Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We thank Ricardo Caballero for comments on the manuscript. This work was supported by a 2018 Health Research Grant from “la Caixa” Foundation; CNIC Intramural Grant Severo Ochoa Program (IGP-SO); R01 Grant HL122352 from the U.S. National Heart Lung and Blood Institute (J.J.) and the Leducq Foundation (J.J.), as well as Ministerio de Economía, Industria y Competitividad (SAF2017-88116-P) and Comunidad de Madrid (S2017/BMD-3738; E.D.).
The current understanding of the relationship between the sodium inward current (I Na ) and the inward rectifier potassium current (I K1 ), the two most important ionic currents that control ventricular excitability, began in 1955 with the seminal work of Silvio Weidmann. It also derives from the basic and clinical studies on arrhythmogenesis in ion channel diseases and heart failure (HF), which have demonstrated that the modification in the peak density of either I Na or I K1 changes cell excitability and conduction velocity (CV). Until recently, however, the pathophysiologic consequences of a molecular interplay between the individual channels at the center of such diseases had not been investigated. In the heart, I K1 is the major current responsible for the maintenance of the resting membrane potential (RMP), whereas I Na provides the largest fraction of the inward depolarizing current that flows during an action potential (AP). It is well known that by controlling RMP and AP duration (APD) at the end of repolarization, I K1 modifies the Na + channel availability and therefore cell excitability. In addition, I K1 -I Na interactions are important for stabilizing and controlling the frequency of the electrical rotors that are responsible for the most dangerous cardiac arrhythmias, including ventricular tachycardia (VT) and ventricular fibrillation (VF). ,
Data obtained from adult transgenic mice, single adult rat ventricular myocytes (ARVMs), human cardiomyocytes derived from induced pluripotent stem cells (hiPSC-CM), neonatal rat ventricular myocyte (NRVM) monolayers, and heterologous expression systems (HEK293 and CHO cells) have demonstrated that the I Na -I K1 interplay is much more complex than previously thought. , It comprises model-independent, reciprocal modulation of the expression of their respective channel proteins (Na V 1.5 and Kir2.1) within a macromolecular complex that involves the membrane-associated guanylate kinase (MAGUK)-type protein synapse-associated protein 97 (SAP97),2 α1 syntrophin, and possibly additional scaffolding proteins. In cardiomyocytes from adult transgenic mice overexpressing Kir2.1 (Kir2.1 OE), I K1 is increased and peak I Na density is twice as large as in cardiomyocytes from control hearts. Conversely, in heterozygous Kir2.1 knockout (KO) mice (Kir2.1–/+), in addition to I K1 , both Na V 1.5 protein and I Na are significantly reduced. Similarly, in single ARVMs and hiPSC-CMs, I K1 increased significantly on adenoviral transfer of Na V 1.5. In NRVM monolayers, the co-OE of Na V 1.5 with Kir2.1 increased CV, abbreviated APD, and increased rotor frequency beyond those produced by Kir2.1 OE alone. Furthermore, data in the literature suggest that conditions that result in Na V 1.5 protein reduction, such as those occurring in dystrophin-deficient mdx 5cv mice or in vivo models of Brugada Syndrome (BrS; Scn5a +/– ), are accompanied by a concomitant reduction in Kir2.1 protein levels. ,
This chapter discusses recent results on Kir2.1-Na V 1.5 interactions in the context of cardiac excitability and the mechanisms of reentrant arrhythmias. Sodium and potassium channel interactions depend on more than membrane voltage alone, and rare inherited cardiac diseases that lead to a decrease in Na V 1.5 or Kir2.1 channels concomitantly and unexpectedly inhibit I K1 or I Na , respectively. Altogether, the evidence suggests that cardiac cells undergo model-independent coregulation that involves posttranslational mechanisms underlying Kir2.1 and Na V 1.5 function, with important physiologic and pathophysiologic consequences for myocardial excitation, electrical impulse velocity, and arrhythmogenesis.
In the heart, I Na is the major current that controls excitability in working atrial and ventricular cardiomyocytes and the Purkinje fibers. Normally closed at the resting potential (approximately –85 mV in adult ventricular cardiomyocytes), sodium channels open upon depolarization beyond the threshold, allowing an influx of Na + ions down their electrochemical gradient. This inward current causes “all or none” membrane depolarization at a rapid rate (around 500 V/s in the Purkinje fibers) in a process that moves the membrane potential to positive values. The rapid voltage-dependent activation of the sodium channel is immediately followed by an inactivation process that is also triggered by an initial depolarization. Inactivation causes I Na to be brief and results in the termination of the current. These properties are important for the rapid CV (around 50 cm/s) of the cardiac electrical impulse.
From a clinical standpoint, multiple mutations in the SCN5A gene, which codes for Na V 1.5, have been identified in association with inherited arrhythmogenic diseases, such as long QT syndrome (LQTS), BrS, idiopathic VF, cardiac conduction defects, sick sinus syndrome, and dilated cardiomyopathy associated with atrial fibrillation, as reviewed in subsequent chapters. , Such mutations illustrate the pathophysiologic importance of these channels. Furthermore, several studies have demonstrated that reduced SCN5A expression is associated with common acquired cardiac diseases, such as HF and ischemia/reperfusion injury. , In fact, reduced SCN5A expression by itself is associated with severe HF disease and mortality and, vice versa, HF and ischemia/reperfusion injury produce a significant reduction of Na V 1.5 channel expression. Thus it would be reasonable to expect a significant decrease in Kir2.1 (and its respective ionic current I K1 ) and RMP depolarization of adult ventricular myocytes as a consequence of inherited or acquired reduced SCN5A expression . We assessed this possibility extensively using transgenic mouse models, as detailed later.
Kir2.x channels are responsible for I K1, and they are involved in the depolarization, repolarization, and resting phases of the cardiac AP. , Among the three strong inward rectifier potassium channel proteins (Kir2.1, 2.2, and 2.3) that are expressed in the heart, Kir2.1 (encoded by the KCNJ2 gene) is the most abundant in the human ventricles. It is usually accepted that, near the resting potential, the ventricular I K1 conductance is much larger than that of any other current, with the exception of the adenosine triphosphate (ATP)-sensitive potassium current (I KATP ), which, however, is generally not active because KATP channels are not open under normal conditions. It is thus likely that the physiologic and/or pathophysiologic modulation of I K1 will significantly affect excitability. Kir2.1 channels show strong rectification between –50 and 0 mV, which means they remain closed during the AP plateau; they open only when the membrane potential repolarizes to levels between –30 and –80 mV, which occurs during the late phases of the AP. , Rectification is achieved by a voltage-dependent blockade by intracellular polyamines (spermine, spermidine, putrescine) and magnesium, which are known to interact with at least three amino acid residues located inside the pore of the channel complex. As shown by Zartizky and colleagues, ventricular myocytes from Kir2.1 KO ( Kcnj2 –/– ) mice lack I K1 in whole-cell recordings under physiologic conditions, which demonstrates that Kir2.1 is the major determinant of I K1 . Loss-of-function mutations in the KCNJ2 gene have been identified in patients affected by Andersen-Tawil syndrome (ATS), also known as LQTS type 7. In the heart, I K1 reduction leads to QT prolongation and predisposes the patient to arrhythmias; however, QT prolongation is less prominent in patients with ATS than in those with other LQTS types. Nevertheless, patients with ATS develop ventricular tachyarrhythmias, including torsades de pointes and sudden cardiac death. In fact, recently released clinical research has upgraded the severity of this disease. In addition to being expressed in the heart, Kir2.1 is expressed in other organs, such as skeletal muscle. As a result, ATS is associated with hypokalemic periodic paralysis and skeletal developmental abnormalities. Importantly, common acquired cardiomyopathies, such as HF, are also associated with KCNJ2 decreased expression. Indeed, cardiomyocytes from patients with HF or idiopathic dilated cardiomyopathy exhibited a reduced I K1 density secondary to a decrease in Kir2.1 expression. ,
Gain-of-function mutations (e.g., D172N, E299V, and M301K) in the KCNJ2 gene have been demonstrated in patients with short QT syndrome type 3. The mutations cause a significant increase in the outward component of the current-voltage (I–V) relationship of I K1 and are associated with an accelerated repolarization, which can be highly arrhythmogenic. Nevertheless, the direct involvement of I K1 in arrhythmia mechanisms was not demonstrated in affected patients; therefore the Kir2.1 OE mouse model was used to study the effect of I K1 increase on VF at the molecular level. An increase of I K1 was shown to shorten repolarization and the QT interval and to exert a proarrhythmic effect in both atria and ventricles of this transgenic mouse model. , Optical mapping and numerical studies in these mice demonstrated that, by hyperpolarizing the RMP, I K1 OE enhanced the availability of sodium channels during sustained reentry, which contributed to the observed increase in the frequency and stability of rotors and VF.
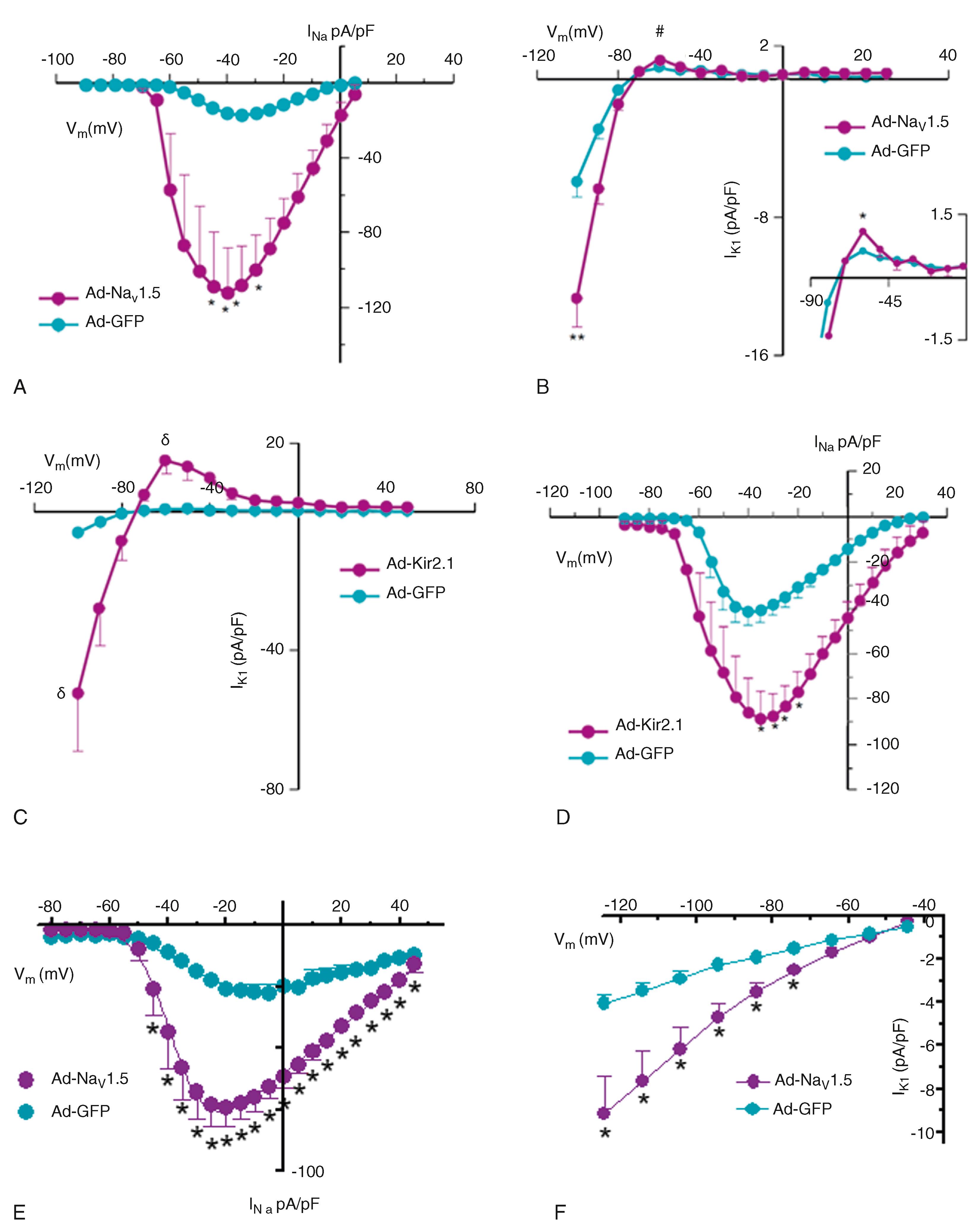
The voltage-clamp experiments presented in Fig. 21.1 illustrate the reciprocal regulation of Na V 1.5 and Kir2.1 in ARVMs. Na V 1.5 was overexpressed using the adenoviral (Ad) construct (Ad-Na V 1.5) 2 ; a control group of myocytes was infected with an adenovirus-encoding green fluorescent protein (Ad-GFP). Average I Na -density–voltage (I–V) plots are shown in Fig. 21.1A . There was a significant increase ( P < .005) in I Na density compared with that in control cells. There was no significant difference for V 1/2 values between control and Ad-Na V 1.5–treated cells. 2 Na V 1.5 OE also resulted in a significant increase in I K1 density in both the inward ( P < .01) and outward ( P < .05) directions (see Fig. 21.1B ). Next, the possibility that Kir2.1 OE would increase Na V 1.5 functional expression was considered. 2 Infection with Ad-Kir2.1 resulted in an increase of I K1 in ARVMs (see Fig. 21.1C ). Peak inward (−100 mV) and outward (−60 mV) currents were significantly larger ( P < .01) than those in control (Ad-GFP) cells. As hypothesized, peak I Na density was also increased ( P < .005; see Fig. 21.1D ) with no changes in the voltage dependence of activation or inactivation. 2 Similar results were obtained in heterologous expression systems and in hiPSC-CMs. , Voltage-clamp experiments in hiPSC-CMs infected with Ad-NaV1.5 show a significant increase in INa density and, concurrently, in IK1 (see Fig. 21.1E –F). Therefore different models provided consistent evidence of the occurrence of the reciprocal positive modulation between NaV1.5 and Kir2.1 channels.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here