Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter will concentrate on the principles and concepts of bone repair, grafting, and reconstruction.
The embryology, physiology, microanatomy, and histochemistry of bone will be reviewed.
Principles of mechanotransduction and cellular mechanisms of bone turnover will also be discussed.
Pathophysiology of traumatic injury to bone (fractures, segmental loss, defects) will be categorized and reviewed.
Bone-remodeling mechanisms (osteoconduction, osteoinduction, osseointegration) will be described.
After a brief history of autogenous bone grafting, the clinical application of bone transfer and transplantation will be captured by a brief atlas of harvest of each subtype.
The reader will also be provided with a brief overview of bone substitutes.
Access video lecture content for this chapter online at Elsevier eBooks+ ![]()
Bone is a complex structure that serves many roles within the body. It is crucial for maintaining mineral homeostasis, provides protection to internal organs, and offers structural support for locomotion and stature. Osseous tissue undergoes continuous remodeling, during which old bone is degraded and replaced by new bone. Most organs in the adult human undergo repair with scar tissue after injury. Bone, however, regenerates, making it a unique organ system.
The formation of bone occurs by either intramembranous ossification or endochondral ossification. Direct condensations of mesenchymal stem cells (MSCs) initiate the process of intramembranous ossification to create flat bones of the craniofacial skeleton. During intramembranous ossification, osteoblasts lining skeletal surfaces deposit new bone upon previously laid bone in a process called apposition . Alternatively, endochondral ossification is characterized by the formation of an MSC-derived hyaline cartilage template that is subsequently replaced by bone. Endochondral ossification is responsible for the development of tubular long bones of the appendicular skeleton as well as the bones of the vertebral column and pelvis.
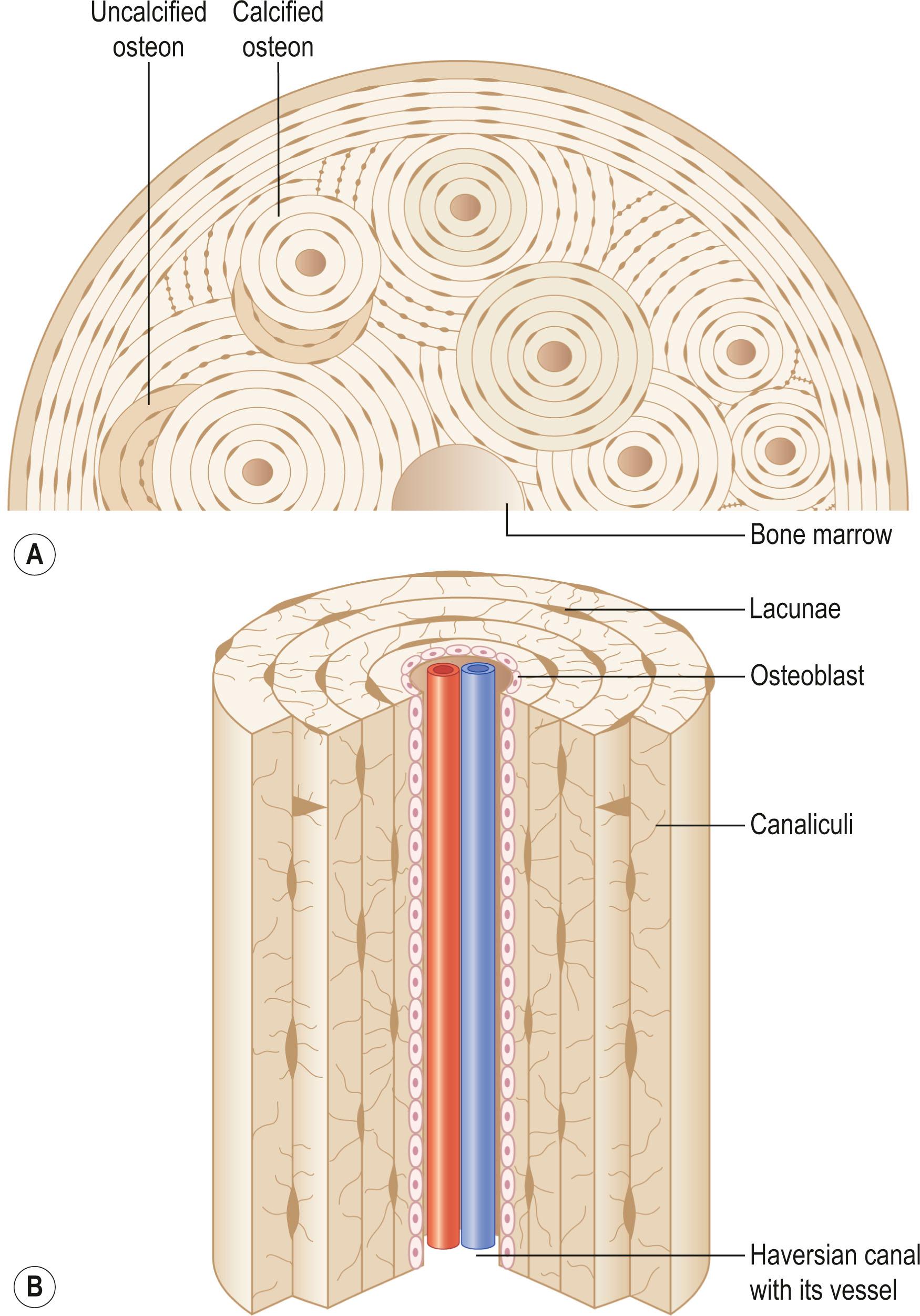
Bony tissue contains osteocytes located within open cavities called lacunae . Osteocytes project radiating processes through microscopic canaliculi that adjoin neighboring lacunae. Osteocytic cytoplasmic processes are linked to one another via gap junctions, through which osteocytes communicate.
The skeleton is comprised of cortical and cancellous bone ( Fig. 20.1 ). Cortical bone is compact and forms the outer skeleton. It accounts for 80% of osseous tissue. Cortical bone is dense and strong to support the body and protect organs. A bilayered cellular membrane ( periosteum ) covers cortical bone. The outer layer is composed of a dense fibrous membrane containing flat fibroblast-like cells and serves as an attachment for muscles and tendons. Large plump cells line the inner periosteal layer ( cambium layer ) and can differentiate into osteoblasts upon stimulation. Bone also contains a thin layer of vascular connective tissue ( endosteum ) that lines the medullary cavity.
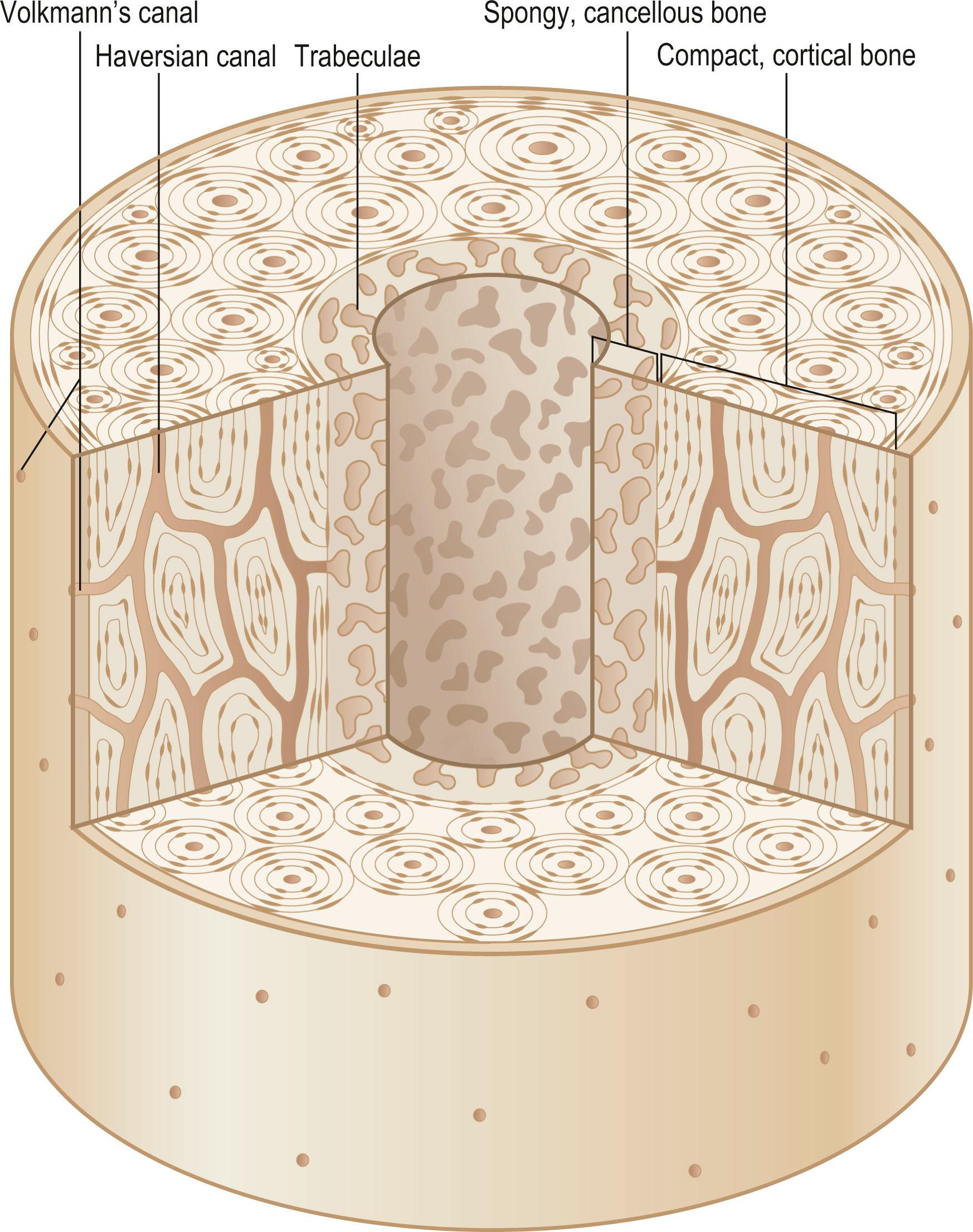
The primary functional unit of cortical bone is the osteon or haversian system ( Fig. 20.2 ). An osteon contains concentric layers of cortical tissue (lamellae) that surround a central haversian canal that contains vascular and nervous tissue. Nutrient vessels within haversian canals anastomose to blood vessels in bone marrow and periosteum via the perpendicular-running Volkmann's canals.
Cancellous or trabecular bone comprises approximately 20% of the skeleton. Relative to cortical bone, cancellous bone is porous and is located deep within the medullary cavity, typically at the ends of long bones. It consists of bony trabeculae oriented along the lines of stress. Cancellous and cortical bone are chemically equivalent; however, cancellous bone has increased metabolic activity due to its greater surface area for remodeling. Cancellous bone provides internal support for bone marrow elements and cortical bone.
Bone is a calcified tissue composed of 60% inorganic matter, 30% organic matter, and 10% water. The inorganic component of bone makes up approximately 40% of bony volume, while the organic component and water comprise 35% and 25%, respectively. The inorganic phase primarily consists of the mineral crystal hydroxyapatite (Ca 10 (PO 4 ) 6 (OH) 2 ), which measures 20–25 nm in length, 15 nm in width, and 2–5 nm in thickness. It is the hydroxyl endmember of the apatite group; however, impurities arise when the hydroxyl group is replaced by potassium, magnesium, or sodium; or when carbonate replaces the phosphate group. By storing them as a mixture within impure hydroxyapatite, bone provides a reservoir of various minerals within the body.
The organic component of bone (demineralized organic bone matrix or osteoid) is deposited by osteoblasts during bone formation. Osteoid is primarily composed of type I collagen, but also includes many noncollagenous proteins. Collagen is a trilaminar protein consisting of two α1 chains and one α2 chain that form a distinct right-handed helical structure. Within the rough endoplasmic reticulum of osteoblasts, procollagen undergoes hydroxylation and glycosylation before transfer to the Golgi apparatus, where it is packaged and secreted. Extracellularly, procollagen peptides are cleaved into tropocollagen. The copper-dependent enzyme lysyl oxidase mediates covalent cross-linking within and between tropocollagen molecules to create mature collagen fibers. The strength of bone is derived from interactions between collagen fibers and inorganic minerals.
Three cell types are predominantly found in bone: osteoblasts, osteocytes, and osteoclasts ( Table 20.1 ).
| Cell type | Morphology | Location | Function | Source | Precursor cell | Differentiation product |
|---|---|---|---|---|---|---|
| Osteoblast |
|
|
Produce bone matrix |
|
Preosteoblast Bone-lining cells? | Osteocyte |
| Osteocyte | Stellate cells with thin cytoplasmic processes | Embedded in lacunae |
|
Osteoblasts that become embedded in osteoid | Osteoblasts | Terminally differentiated cell |
| Osteoclast |
|
|
Bone resorption | Bone marrow Spleen? Lung, peritoneum, peripheral blood | Hematopoietic stem cell | None |
Osteoblasts are derived from MSCs that have the capacity to differentiate into several connective tissue cell types. Within the appropriate osteogenic environment, MSCs differentiate into osteoprogenitor cells, thereby committing to an osteoblastic lineage. Osteoprogenitor cells are ubiquitous in bone, located within Volkmann's and haversian canals, the cambium layer of periosteum, endosteum, and within perivascular tissue adjacent to bone.
Osteoblasts are plump, basophilic cells located on the external surfaces of bone at areas of active bone formation during development and fracture repair. They have a vast endoplasmic reticulum for robust collagen production and contain many mitochondria and a large Golgi apparatus to package and secrete procollagen. Osteoblasts are responsible for the production of bony matrix and facilitate the mineralization of osteoid. Some osteoblasts will eventually become surrounded with bony matrix and terminally differentiate into osteocytes.
During osteoprogenitor cell differentiation, cells halt proliferation and start expressing proteins characteristic of an osteoblastic lineage. Preosteoblasts secrete proteins (e.g., alkaline phosphatase (ALP)) that denote early osteogenesis, and also begin to produce bony matrix proteins. As the differentiation process ensues, cells display a basophilic appearance, stain strongly for ALP, and lose the ability to proliferate. Mature osteoblasts deposit significant osteoid. They also play a role in many other processes as indicated by their secretion of numerous cytokines and noncollagenous proteins that have pleiotropic effects, as well as factors important for myelopoiesis. Differentiated osteoblasts affect bone metabolism and mineral homeostasis by interacting via surface receptors with parathyroid hormone and 1,25-dihydroxyvitamin D. Most osteoblasts ultimately undergo apoptosis or become bone-lining cells. However, 20% differentiate into osteocytes.
Under the control of multiple signaling pathways, preosteoblasts differentiate into mature osteoblasts. The Wnt pathway, the TGF-β/BMP (transforming growth factor beta/bone morphogenetic protein) superfamily, notch signaling, , the JAK/STAT (Janus kinase/signal transducer and activation transcription) pathway, PERK (protein kinase RNA-like endoplasmic reticulum kinase) signaling, Hedgehog proteins, and FGFs (fibroblast growth factors) have all been implicated in the molecular signaling of osteogenesis. Although the exact mechanisms of osteogenesis are complex and only partially elucidated, advances have been made regarding the initiation and molecular control of this process. Wnt/β-catenin signaling has been shown to control the differentiation of both osteoblasts and osteoclasts, which may be important in postnatal bone acquisition. Wnt/β-catenin signaling is also important during skeletogenesis in the fetus, and is considered to be partially responsible for both osteoblast and chondrocyte differentiation. Notch signaling is a highly conserved signaling system involving cell–cell communication. In addition to cell fate division and homeostatic maintenance, the notch pathway is believed to be important in osteogenesis because of notch1–BMP-2 interactions that promote osteogenic differentiation. Additionally, Engin et al . showed with osteoblast-specific gain of Notch function studies that Notch not only stimulates terminal osteoblast differentiation, but that it also plays a role in the proliferation of immature osteoblasts. In addition, dysregulation of notch signaling is associated with many congenital disorders and acquired diseases of the skeleton. Similar to Wnt/β-catenin, the JAK/STAT pathway influences osteoblast and osteoclast activity through various cytokines and their receptors, though many of the mechanistic underpinnings remain to be elucidated. By way of regulating osteoblast and osteoclast activity, JAK/STAT is also important for guiding skeletal development and regulating bone homeostasis.
The RNA-dependent protein kinase PERK is a key regulator of endoplasmic reticulum stress. Increasing evidence has demonstrated its role in bone metabolism and osteoblast and osteoclast differentiation by influencing downstream targets elF2α, FOXO1, CaN, Nrf2, and DAG. Hedgehog signaling plays a role in many embryonic processes and also is involved with the maintenance of stem cells in adults. There are three mammalian Hedgehog orthologs: Desert, Sonic, and Indian. Sonic Hedgehog and Indian Hedgehog both have important roles in osteogenesis. Specifically, Indian Hedgehog is critical for endochondral bone formation, whereas Sonic Hedgehog appears to be important for skeletal patterning.
Many transcription factors play a significant role in osteogenesis. Runt-related transcription factor 2/core-binding factor alpha 1 (RUNX2/CBFα-1) and osterix have important effects on osteoblast differentiation. Several SOX genes are also expressed within the osteoblast lineage that play an inhibitory role upstream of RUNX2/CBFα-1 and osterix. RUNX2 is the mammalian homolog of the Drosophila transcription factor Runt and is believed to be evolutionarily conserved in humans to serve a critical role throughout the many steps of osteoblastogenesis, including osteoblast induction, proliferation, and maturation. Homozygous deletions of the Runx2 gene are uniformly lethal in mice due to a complete lack of mineralized bone matrix. Runx2 haploinsufficiency causes cleidocranial dysplasia, an autosomal-dominant disease in humans characterized by hypoplastic clavicles, dental deformities, shortened stature, brachycephaly, hypertelorism, and other skeletal defects. While the exact mechanisms that control the regulation of Runx2 have not been fully decoded, studies have shown that various histone acetyltransferases are important Runx2 cofactors. Additionally, microRNAs, lncRNAs, and circRNAs appear to regulate Runx2 protein expression. MicroRNAs play critical roles in stem cell function, and thus may have important clinical implications for diseases due to dysfunctional MSC/Runx2 interactions. Many other regulators also play an important role in Runx2 function ; however, their exact roles are currently under study.
The zinc finger-containing protein osterix (Osx) is also a key transcription factor to bone formation. Osx-null mice form neither cortical nor cancellous bone. Unlike Runx2-null mice, however, Osx-null mice still produce normal cartilage. As a result of the findings that Osx expression is absent in Runx2 knockout mice, whereas Runx2 expression is normal in Osx-null mice, the Osx gene is thought to be downstream to Runx2. Nishio et al . confirmed this using overexpression experiments to show that Runx2 transactivates the Osx gene promoter significantly, indicating that there is a Runx2-binding element within the promoter region.
The signaling pathways and transcription factors responsible for osteoblastogenesis are exquisitely detailed elsewhere.
Osteocytes are terminally differentiated cells of the osteoblast lineage and comprise 90–95% of all bone cells. Despite being the most abundant cell in bone, osteocytes are the least well characterized. Osteocytes are located within lacunae. Multiple cytoplasmic processes radiate from osteocyte somas and travel through canaliculi between lacunae. Gap junction connections between adjacent cytoplasmic processes enable osteocytes to communicate with one another. Osteocytes communicate with osteoblasts by way of this canalicular network as well. Processes also extend to the haversian canals so that osteocytes can rid themselves of waste products and receive nutrients necessary for survival. Relative to osteoblasts, osteocytes have qualitatively similar organelles, but the organelles are smaller in both size and number.
Bone is a dynamic substance that maintains its viability by constantly adapting to the environment in which it lives. Victim to mechanical loading and various traumas, bone must have a way to recognize such stressors. It is believed that osteocytes are mechanosensory cells that translate (via mechanotransduction) physical stress into chemical and/or electrical signals that stimulate bone remodeling. This hypothesis has been derived from various lines of evidence. Anatomically, osteocytes form a syncytium with surrounding cells via their cellular processes. These connections may provide communication between mechanical stimuli and effector cells (osteoblasts and osteoclasts). Recent evidence has shown that osteocyte processes also attach to extracellular matrix (ECM) proteins and may mediate mechanotransduction by the amplification of shear stress experienced at the ECM. In addition, mechanical loading alters osteocyte gene expression. Osteocytes rapidly produce increased levels of c-fos , insulin-like growth factor (IGF)-1, prostaglandin (PG), and nitrous oxide (NO) when mechanically stimulated. These and many other molecules (see below) have numerous effects on bone turnover. Molecular studies have implicated the activation of the Wnt/β-catenin pathway in response to loading. The osteocytic Wnt/β-catenin pathway appears to be triggered by crosstalk with various signaling pathways when the osteocyte senses a load strain, thereby decreasing negative regulators of the Wnt/β-catenin pathway, an important regulator of bone mass. Finally, Tatsumi et al . have shown that targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction.
One potential function of the osteocyte, termed periosteocytic osteolysis, has remained elusive. Osteocytes may fulfill an osteoclast-like activity by resorbing varying amounts of calcified bone matrix surrounding their lacunae. Evidence for this comes from studies that have shown a larger than expected size of lacunae in conditions characterized by increased bone resorption. Additionally, the administration of parathyroid and thyroid hormone has been shown to induce microradiographic enlargement of lacunae, thought to be the result of the hormones' actions on osteocytes. Wysolmerski suggested that osteocytes have a role in coordinating bone and mineral metabolism during reproductive cycles. Specifically, osteocytes reversibly remodel perilacunar and pericanalicular bone during lactation by removing mineralized tissue in order to liberate calcium (and potentially phosphorous) for milk production. This tissue is then replaced during the recovery period after weaning.
Osteoclasts are multinucleated cells primarily responsible for bone resorption, a process necessary for bone growth, tooth eruption, fracture repair, and calcium homeostasis. Osteoclasts typically have between three and 25 nuclei per cell and are approximately 40 µm in diameter. Histologically, osteoclast cytoplasm has a characteristic homogeneous, “foamy” appearance due to a high concentration of vesicles and vacuoles. Osteoclasts are derived from hematopoietic progenitor cells and are related to the monocyte/macrophage lineage. Osteoclasts are found on the endosteal and periosteal surfaces of bone in areas of active remodeling.
Bone resorption is a complex process by which crystalline hydroxyapatite is dissolved and organic bone matrix is degraded. In vitro bone resorption models using primary osteoclast isolates provided the basis for the resorption cycle. The cycle is a complex series of actions which includes osteoclast migration to the resorption site and attachment to bone, membrane polarization, dissolution of hydroxyapatite followed by degradation of organic bone matrix, removal of degradation products, and osteoclast inactivation or apoptosis. Clinically, bone resorption is an extremely important process, as evidenced by pathologic conditions characterized by dysfunctional resorption. For example, increased osteoclastic function leads to excess bone resorption, thereby causing osteoporosis. In contrast, osteoclast underactivity decreases bone resorption, which causes osteopetrosis.
Bone resorption is key for both bone remodeling at sites of injury and also as a mechanism of calcium homeostasis. Osteoclasts are stimulated to resorb bone by growth factors and cytokines secreted by local osteoblasts. Once osteoclasts localize to the area in need of resorption they form a tight seal that separates the area of bone to be resorbed from the extracellular environment. Evidence has pointed to integrins (in particular integrin α v β 3 ) and cadherins as playing a prominent role in the attachment of this sealing zone. Myosin and actin-binding proteins are also important for such attachments. After sealing zone attachments are completed, osteocytes polarize such that the area adjacent to the resorbing bone surface becomes ruffled. The ruffled border is a specialized membrane domain formed by the fusion of intracellular acidic vesicles and functions as the osteocyte's resorbing organelle. Finger-like projections from the ruffled border increase the effective surface area of resorption. Before proteolytic cleavage of the organic matrix occurs, osteoclasts dissolve hydroxyapatite crystals by targeted secretion of hydrochloric acid through the ruffled border toward the resorption pit (Howship's lacuna). After the dissolution of inorganic matter, many proteolytic enzymes, including matrix metalloproteinase-9 and cathepsin K, are secreted into the resorption lacuna to degrade the organic components of bones. Both phases of bone are subsequently removed from the resorption lacuna via transcytosis through the ruffled border and sent to the secretory domain of the osteoclast, which then expels these degradation products into the extracellular space outside the osteoclast. The osteocytic marker tartrate-resistant acid protease (TRAP) has been localized to transcytotic vesicles of resorbing osteoclasts, and is believed to generate reactive oxygen species that degrade matrix degradation products within these vesicles.
The mechanisms surrounding osteoclast differentiation are complex and have been the subject of intense study. Depending on local stimuli, hematopoietic stem cells may differentiate into osteoclasts, macrophages, or dendritic cells. Macrophage colony-stimulating factor (M-CSF) and receptor activator for nuclear factor κB (RANK) and RANK ligand (RANKL) are early mediators that signal osteoclastogenesis. In murine studies, M-CSF is functionally lacking in op/op mice, which express an osteopetrotic phenotype. In addition, in order for osteoclastogenesis to commence, RANKL (found on the cellular membrane of osteoblasts) must interact with RANK, a receptor found on osteoclast precursors. RANK–RANKL interaction must occur in order for osteoclast precursor cells to begin expressing phenotypic markers (e.g., TRAP) that characterize osteoclasts. Similar to op/op mice with functionally absent M-CSF, RANK and RANKL knockout mice also express an osteopetrotic phenotype.
Osteoprotegerin (OPG), a TNF-related soluble protein secreted by osteoblasts, is also an important regulator of osteoclastogenesis. OPG has effects on bone density and bone mass by acting as a decoy receptor for RANK, thereby blocking RANK–RANKL interaction. Transgenic mice that overexpress OPG suffer from an osteopetrotic phenotype, while OPG knockout mice display an osteoporotic phenotype.
The RANK molecule lacks enzymatic activity, and thus must recruit adaptor proteins when stimulated by RANKL to promote differentiation. TNF receptor-associated factor (TRAF) family members appear to be important adaptor proteins, with TRAF6 serving the most critical role. TRAF6 knockout mice develop severe osteopetrosis as a result of either the dysfunction or total absence of osteoclasts. Moreover, TRAF6 activates NF-κB, another essential modulator of osteoclast differentiation. NF-κB enters the nucleus of osteoclasts stimulated with RANKL, and regulates transcription of target genes critical for osteoclastogenesis.
The extracellular matrix (ECM) is the scaffolding from which bone derives much of its strength and architecture. Most molecules found within the ECM are produced by osteoblasts. The ECM contains nearly 30 types of collagen, as well as noncollagenous phospho- and glycoproteins. Type I collagen is the predominant form found within the ECM, making up approximately 90% of bone matrix. The significance of type I collagen is highlighted by patients with osteogenesis imperfecta (OI), a disease characterized by bone fragility and repeated fracture. OI often occurs in patients with pro-α1 or pro-α2-chain gene mutations, or from a mutation in one of the enzymes responsible for post-translational hydroxylation of collagen. In addition, bone mineralization takes place within the ECM. Bone mineralization is a well-orchestrated process during which hydroxyapatite crystals are laid down within the organic matrix of bone and calcify proteins that are secreted by osteoblasts.
In addition to type I collagen, many noncollagenous proteins are found within the ECM ( Table 20.2 ). Osteopontin is a ubiquitous phosphoprotein that exists in multiple forms. It has been localized to most cells within bone, including osteoblasts, osteocytes, osteoclasts, and bone precursor cells. Osteopontin plays a role in the nucleation of hydroxyapatite crystals during matrix mineralization and also in osteoclast adhesion during bone remodeling.
| Molecule | Protein structure | Cell source | Present in non-osseous tissues | Function | Phenotype of knockout animals or deficiency in humans |
|---|---|---|---|---|---|
| Collagen type I | Trilaminar protein consisting of 2 α1 chains and 1 α2 chain | Many Mature and immature osteoblasts in bone | Yes | Primary scaffold of bone 90% of matrix | Osteogenesis imperfecta in humans |
| Alkaline phosphatase | Metalloenzyme |
|
Yes |
|
Hypophosphatemia and defects in skeletal mineralization in knockout mice |
| Osteopontin | Phosphorylated glycoprotein |
|
Yes |
|
Alterations in extracellular matrix remodeling in knockout mice |
| Osteonectin | Glycoprotein |
|
Yes |
|
Unknown |
| Bone sialoprotein | Phosphorylated glycoprotein | Almost exclusively produced by skeletal cells (osteoblasts, osteocytes, hypertrophic chondrocytes) | No |
|
Unknown |
| Osteocalcin | Vitamin K-dependent γ-carboxyglutamic acid-containing protein |
|
No |
|
Osteopetrosis in knockout mice |
| Biglycan | Proteoglycan |
|
Yes |
|
Osteoporosis and small, thin, short limbs in knockout mice |
Osteonectin is a glycoprotein that binds to calcium and type I collagen and also initiates and regulates bone mineralization. BSP is another important noncollagenous protein that, in contrast to osteopontin and osteonectin (both of which are expressed by many tissues throughout the body), is mainly expressed by cells within the skeleton. BSP is an acidic phosphoprotein that binds to collagen and also promotes hydroxyapatite nucleation. BSP-null mice express a phenotype characterized by small and undermineralized bones.
Osteocalcin (bone Gla protein) is a vitamin K-dependent γ-carboxyglutamic acid-containing protein, similar to factors II, VII, IX, and X of the coagulation cascade. The most abundant noncollagenous protein in bone, osteocalcin is secreted by osteoblasts and odontoblasts and is an important marker of increased bone turnover. Osteocalcin plays a role in bone turnover, osteoclast differentiation, and energy metabolism.
Osteoblasts also produce proteoglycans, the most abundant of which is biglycan (BGN). BGN, a leucine-rich noncollagenous protein, is believed to play a role in the mineralization of bone given that Bgn -deficient mice express an osteoporotic phenotype. However, BGN seems to play an important role outside the skeleton as well, as Bgn -knockout mice manifest spontaneous aortic rupture and dissection, dental and muscular abnormalities, thinning of skin, and osteoarthritis.
A variety of enzymes produced by bone cells also function within the ECM. The most prominent is alkaline phosphatase (ALP). Like osteocalcin, ALP is often used as a marker of bone turnover. While the exact function of ALP is unknown, it is believed to be important for bone mineralization, during which it regulates apatite formation.
Unique to bone is its ability to undergo regeneration rather than repair with scar formation, as is typified by most other tissues within the body. As described previously, bone is subject to constant remodeling, a process mediated by osteoclasts and osteoblasts. Physiologically, bone homeostasis is maintained by the counterbalancing of bone resorption with formation. When one of these processes becomes dysfunctional, patients may manifest a distinct pathology. Excessive bone resorption resulting in an excessive loss of bone is the fundamental pathogenesis underlying osteoporosis. Conversely, decreased bone resorption due to decreased osteoclast activity leads to an osteopetrotic phenotype. Decreased bone resorption due to increased osteoblast activity, however, will produce an osteosclerotic phenotype.
The structure of bone is a function of its material composition as well as the genetic blueprint of an individual. In addition, various loads to which bone is subjected define its structure. This process of functional adaptation mediates repair of damaged bone and also facilitates prevention of damage before it may occur. Bone exhibiting suppressed remodeling and resorption suffers increased microdamage and fracture accumulation. It has been demonstrated that sites of microcracks are subsequently associated with new bone remodeling more often than expected by chance, which has led to the belief that bone remodeling and repair are often targeted to specific locations.
Wolff (1892) derived a mathematical formula to describe how changes in the form and function of bone could lead to a change in its internal architecture and external structure. Wolff believed that during the functional adaptation of bone to new loads that occur during one's life (e.g., as a result of trauma), the trabeculae within bone would reorient to align with the new principal stress trajectories of these environmental loads. Wolff's contributions were key to the early understanding of bone adaptation. More recently, authors have recognized that bones are subject to dynamic applied loads.
Considerable research has examined the initial events that stimulate bone adaptation. It is believed that the stimulus for bone remodeling is strain (the physical deformation of bone tissue). Osteocytes, the mediators of mechanotransduction, respond molecularly to mechanical loads. They undergo direct deformation by bending and also are deformed by the electrical potential induced by the flow of surrounding interstitial fluid. In addition, osteocytes are subjected to shear stress by the physical flow of interstitial fluid around their cellular membranes.
When strained, osteocytes convert the mechanical stimulus into chemical or electrical activity in a process thought to require various signaling molecules including NO and PGs. Interestingly, NO synthase, a calcium/calmodulin-dependent enzyme, is activated under conditions of increased intracellular calcium; intracellular calcium concentrations appear to increase in response to fluid flow. Klein-Nulend and colleagues demonstrated that a pulsating fluid flow model led to the release of NO from osteocytes. Taken together, these studies indicate that NO may be important in osteocyte mechanotransduction. Additional studies have shown that pulsating fluid flow treatment also stimulates osteocytes to synthesize increased levels of PGE 2 and PGI 2 , as well as increased levels of cyclooxygenase-2 (COX-2) mRNA. COX-2 production is also stimulated by mechanical loading. Thus, COX-2 induction and the production of PGs are thought to be important mediators in the conversion of mechanical shear forces into signals that trigger bone turnover.
In the last decade, the field of regenerative medicine has exploded with the discovery of new techniques to isolate cells capable of differentiating into many different tissues. In particular, the MSC ( Fig. 20.3 ) has considerable therapeutic potential for fracture repair and other bone pathologies. For concordance, Dominici and colleagues have proposed that MSCs meet the following criteria: (1) isolated MSCs must adhere to plastic in culture; (2) MSCs should express the CD105, CD73, and CD90 surface antigens and not express the CD34, CD45, CD14 or CD11B, CD79A or CD19 and human leukocyte antigen-DR (HLA-DR) surface antigens; and (3) MSCs must have the capacity to differentiate into osteoblasts, adipocytes, and chondroblasts. MSCs and MSC-like cells are derived from bone marrow, but can also be expanded from skeletal muscle, adipose tissue, dental pulp, the circulatory system, synovium, amniotic fluid, urine, the umbilical cord, and fetal tissues.
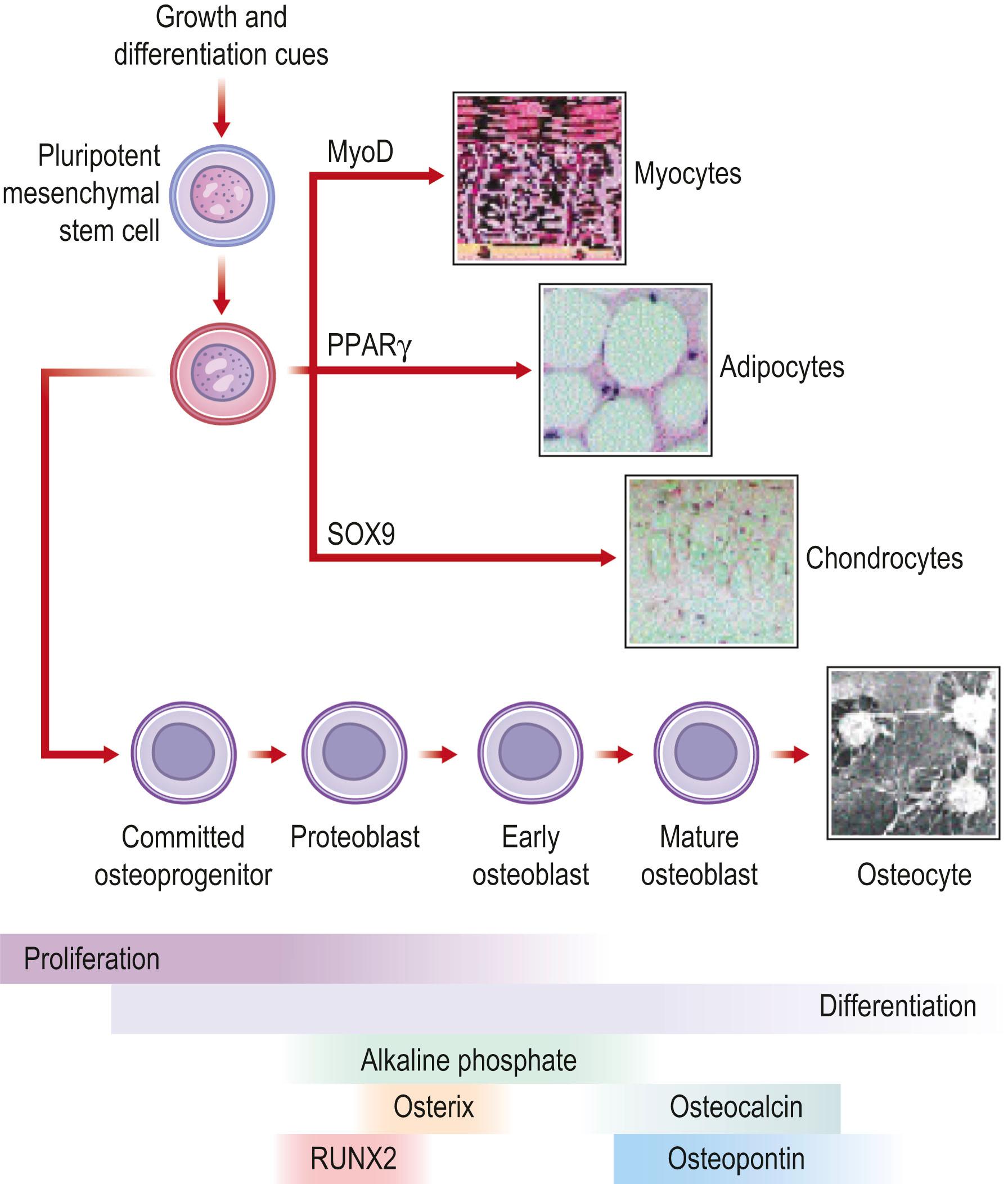
The capability of MSCs to differentiate into and assist with the restoration and repair of bone has been extensively studied. MSCs are also used clinically in patients with various bone disorders. In a clinical trial that examined MSC administration to children with osteogenesis imperfecta (OI), bone mineral density, growth velocity, and ambulation all improved after MSC administration. However, the concentration of MSCs detected in bone, skin, and other tissues was less than 1%. These data and evidence from additional studies have led authors to postulate that MSCs may actually secrete soluble factors that alter tissue microenvironment, which may be an important mechanism by which MSCs repair tissue.
Bone regeneration consists of a complex interplay of molecular processes that promote MSC migration, proliferation, and differentiation. Recently, there has been a great deal of research aimed at the identification of these molecular processes, and advances in our understanding of the molecular mechanisms of bone regeneration have been made. Many have been able to identify important signaling molecules as well as transcriptional regulators of bone regeneration ( Table 20.3 ).
| Molecule | Ectopic bone formation | Segmental defect healing | Effect on fractures | Combination with other growth factors | Effective in lower vertebrates | Effective in primates | Potential clinical use |
|---|---|---|---|---|---|---|---|
| BMPs | Yes |
|
|
Synergistic with TGF-β | Yes | Yes |
|
| TGF-β | No |
|
|
|
Yes | No (limited study) | Role alone unclear |
| FGFs | No |
|
|
Increase VEGF expression? Synergistic with TGF-β | Yes | Yes (limited study) | Potential for augmenting angiogenesis in compromised wounds |
| PDGF | No | Endochondral – Membranous − |
|
|
Yes | No | Unknown |
In the 1960s, Urist discovered the function of BMP from his work with demineralized bone matrix (DBM). To date, approximately 20 BMP isoforms have been characterized. BMPs are pleiotropic members of the TGF-β superfamily. BMPs, while important for brain and bone formation in utero , have generated much of their recognition from their osteoinductive properties. BMPs have also been implicated in human disease.
BMPs are synthesized as large precursor molecules that consist of characteristic subsections. A series of seven cysteine residues is located at the carboxyl termini of BMPs, and is important for proper protein folding. BMPs also contain a signal peptide, a prodomain, and a mature peptide. BMPs are thought to be secreted from cells in their active form.
BMPs are natural ligands for type I and type II serine/threonine kinase transmembrane cellular surface receptors. There are three type I and three type II receptors that are capable of binding BMPs. Upon ligand (BMP) binding, a heterotetrameric complex is formed, after which type II receptor kinase domains phosphorylate Gly-Ser domains in the type I receptor kinases. This process activates type I receptors to recruit and phosphorylate intracytoplasmic SMAD (suppressor of mothers against decapentaplegic) proteins (SMADs 1, 5, and 8). Following SMAD phosphorylation, SMAD 1, 5, or 8 will bind to SMAD 4, and this SMAD4-phosphorylated SMAD 1, 5, or 8 complex will relocate to the cell nucleus. Transcription factor activation occurs, leading to the transcription of bone morphogenetic target genes.
BMP exerts pleiotropic effects throughout the lifespan of an organism. In the early stages of human development, BMP plays a key role in skeletogenesis. Also during this time period, BMP signals epidermal induction, directs the development of neural crest cells, and induces a sympathetic adrenergic phenotype. Bmp-2-null mice typically die between 7 and 10 days of gestation due to cardiac defects, even before the formation of bone begins.
BMP is the only signaling molecule capable of singly inducing de novo bone formation. It has been hypothesized that BMP-2, -6, and -9, and to a lesser extent, -4, and -7, various BMP isoforms, have the greatest osteogenic capacity. Luu and colleagues infected HEK293, C2C12, and C3H10T1/2 cells with adenoviral vectors containing various BMP isoforms (AdBMP). The authors measured the expression of early (ALP) and late (osteocalcin) osteogenic markers in vitro . They also assessed in vivo induction of heterotopic ossification by implanting AdBMPs into nude mice and evaluating radiographic and histologic results at 3 and 5 weeks. BMP-2, -4, -6, and -7 were shown to induce increased ALP elevation compared to other BMPs. In addition, BMP-2, -4-, -6, -7, and -9 stimulated increased osteocalcin expression compared to others. The authors also found that BMP-2, -6, -7, and -9 induced the greatest degree of osteogenesis in vivo . The authors concluded that BMP-2, -6, and -9 have the greatest osteogenic potential, and that BMP-4, and -7 also have a significant degree of osteogenic potential.
BMP-2 promotes osteoblast differentiation by stimulating Runx2 in a process mediated by SMAD proteins. In addition, BMP-2 affects osteoblast differentiation via activation of the β-catenin signaling pathway. Interestingly, endogenous activation of β-catenin induces ALP mRNA expression; however, BMP-2 must be present for β-catenin signaling to induce osteocalcin expression as well. Thus, β-catenin-dependent differentiation processes likely require BMP-2 to promote the later stages of osteoblast differentiation. Recently, it has been demonstrated that BMP-2-mediated osteoblast differentiation is also dependent upon Akt2 selective signaling. IGFs are important for osteoblast differentiation and normal bone growth, and Akt2 is a key molecule within the insulin signaling pathway. Mukherjee et al . showed that, although BMP-2 signaling is normal, osteoblast differentiation does not occur in Akt2-knockout mice. Delivery of Runx2 to Akt2-deficient mice restores osteoblast differentiation; thus, Akt likely serves a specific role in osteoblast differentiation through its regulation of Runx2 gene expression.
Furthermore, BMP-2 appears to improve healing of large osseous defects. Many studies have evaluated recombinant human BMP-2 (rhBMP-2) in in vivo animal models. In nonhuman primates and other mammalian models, rhBMP-2 has been shown to augment bone growth successfully and also close critical-sized osseous defects. The efficacy of rhBMP-2 in humans has also been assessed. For most applications in craniofacial surgery, rhBMP-2 requires further investigation to fully understand the risk–benefit ratio. In contrast, Slosar et al . demonstrated that anterior lumbar allografts filled with rhBMP-2 experienced higher fusion rates than lumbar allografts without rhBMP-2 in patients who underwent lumbar fusion. The authors demonstrated that at 6 months and at 2 years, allografts with rhBMP-2 shortened the timeframe for expected improvement, and also improved patients' Oswestry Disability Index scores. Thus, it has been concluded allografts with rhBMP-2 can offer reliable and clinically significant benefits.
Adenoviral delivery of BMP (AdBMP) is also effective in stimulating osteogenic differentiation of mesenchymal progenitor cells. Our group isolated and subsequently reversibly immortalized primary murine calvarial cells that retained a progenitor cell phenotype. Immortalized calvarial cells (iCALs) treated with AdBMP-2 demonstrated an increase in early (e.g., alkaline phosphatase activity, osteocalcin mRNA transcription) and late (e.g., matrix mineralization) marker expression of osteogenic differentiation compared to control iCALs treated with AdGFP (green fluorescent protein). Additionally, in vivo stem cell implantation assays revealed increased ectopic bone production from iCALs exposed to BMP-2 compared to control. In a similar study that evaluated the capacity of BMP-9 to induce osteogenic differentiation of calvarial progenitor cells, Teven and colleagues demonstrated that AdBMP-9-treated iCALs display enhanced osteogenic differentiation compared to AdGFP-treated cells (control) in both the in vitro and in vivo settings. The translational utility of these experiments relates to identifying effective agents for bony (e.g., calvarial) tissue regeneration. However, the clinical use of adenovirally-delivered BMPs has been limited and therefore further investigation is warranted.
Presently, rhBMP-2 (INFUSE, Medtronic, Inc.) is the only rhBMP product approved by the US Food and Drug Administration (FDA) for human application. A second product, rhBMP-7 (rhOP-1, Stryker, Leibinger), received limited FDA approval but was subsequently removed from the market after failing to gain FDA premarket approval in 2009. As described, rhBMP-2 is often used in spinal fusion, as well as orthopedic trauma and dental procedures. Interestingly, although BMP-2 may be more osteoinductive than BMP-7 based on in vitro analyses, there has been relatively little in vivo data comparing the two. Barr and colleagues have found that rhBMP-7 promotes similar bone quality but greater bone volume induction than rhBMP-2 in murine muscle pouch assays analyzed by microscopic computed tomography and histology. However, other studies have found different results. Conflicting results may be due to differences in experimental design.
TGF-β has protean effects on cellular processes within the human body. TGF-β has been implicated in cell cycle regulation, angiogenesis, wound healing, and skeletogenesis. TGF-β also appears to have significant roles in human disease. There are three distinct human TGF-β isoforms (TGF-β1, -β2, and -β3). Though they exhibit differences, each isoform shares similar functions with one another, including functions pertaining to the regulation of osteogenesis and osseous repair.
TGF-β, like BMP, contains a cluster of conserved cysteine residues. Each isoform is produced as a precursor molecule consisting of TGF-β and a propeptide region. In contrast to BMPs, however, TGF-β is secreted as an inactive molecule and stored in its latent form within the ECM. TGF-β becomes active after the noncovalent disulfide bond linking TGF-β and its propeptide region is broken.
There are four TGF-β receptors identified: types I, II, and III, and endoglin. TGF-β-initiated signaling, like BMP-mediated signaling, may use SMAD protein intermediates. Active TGF-β binds to either type III or type II receptors, both of which promote the phosphorylation of type I receptors. Phosphorylation of SMAD2 or SMAD3 follows, which in turn leads to the binding of SMAD4 to the phosphorylated SMAD2 or SMAD3 proteins. This complex translocates to the cellular nucleus, where transcription factors activate specific target gene transcription, thereby mediating the effects of TGF-β at the cellular level.
Evidence that TGF-β may play a role in osteogenesis has come from experiments demonstrating that TGF-β is produced by osteoblasts and stimulates the production of collagen and osteopontin. In vivo studies have corroborated the osteoinductive effect of TGF-β. TGF-β1, in particular, has been the subject of a considerable body of literature with respect to its osteogenic potential, in part because it is the dominant TGF-β isoform expressed by bone cells. In vitro analyses have demonstrated that TGF-β1, by recruiting and stimulating the proliferation of osteoblast progenitors, increases bone formation. Interestingly, TGF-β1 seems to have a differential effect on osteoblast differentiation. Early phases of differentiation appear to be promoted by TGF-β1, while later phases are blocked.
In addition, TGF-β1 modulates the processes of osteoclastogenesis and bone resorption. Mediated by Smad1 and Smad3, TGF-β has been shown to exhibit both inhibitory and stimulatory effects on osteoclast differentiation. TGF-β1 binds to its receptor on osteoblasts and augments the expression of OPG while also decreasing RANKL expression. A lower RANKL : OPG ratio results in maximal RANKL occupation by OPG, thus decreasing maturation of osteoclasts. TGF-β1 appears to serve a bifunctional role, however, as low concentrations are associated with high levels of both RANKL and RANK, and subsequent osteoclastogenesis.
TGF-β1 may also be important for the coupling of bone resorption and formation, as is found at bone resorption sites. Tang and colleagues injected GFP-labeled osteogenic bone marrow stromal cells (BMSCs) into the femur cavity of Tgfb1 +/+ and Tgfb1 −/− immunodeficient mice. Using anti-GFP antibodies, injected BMSCs were detected at both 1 and 4 weeks. After 1 week, BMSCs were localized to the bone surfaces in Tgfb1 +/+ mice; BMSCs were widely dispersed in the bone marrow (i.e., not at bone surfaces) of Tgfb1 −/− mice. After 4 weeks, BMSCs were embedded in trabecular bone in Tgfb1 +/+ mice but not in Tgfb1 −/− mice. The authors concluded that TGF-β1 plays an essential role in the coupling of bone resorption to bone formation by their induction of osteogenic BMSCs to sites of resorption.
In addition, TGF-β expression is elevated during fracture repair and has been considered for exogenous use to augment bone growth and repair. Studies support that TGF-β isoforms significantly and rapidly induce the formation of endochondral bone at extracranial sites. Studies on the effects of TGF-β on calvarial tissue are inconclusive. Many authors have found little osteoinductive potential associated with TGF-β. More recently, ectopic TGF-β was shown to augment calvarial defect healing in rabbits and promote normal suture reformation. Interestingly, combination therapy of BMP-2 and TGF-β did not demonstrate increased calvarial defect repair compared to either therapy individually. In contrast, Senartha-Yapa et al. demonstrated that small molecule induced inhibition of TGF-β enhanced calvarial regeneration by activation of the BMP pathway in vivo.
The FGF family is comprised of structurally related cytokines that mediate several physiologic processes including cellular proliferation, migration, and differentiation; mitogenesis; angiogenesis; embryonic development; and wound healing. There are at least 18 known members of the FGF family, all possessing a characteristically high affinity for heparin. Most isoforms share a similar internal core region that has important FGF receptor (FGFR)-binding properties. Mutations in FGFs or FGFRs are involved in the development of various skeletal dysplasias, including achondroplasia and craniosynostosis. Significant evidence exists demonstrating that FGF–FGFR signaling is essential to the developing axial skeleton. Additionally, the signaling cascade is necessary for cranial bone intramembranous ossification as well as for the maintenance of cranial suture homeostasis. A review of the roles of FGF signaling in physiologic development and in the development of skeletal diseases can be found elsewhere.
There are four known FGFRs (FGFR-1, -2, -3, -4). Following FGF ligand binding, receptor dimerization occurs, which promotes the transphosphorylation of each receptor monomer by an intrinsic tyrosine kinase domain. Phosphorylated tyrosine residues on FGFRs become docking sites for adaptor proteins necessary for downstream signaling. Human gene studies have identified associations between skeletal disorders and FGFR mutations, including Pfeiffer syndrome (FGFR1), Crouzon syndrome (FGFR2), and achondroplasia (FGFR3).
FGF has been implicated in a variety of osteogenic mechanisms. Coffin et al . found that increased concentrations of FGF-2 are found at epiphyseal growth plates of endochondral bone, specifically within the proliferation and hypertrophic zones. FGF-2 also accelerates fracture repair and closure of critical-sized defects. Interestingly, there seems to be a differential effect of FGF-2 treatment based on delivery. Continuous FGF-2 treatment stimulates osteoblast proliferation; however, it decreases levels of differentiation markers (e.g., ALP) and augments osteoclast formation, thus resulting in net bone resorption. In contrast, intermittent FGF-2 treatment enhances bone formation.
The effects of FGF-2 in combination with other growth factors have also been reported. Maegawa and colleagues examined MSCs deposited in osteogenic media supplemented with BMP-2, FGF-2, or both. MSCs supplemented by both FGF-2 and BMP-2 concurrently expressed the highest levels of ALP, osteocalcin mRNA, and bone matrix formation. In addition, Sabbieti et al . investigated whether FGF-2 modulates the anabolic response of bone to PTH. Primary calvarial osteoblasts were isolated from Fgf-2 +/+ (wild-type) and Fgf-2 −/− mice. By immunocytochemistry, Fgf-2-null osteoblasts expressed decreased Runx2 protein synthesis as well as significantly less Runx2 expression within perinuclear and nuclear spaces than wild-type variants when exposed to PTH. Given the importance of Runx2 on osteogenesis, it was concluded that Fgf-2 expression is required for PTH to promote an anabolic response by bone.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here