Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advances in neuroimaging have had a remarkable impact on the diagnosis and treatment of neurologic diseases ranging from earlier detection and treatment of stroke to a more timely diagnosis of dementia, from the rapid detection and treatment of cerebral aneurysms to the ability to diagnose multiple sclerosis after a single attack.
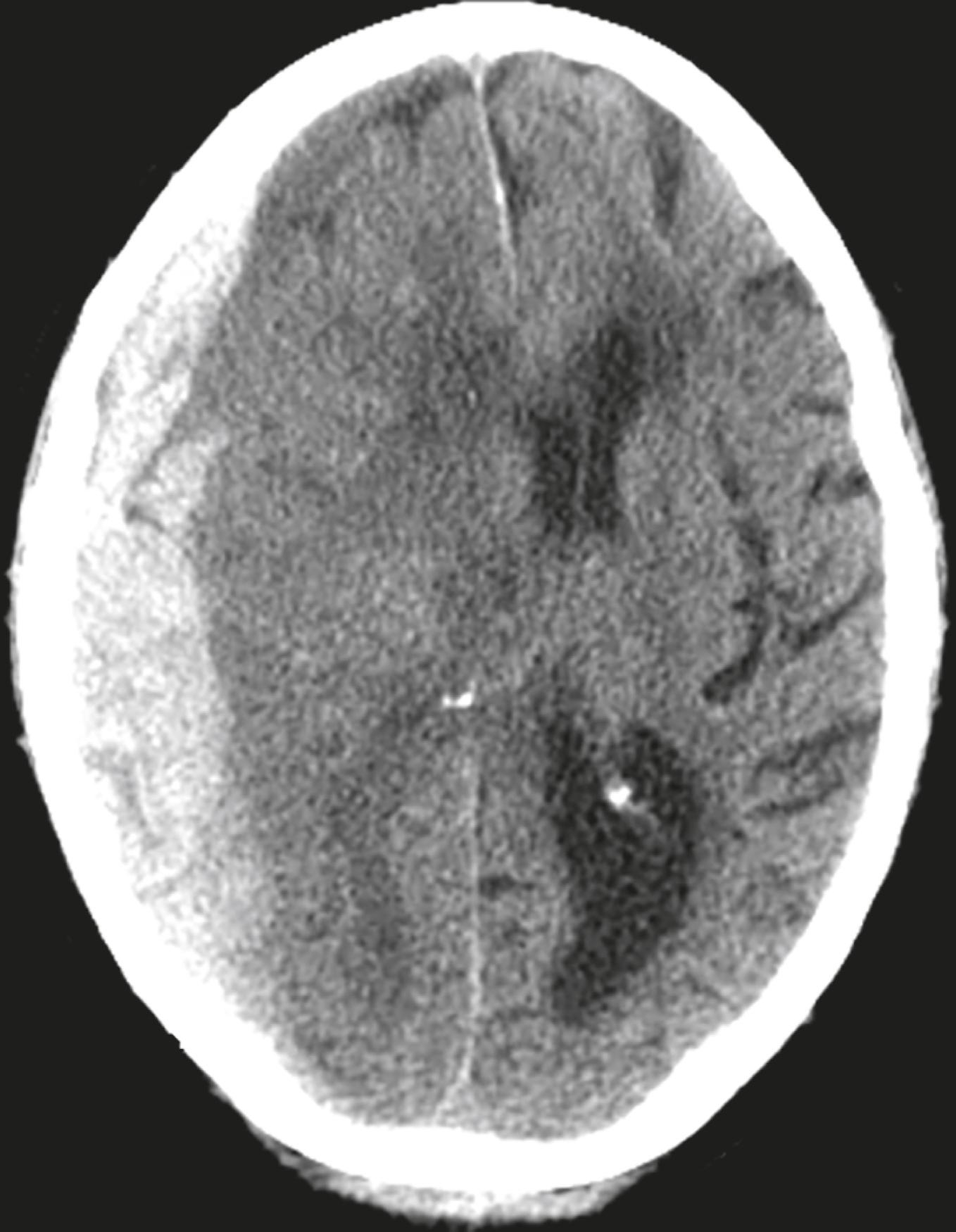
This is an image from an unenhanced head CT on a 68-year-old male who fell and struck his head earlier in the same day and has become progressively disoriented. Pertinent clinical history is the current use of anticoagulants for heart disease. What is the diagnosis? See the answer at the end of this chapter.
Both CT and MRI are utilized for studying the brain and spinal cord, but MRI is the study of first choice in most clinical scenarios ( Table 26.1 ). Conventional radiography has no significant role in imaging intracranial abnormalities.
| Abnormality | Study of First Choice | Other Studies |
|---|---|---|
| Acute stroke | Diffusion-weighted imaging (see Table 26.8 ) for acute or small strokes, if available | Noncontrast CT can differentiate hemorrhagic from ischemic infarct |
| Headache, acute and severe | Noncontrast CT to detect subarachnoid hemorrhage | MR angiography (MRA) or CT angiography (CTA) if subarachnoid hemorrhage is found in order to detect aneurysm |
| Headaches, chronic (with new features) | MRI, without and with contrast, or MRI without contrast | CT, without and with contrast |
| Seizures | MRI, without and with contrast. Add thin section images through hippocampi in patients with history of childhood seizures | CT, without and with contrast, can be substituted if MRI not available |
| Blood | Noncontrast CT | Ultrasound for infants |
| Head trauma | Nonenhanced CT is readily available and the study of first choice in head trauma | MRI is better at detecting diffuse axonal injury but requires more time and is not always available |
| Extracranial carotid disease | Doppler ultrasonography | MRA excellent study; CTA best for preoperative stenosis evaluation |
| Hydrocephalus | MRI as initial study | CT for follow-up |
| Vertigo and dizziness | Contrast-enhanced MRI | MRA if needed and/or thin section images through internal auditory canals as needed |
| Masses | MRI, without and with contrast | Contrast-enhanced CT if MRI not available |
| Change in mental status | MRI, without or with contrast | CT without contrast |
We will look at the normal anatomy of the brain using CT.
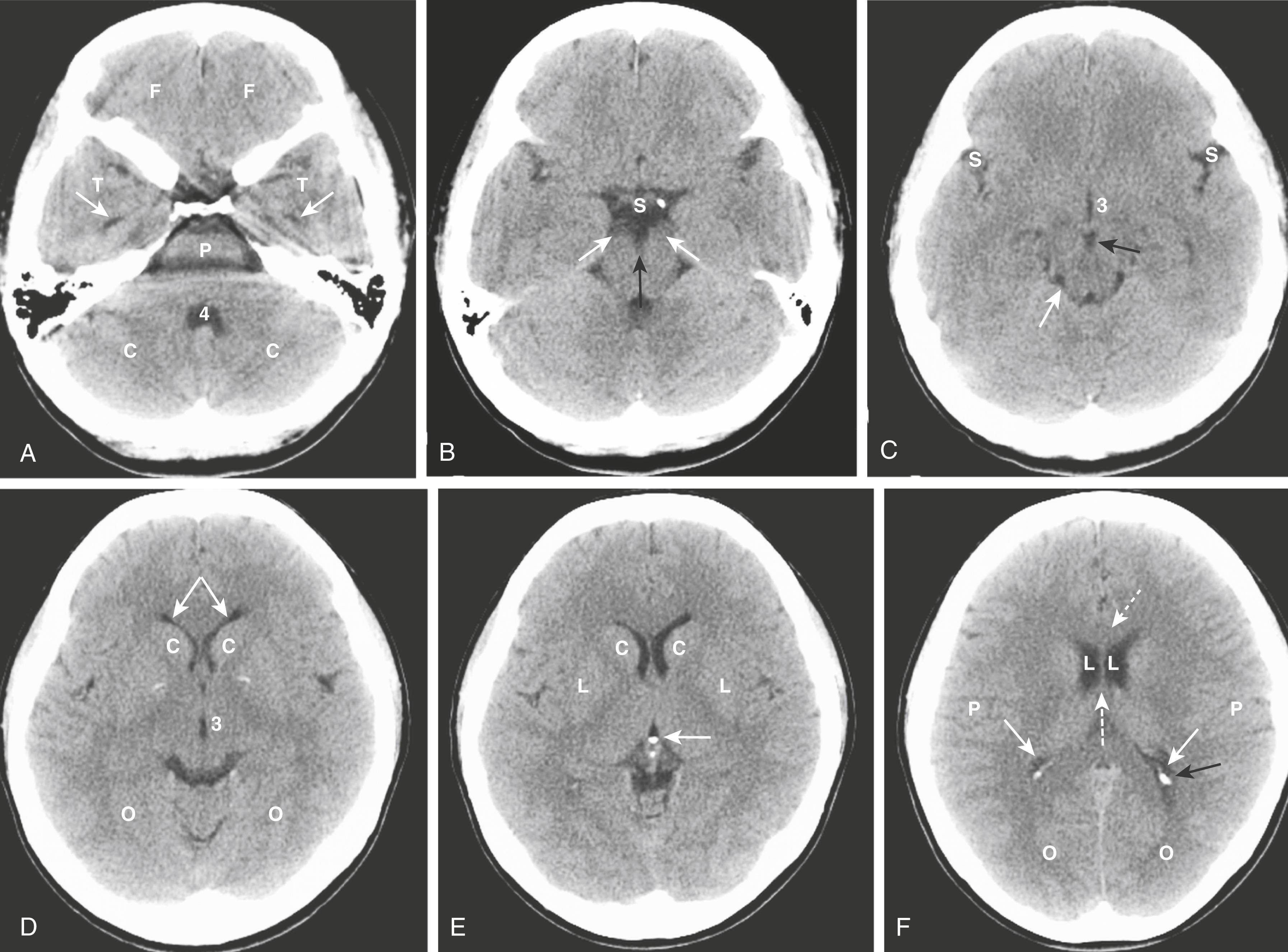
In the posterior fossa, the fourth ventricle appears as an inverted U-shaped structure. Like all cerebrospinal fluid-containing structures on CT, it normally appears black. Posterior to the fourth ventricle are the cerebellar hemispheres, anteriorly lie the pons and medulla oblongata. The tentorium cerebelli separates the infratentorial components of the posterior fossa (cerebellum and fourth ventricle) from the supratentorial compartment.
The interpeduncular cistern lies in the midbrain and separates the paired cerebral peduncles (which emerge from the superior surface of the pons). The suprasellar cistern is anterior to the interpeduncular cistern and usually has a five- or six-point star-like appearance.
The Sylvian fissures are bilaterally symmetric and contain cerebrospinal fluid (CSF). They separate the temporal from the frontal and parietal lobes.
The lentiform nucleus is composed of the putamen (laterally) and globus pallidus (medially). The third ventricle is slit-like and midline. At the posterior aspect of the third ventricle is the pineal gland. Farther posterior is the quadrigeminal plate cistern.
The corpus callosum connects the right and left cerebral hemispheres and forms the roof of the lateral ventricle. The anterior end is called the genu and the posterior end is called the splenium.
The basal ganglia are represented by the subthalamic nucleus and the substantia nigra, globus pallidus, putamen, and caudate nucleus. The putamen and caudate nucleus are called the striatum.
The frontal (also known as anterior ) horns of the lateral ventricles hug the head of the caudate nucleus. The two frontal horns are separated by the midline septum pellucidum. The temporal horns, which are normally very small, are more inferior and contained in the t emporal lobes. The posterior horns ( occipital horns ) of the lateral ventricle lie in the occipital lobes. The most superior portion of the ventricular system are the bodies of the lateral ventricles.
The falx cerebri lies in the interhemispheric fissure , which separates the two cerebral hemispheres , and is frequently calcified in adults.
The surface or cortex of the brain is composed of gray matter convolutions made up of sulci (grooves) and gyri (elevations). The medullary white matter lies below the cortex.
On an unenhanced CT scan of the brain, anything that appears white will generally either be bone (calcium) density or blood, in the absence of a metallic foreign body ( Table 26.2 ).
| Hypodense (Dark) | Isodense | Hyperdense (Bright) |
|---|---|---|
| Fat (not usually present in the head) | Normal brain | Metal (e.g., aneurysm clips or bullets) |
| Air (e.g., sinuses) | Some forms of protein (e.g., subacute subdural hematomas) | Iodine (after contrast administration) |
| Water (e.g., CSF) | Calcium | |
| Chronic subdural hematomas/hygromas | Hemorrhage (high protein) |
Physiologic calcifications that may be seen on CT of the brain:
Pineal gland ( Fig. 26.2A )
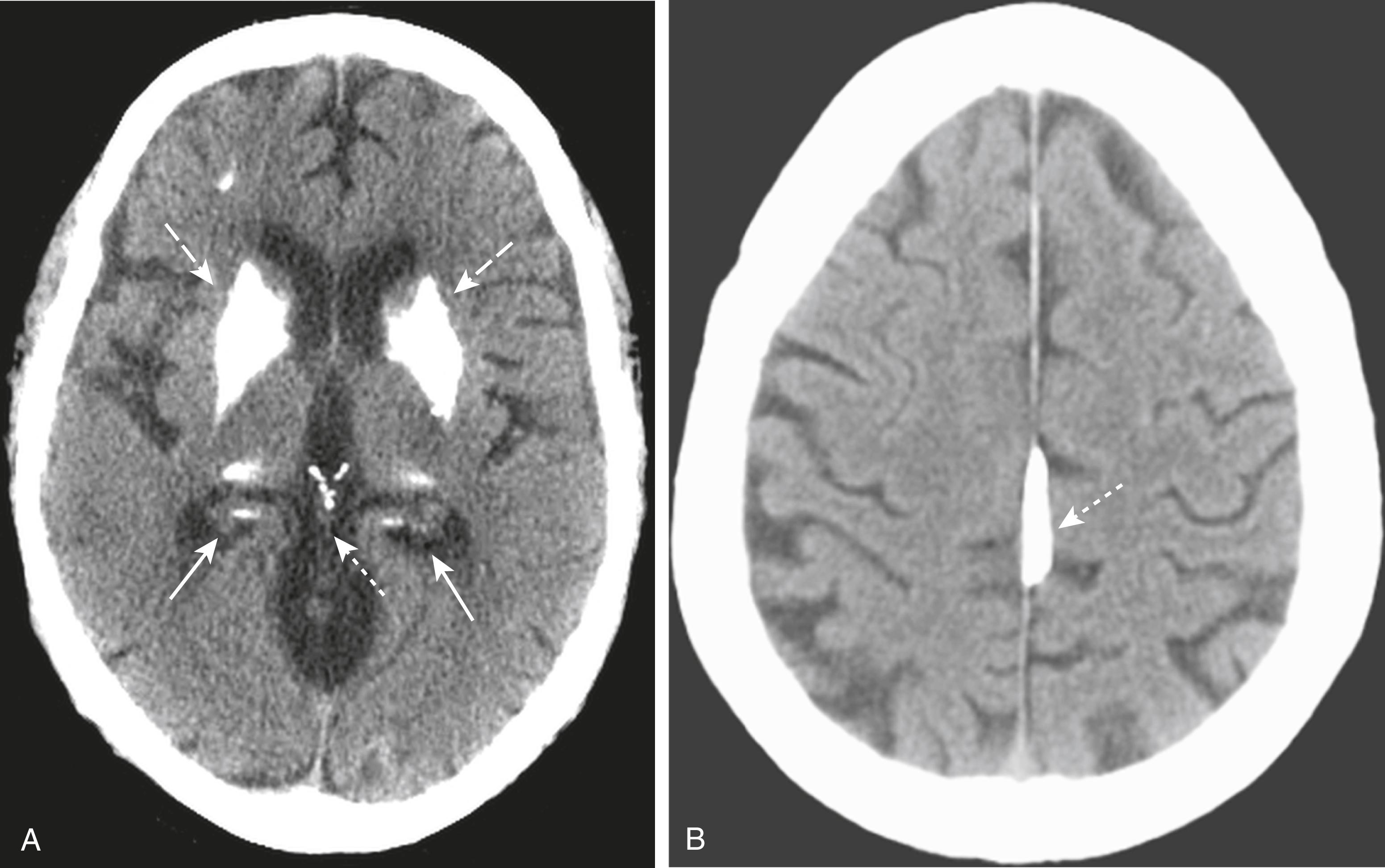
Basal ganglia (see Fig. 26.2A )
Choroid plexus (see Fig. 26.2A )
Falx and tentorium ( Fig. 26.2B )
Normal structures that can enhance after administration of iodinated intravenous contrast :
Venous sinuses
Choroid plexus
Pituitary gland and stalk
Metallic densities in the head can cause artifacts on CT scans. Dental fillings, aneurysm clips, and bullets can all cause streak artifacts .
In general, MRI is the study of choice for detecting and staging intracranial and spinal cord abnormalities. It is usually more sensitive than CT because of its superior contrast and soft-tissue resolution. It is, however, less sensitive to CT in detecting calcification in lesions or in evaluating cortical bone , which appear as signal voids with MR. It may be contraindicated in some patients with pacemakers.
MRI is more difficult to interpret in part because the same structure or abnormality may appear differently on the same study, depending on the pulse sequence, the scan parameters, and the fact that MRI is more variable in its depiction of differences that occur over the time course of some abnormalities (e.g., hemorrhage) than is CT.
Initial evaluation of an MRI of the brain might start with a T1-weighted sagittal sequence of the brain. On this sequence, the brain looks more like the anatomic specimens or diagrams that you are more accustomed to seeing ( Fig. 26.3 ). Many structures in the brain are paired , so remember to compare one side with the other on axial scans of the brain ( Fig. 26.4 ).
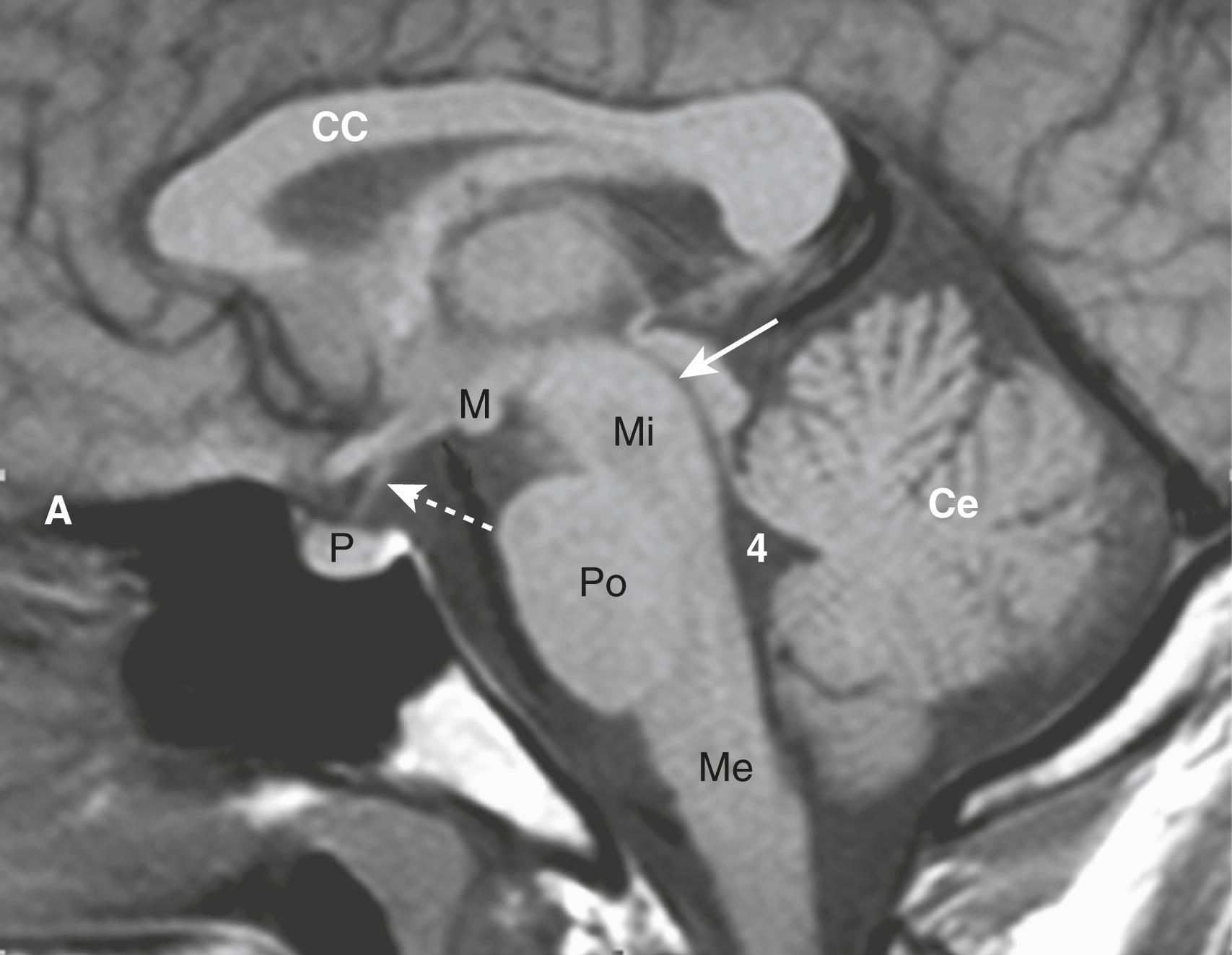
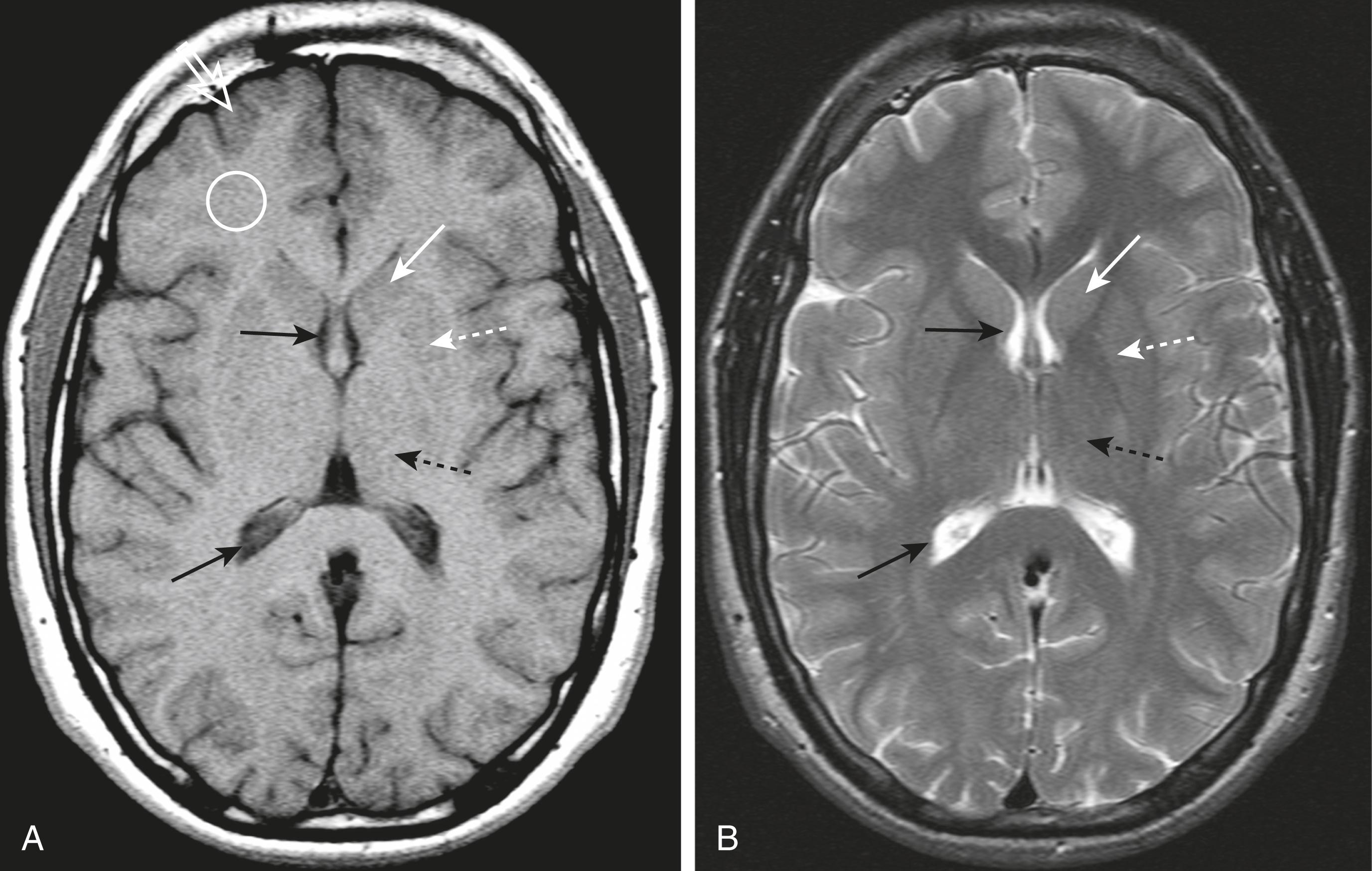
Table 26.3 summarizes the signal characteristics of various tissues seen on MRI.
| Bright on T1 | Dark on T1 | Bright on T2 | Dark on T2 |
|---|---|---|---|
| Fat | Calcification | Water (edema, CSF) | Fat |
| Gadolinium | Air | Calcification | |
| High protein | Chronic hemorrhage | Hyperacute hemorrhage | Air |
| Subacute hemorrhage | Acute hemorrhage is isointense to hypointense on T1 | Late subacute hemorrhage | Early subacute hemorrhage |
| Melanin | Water (edema, CSF) | Chronic hemorrhage | |
| Acute hemorrhage | |||
| High protein |
Traumatic brain injuries extract a huge cost to the patient and society, not only as a result of the acute injury but for the long-term disability they produce. In the United States, motor vehicle accidents account for nearly half of traumatic brain injuries.
Unenhanced CT is the study of choice in acute head trauma. The primary goal in obtaining the scan is to determine whether there is a life-threatening, but treatable, lesion. One of the principle advances in the advent of CT scanning of the head was its ability to detect surgically amenable lesions in a timely fashion.
Initial CT evaluation of the brain in the emergency setting focuses on whether there is (1) mass effect and (2) blood.
To determine whether there is mass effect, look for a displacement or compression of key structures from their normal positions by analyzing the location and appearance of the ventricles , basal cisterns, and the sulci.
Blood will usually be hyperattenuating (bright) and might collect in the basal cisterns, Sylvian and interhemispheric fissures, ventricles, subdural or epidural spaces, or in the brain parenchyma (intracerebral).
Skull fractures are usually produced by direct impact to the skull, and they most often occur at the point of impact. They are important primarily because their presence implies a force substantial enough to cause intracranial injury.
In order to visualize skull fractures, you must view the CT scan using the bone window settings that optimize visualization of the osseous structures ( Fig. 26.5 ).
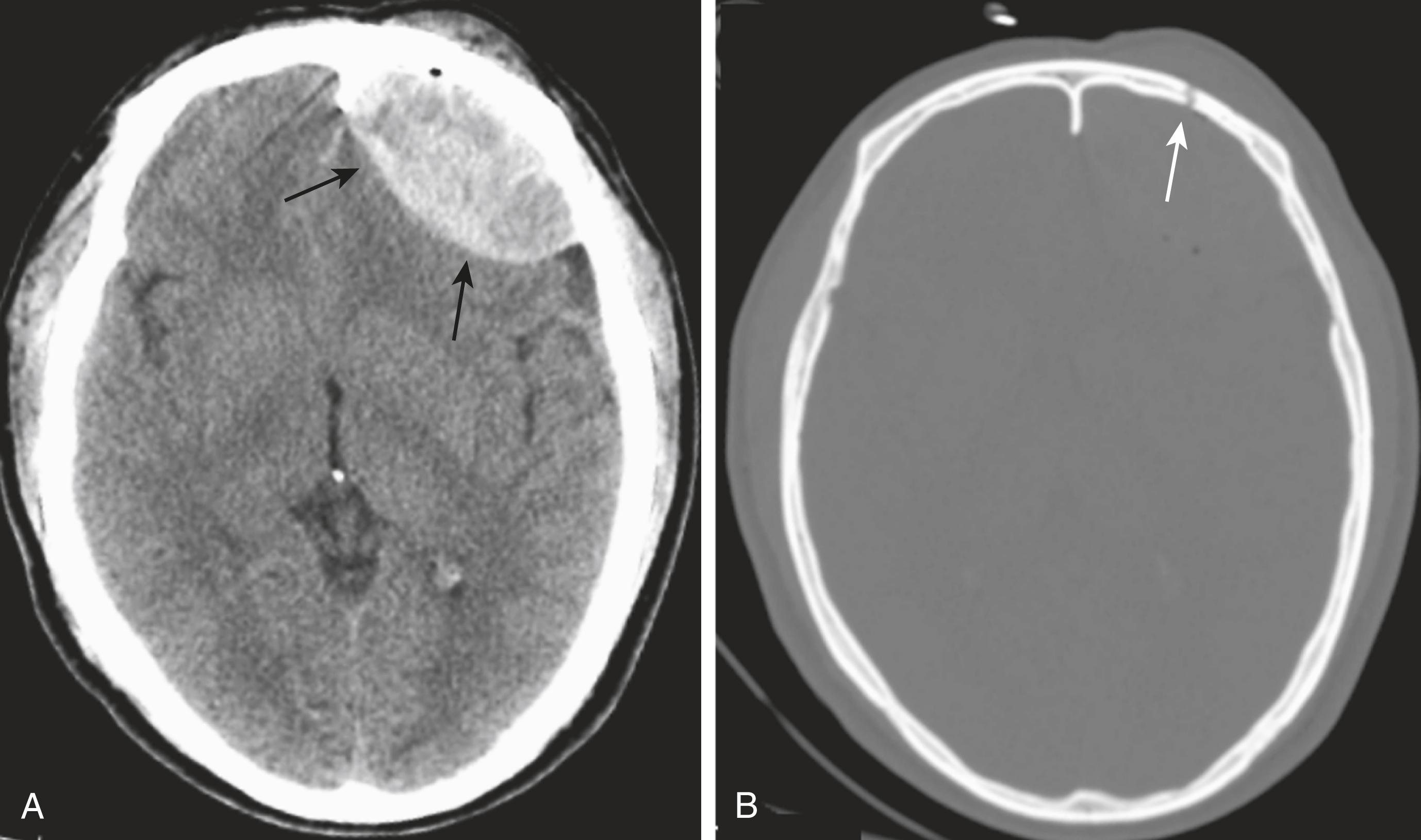
Skull fractures can be described as linear, depressed, or basilar.
Linear skull fractures are the most common, and their primary importance lies in the intracranial abnormalities that may have occurred at the time of the fracture, such as an epidural hematoma. Fractures of the cranial vault most likely occur in the temporal and parietal bones (see Fig. 26.5B ).
Depressed skull fractures are more likely to be associated with underlying brain injury. They result from a high-energy blow to a small area of the skull (e.g., from a hammer), most often in the frontoparietal region, and are usually comminuted. They may require surgical elevation of the depressed fragment when the fragment lies deeper than the inner table adjacent to the fracture ( Fig. 26.6A ).
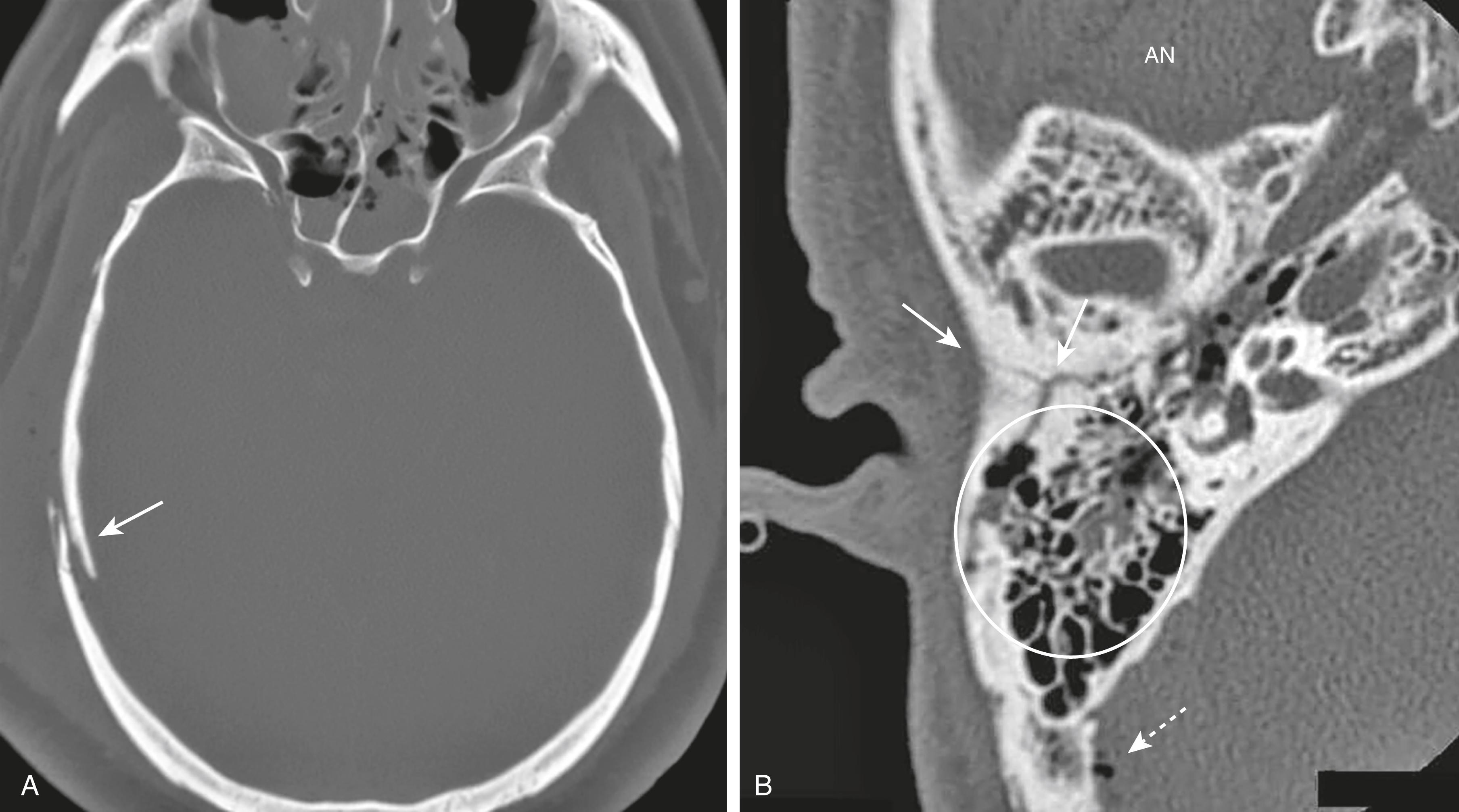
Basilar fractures are the most serious and consist of a linear fracture at the base of the skull. They can be associated with tears in the dura mater with subsequent CSF leak, which can lead to CSF rhinorrhea and otorrhea. They can be suspected if there is air seen in the brain (traumatic pneumocephalus), fluid in the mastoid air cells, or an air-fluid level in the sphenoid sinus ( Fig. 26.6B ).
CT is the imaging study of choice for evaluating facial fractures. Multislice scanners allow for reconstruction of the images in the sagittal and coronal planes so the patient does not have to be repositioned in the scanner.
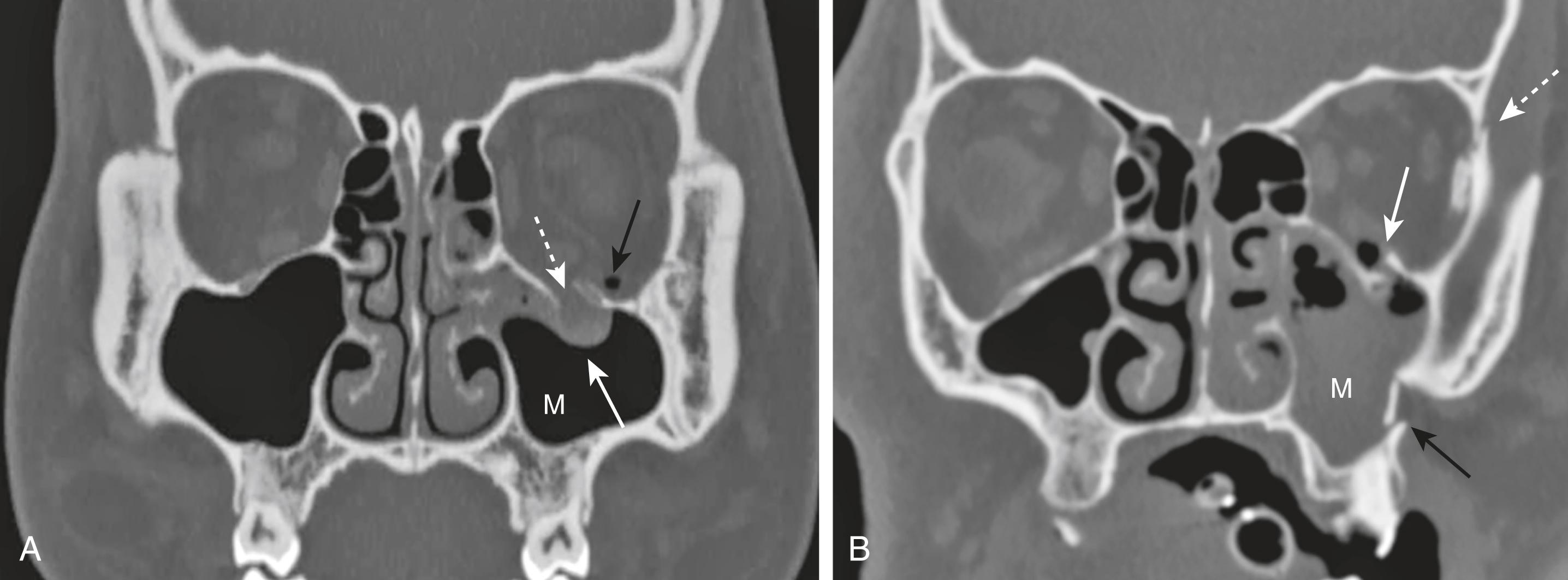
The most common orbital fracture is the blow-out fracture, which is produced by a direct impact on the orbit (e.g., a ball strikes the eye). The impact causes a sudden increase in intraorbital pressure leading to a fracture of the inferior orbital floor (into the maxillary sinus) or the medial wall of the orbit (into the ethmoid sinus). Sometimes the inferior rectus muscle can be trapped in the fracture, leading to restriction of upward gaze and diplopia as presenting symptoms.
Recognizing a blow-out fracture of the orbit ( Fig. 26.7A ).
Orbital emphysema. Air in the orbit from communication with one of the adjacent, air-containing sinuses, either the ethmoid or maxillary sinus.
Fracture through either the medial wall or floor of the orbit.
Entrapment of fat and/or extraocular muscle that projects downward as a soft-tissue mass into the top of the maxillary sinus.
Fluid (blood) in the maxillary sinus.
A tripod fracture, usually a result of blunt force to the cheek, is another relatively common facial fracture. This fracture involves separation of the zygoma from the remainder of the facial bones through the combination of separation of the frontozygomatic suture , fracture of the floor of the orbit, and fracture of the lateral wall of the ipsilateral maxillary sinus ( Fig. 26.7B ).
Skull fractures may be accompanied by intracranial hemorrhage and/or diffuse axonal injury.
There are four types of intracranial hemorrhages that can be associated with head trauma ( Fig. 26.8 ):
Epidural hematoma
Subdural hematoma
Intracerebral hemorrhage
Subarachnoid hemorrhage (discussed with aneurysms)
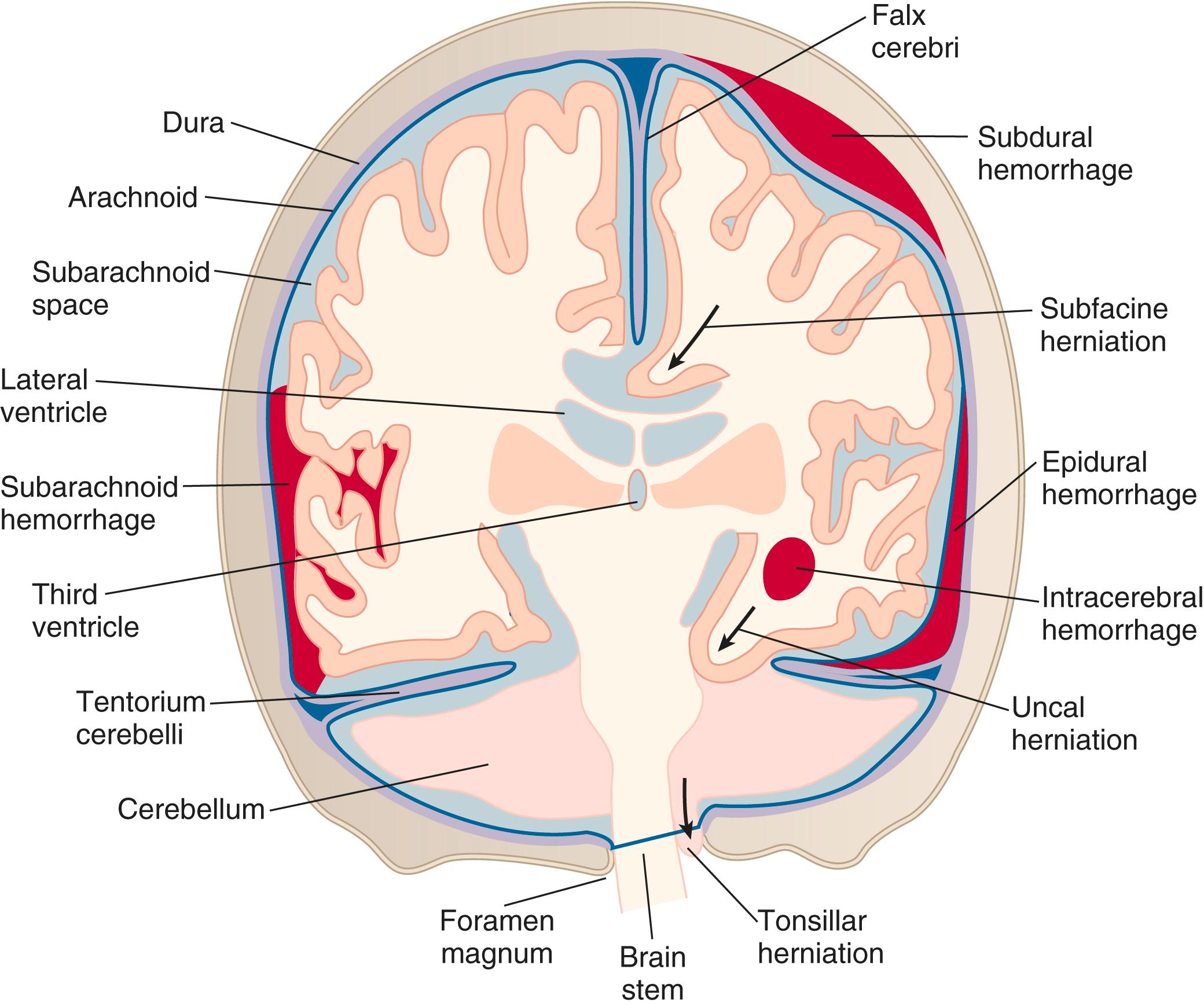
Epidural hematomas represent hemorrhage into the potential space between the dura mater and the inner table of the skull ( Table 26.4 ).
| Layer | Comments |
|---|---|
| Dura mater | Composed of two layers, an outer periosteal layer that cannot be separated from the skull and an inner meningeal layer; the inner meningeal layer enfolds to form the tentorium and falx. |
| Arachnoid | The avascular middle layer is separated from the dura by a potential space known as the subdural space. |
| Pia mater | Closely applied to the brain and spinal cord, the pia mater carries blood vessels that supply both; separating the arachnoid from the pia is the subarachnoid space; together the pia and arachnoid are called the leptomeninges. |
Most cases are due to injury to the middle meningeal artery or vein from blunt head trauma, typically from a motor vehicle accident.
Almost all epidural hematomas (95%) have an associated skull fracture, frequently in the temporal bone. Epidural hematomas may also be caused by disruption of the dural venous sinuses adjacent to a skull fracture.
Recognizing an epidural hematoma:
They appear as a hyperintense, extraaxial, biconvex, lens-shaped density most often found in the temporoparietal region of the brain ( Fig. 26.9 ).
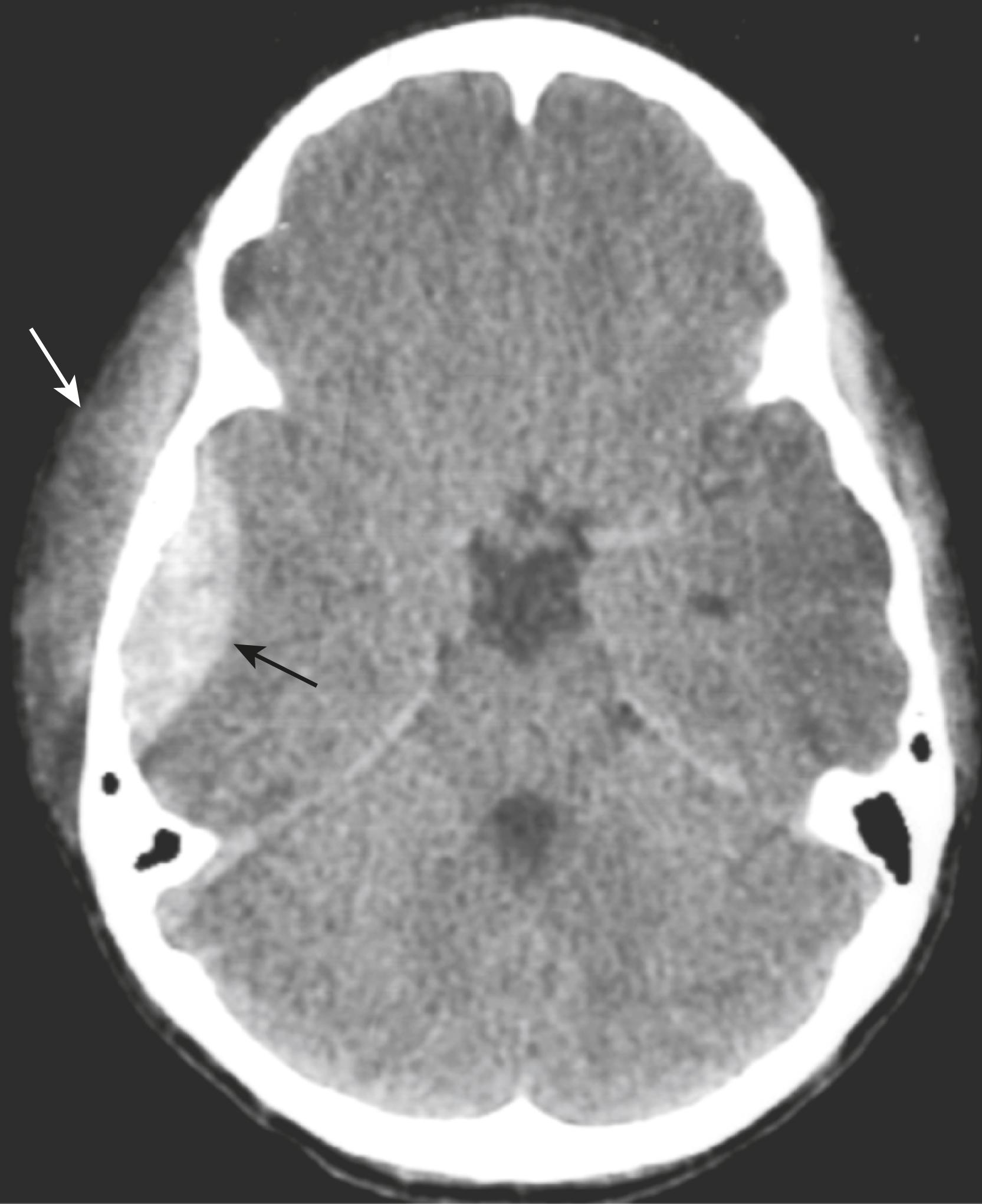
Because the dura is normally fused to the calvarium at the margins of the sutures, it is impossible for an epidural hematoma to cross suture lines (subdural hematomas can cross sutures).
Epidural hematomas can cross the tentorium, but subdural hematomas do not.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here