which it shares many common antigens that generate cross-reactive antibodies.
Mediterranean spotted fever resembles Rocky Mountain spotted fever but has some distinguishing features. A malignant form of the disease that includes purpuric rash, shock, and multiple organ dysfunction has been described in elderly, debilitated patients and in patients with liver disease, diabetes, human immunodeficiency virus (HIV) or G6PD deficiency. The typical clinical presentation is that of a patient with fever, a rash, and a tache noire (i.e., an inoculation eschar at the site of the tick bite). A tache noire is found in 50 to 80% of cases. Multiple eschars are rare because the dog tick vector, R. sanguineus , seldom bites humans. The rash is frequently maculopapular and involves the palms and soles but spares the head.
Israeli tick-bite fever and Astrakhan fever appear to be milder than typical Mediterranean spotted fever. A tache noire is usually lacking.
African tick-bite fever differs from Mediterranean spotted fever in that it is much milder. Fever is frequently absent. Rash, which is observed in only about 50% of patients, may be vesicular (which has never been reported in confirmed Mediterranean spotted fever). Moreover, several eschars are frequently observed, often on the lower limbs and associated with draining lymphadenopathy in the groin.
Japanese spotted fever (caused by Rickettsia japonica ) and Siberian tick typhus (caused by R. sibirica ) resemble Mediterranean spotted fever. Lymphangitis-associated rickettsiosis, caused by R. sibirica mongolitimonae , also resembles Mediterranean spotted fever but in some cases exhibits distinguishing clinical features, including an eschar, groin lymphadenopathy, and lymphangitis joining these two lesions. Rickettsia australis (Queensland tick typhus) and Rickettsia honei (Flinders Island spotted fever) cause diseases resembling Mediterranean spotted fever, but their rash can be vesicular.
R. slovaca and R. raoultii cause a common disease in Europe named scalp eschar and neck lymphadenopathy transmitted by ticks (SENLAT, reported in the Czech Republic, France, Hungary, Germany, Italy, Lithuania, Romania, Spain, Turkey). Its tick vectors, Dermacentor marginatus and Dermacentor reticulatus , preferentially bite in cold months and bite the scalp. In contrast to other tick-borne rickettsioses, the disease is more prevalent in children and women. It is rarely exanthematic; the typical clinical picture consists of an inoculation eschar at the site of the tick bite on the scalp and draining neck lymphadenopathy (which may be painful). Rarely, patients may exhibit fever and a rash. Deep postinfectious asthenia and residual alopecia at the site of the tick bite can be observed. Other Rickettsia species that rarely have been identified as causing SENLAT include Rickettsia massiliae , Rickettsia sibirica mongolitimonae , and Candidatus Rickettsia rioja.
The diagnosis of other tick-borne rickettsioses is similar to that of Rocky Mountain spotted fever, mainly by serology, although PCR testing is becoming increasingly available. An exception is R. slovaca infection, in which the serologic response is weak, possibly because of its lack of general infection; in this case, PCR of a skin eschar sample by a swab or a lymph node aspirate provides the highest diagnostic yield. In R. africae infection, the serologic response occurs later than in Rocky Mountain spotted fever and Mediterranean spotted fever; late serum samples (i.e., 4 weeks after onset of symptoms) are therefore recommended.
Doxycycline (100 mg twice daily for adults or 4.4 mg/kg body weight per day in two divided doses for children under 45.4 kg [100 lb]) is the drug of choice. A single day of therapy usually suffices; in adults with more severe disease, however, it should be administered until the patient is afebrile for 24 hours. Quinolones (e.g., ciprofloxacin 1 g daily) and newer macrolides (e.g., azithromycin 500 mg daily for 5 days) provide results that are comparable to those of doxycycline but require longer treatment courses. In pregnant women, josamycin, a macrolide antimicrobial, has proved efficient at a dose of 3 g daily for 7 days for Mediterranean spotted fever.
Most patients recover completely with treatment, but hospitalized patients have a fatality rate of 1.5 to 2.5%.
Fleas ( Chapter 98 ) can harbor three rickettsial species: Rickettsia typhi , which is the agent of murine typhus; Rickettsia felis , which is the agent of flea-borne spotted fever; and R. asembonensis . Both R . typhi and R. felis can be transmitted transovarially in the flea. Vectors are Xenopsylla cheopis and Pulex irritans but also Ctenocephalides felis , which is a cat flea. Rats, cats, opossums, and dogs can propagate infected fleas. These reservoirs and vectors are distributed worldwide, so these diseases have a global distribution. Fleas can be infected by both species at the same time. R. asembonensis , the agent of an unnamed rickettsiosis, has been associated with cat fleas worldwide but detected in humans only in Peru and Zambia.
In the United States, 50 to 100 cases are reported yearly, mainly in Southern California and southern Texas. In California, a transmission cycle involving opossums and cat fleas has been demonstrated. Murine typhus is extremely common in China, Southeast Asia, and North Africa, and it also is a common cause of fever in travelers to those areas.
Fleas are usually infected by R. typhi when feeding on apparently healthy rats that have blood-borne infection. Humans and other mammals are infected through autoinoculation by scratching a flea bite that is contaminated with feces from an infected flea. Murine typhus, because of its cycle, is more prevalent in hot and humid areas, when rats proliferate.
The incubation period is generally 8 to 16 days. The disease begins with abrupt fever, nausea, vomiting, myalgias, arthralgias, and headache. A rash, which develops in 40 to 50% of patients about 6 days after the onset, is detected less frequently in patients with dark skin. The rash begins as pink maculae that can evolve to be maculopapular. The rash is often discrete, starting in the axilla and spreading to the trunk but not usually to the face, palms, or soles. In severe cases, it can become purpuric.
The most frequently involved organ is the lung. One third of patients have a cough, and about 25% of patients develop a nonspecific interstitial pneumonia that is sometimes associated with a pleural effusion. In severe forms, respiratory failure occurs. Digestive involvement can manifest as vomiting, abdominal pain, jaundice, and, in severe cases, hematemesis. In patients with severe disease, neurologic symptoms range from confusion and stupor to coma and seizures. Cerebral hemorrhages may occur.
The white blood cell count shows leukopenia and then leukocytosis. Thrombocytopenia can be noted as well as anemia, specifically when hemolysis is observed (frequently in patients with G6PD deficiency). A moderate increase in serum liver enzymes is common. In patients with severe disease, hyponatremia and hypoalbuminemia are observed.
The diagnosis of murine typhus, as with Rocky Mountain spotted fever, is based mainly on serology immunofluorescence antibody assay. R. typhi cross-reacts with R. prowazekii , but it can be differentiated by additional testing. Skin biopsies and blood samples for culture and PCR may be valuable but cannot currently substitute for serologies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here