Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
The basal ganglia, thalamus, and cortex are arranged in topographically organized circuits that remain segregated throughout their cortical-subcortical course. These circuits subserve different functions (e.g., motor, limbic, associative). Many movement disorders involve motor circuit dysfunction that can be addressed by surgical interventions that target the specific motor regions of the basal ganglia.
Newly discovered bidirectional subcortical connections between the basal ganglia and the cerebellum suggest that normal motor function relies on tight interactions between the basal ganglia–thalamocortical and the cerebellothalamocortical systems and that most movement disorders may involve both systems.
A key rationale for the use of functional surgery in the treatment of movement disorders is the lack of effective medical treatments or the presence of significant side effects of these treatments.
While ablative surgery (e.g., pallidotomy or thalamotomy) was frequently used in the past, surgeons and patients currently prefer the less invasive technique of deep brain stimulation because it can be adjusted or reversed as needed.
The pathophysiologic abnormalities underlying movement disorders such as parkinsonism, dystonia, or tremor are now more clearly understood than in the past, showing intricate changes of firing rates, oscillatory firing patterns, and greater synchrony of the activity of neurons in the basal ganglia–thalamocortical circuit and elements of the cerebellothalamocortical circuit.
It is unlikely that surgery restores normal activity patterns in motor systems. Instead, improvements of function through ablation or deep brain stimulation may be due to release of relatively intact circuit components in the brainstem, thalamus, and cerebral cortex from the disruptive basal ganglia or cerebellar output, allowing the relatively intact components of the motor circuitry to function more normally.
Many open questions remain in the field of functional surgery for movement disorders. For example, researchers are exploring (1) the possible benefits of conducting surgery earlier than traditionally done in patients with Parkinson disease and dystonia, (2) the use of closed-loop or on-demand stimulation approaches that adjust stimulation to the current needs of the patient, and (3) the use of new surgical targets for improved symptom control.
Ablative approaches targeting the basal ganglia and thalamus for patients with severe tremor and involuntary movements were developed empirically, beginning in the 1930s. Radiofrequency lesioning (pallidotomy and thalamotomy) was widely used in the 1950s and 1960s for treatment of Parkinson disease (PD) and tremor. These procedures were largely abandoned other than for intractable tremor, however, following the introduction of levodopa treatment for PD in the 1960s. As a result of several factors, the use of neurosurgical approaches for movement disorders has increased again over the last two decades. The first is that considerable progress was made in the 1980s and 1990s with regard to our understanding of the functional organization of the basal ganglia and the pathophysiology of disturbances in basal ganglia function in animal models of PD and hemiballismus. These studies led to the development of circuit (network) models of movement disorders, which provided a rationale for ablative approaches and resulted in the discovery of a novel surgical target, the subthalamic nucleus (STN), for treatment of PD. Although there was a brief return to ablative procedures such as pallidotomy for PD and dystonia in the early 1990s, this was rapidly replaced by the introduction of the less invasive and modifiable technique of chronic high-frequency deep brain stimulation (DBS), targeting initially the thalamus for tremor and subsequently the STN and the internal globus pallidus (GPi) for treatment of PD and dystonia.
The effectiveness and versatility of DBS have led to a renaissance of functional surgery for movement disorders and to exploration of its use in the treatment of a number of other neuropsychiatric disorders. Because of its reduced invasiveness (compared with ablative procedures) and its reversibility and adjustability, the use of DBS has also made it possible to explore novel targets for therapeutic responses and dysfunctional network activity. Recently developed DBS devices allow investigators to collect physiologic information that may be used for closed-loop approaches. DBS has now largely replaced lesioning, although the emergence of imaging-guided focused ultrasound lesioning techniques may again lead to increased use of ablative strategies for some cases. In the following sections, we briefly review anatomic and physiologic features of brain motor networks, followed by a discussion of the rationale and possible mechanisms of action of ablative and chronic stimulation approaches in the treatment of movement disorders.
Over the past several decades we have come to a clearer understanding of anatomic and physiologic features of the motor system, including the cortical, basal ganglia, cerebellar, and brainstem networks that are relevant for the pathophysiology of movement disorders. Traditionally the interactions between the cerebral cortex and the basal ganglia and cerebellum were thought to be segregated at the subcortical level, with sharing of information only at the cortical level. Research in recent years has shown, however, that this categorical view is no longer tenable: as shown in Fig. 103.2A , one of the components of the basal ganglia circuitry, the STN, was shown to send disynaptic output to the cerebellar cortex, via the pontine nuclei, and the deep cerebellar nuclei have been found to project to the striatum via a disynaptic pathway involving the thalamus. The existence of these subcortical interactions between basal ganglia and cerebellar circuits indicates that the motor system is a highly integrated network involving the cortex, basal ganglia, cerebellum, and portions of the brainstem with downstream connections to the spinal cord. One behaviorally relevant function where this may come into play is motor learning: the cortex, basal ganglia, and cerebellum may play particular roles in unsupervised (hebbian), reward-based, and error-based learning, respectively. While cortex, basal ganglia, and cerebellum may work together to facilitate motor control, the motor, associative, and limbic networks and functions remain separate throughout both of the cortical-subcortical networks ( Fig. 103.1 and see later).
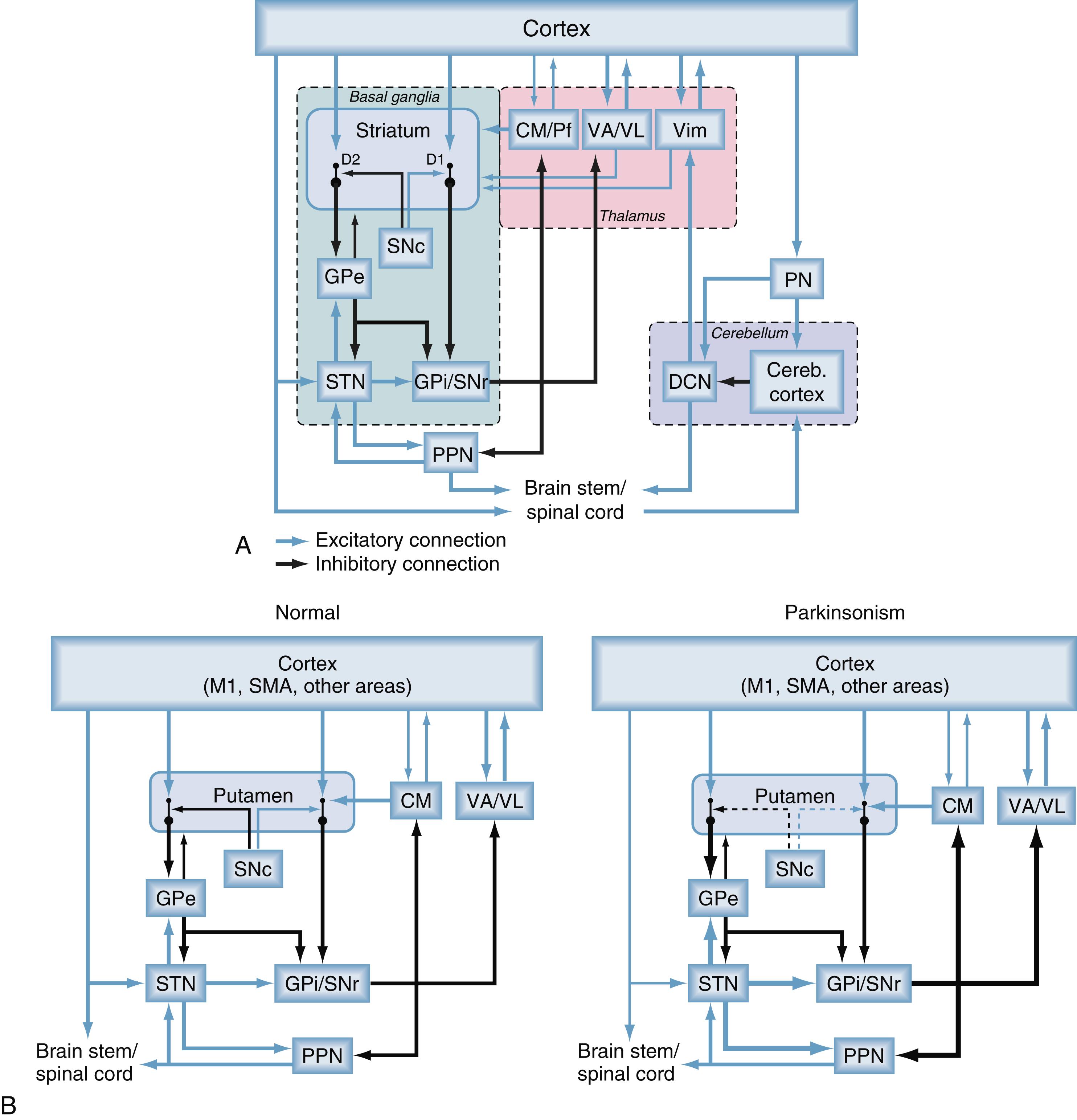
The existence of subcortical interactions between the cerebellar and basal ganglia circuits indicates that disturbances in either circuit may cause disturbances in the other. These findings have high relevance for our understanding of the pathophysiology of movement disorders as well as the mechanism of action of surgical interventions and may lead to the development of novel surgical approaches and targets for neuropsychiatric disorders. However, the pathophysiologic role of the cerebellum remains unknown in most movement disorders, and exploration of surgical interventions targeting the cerebellum and its outflow pathways remains experimental. We therefore focus our discussion largely on surgical approaches targeting the basal ganglia and thalamic nuclei because these remain the targets for most current surgical procedures.
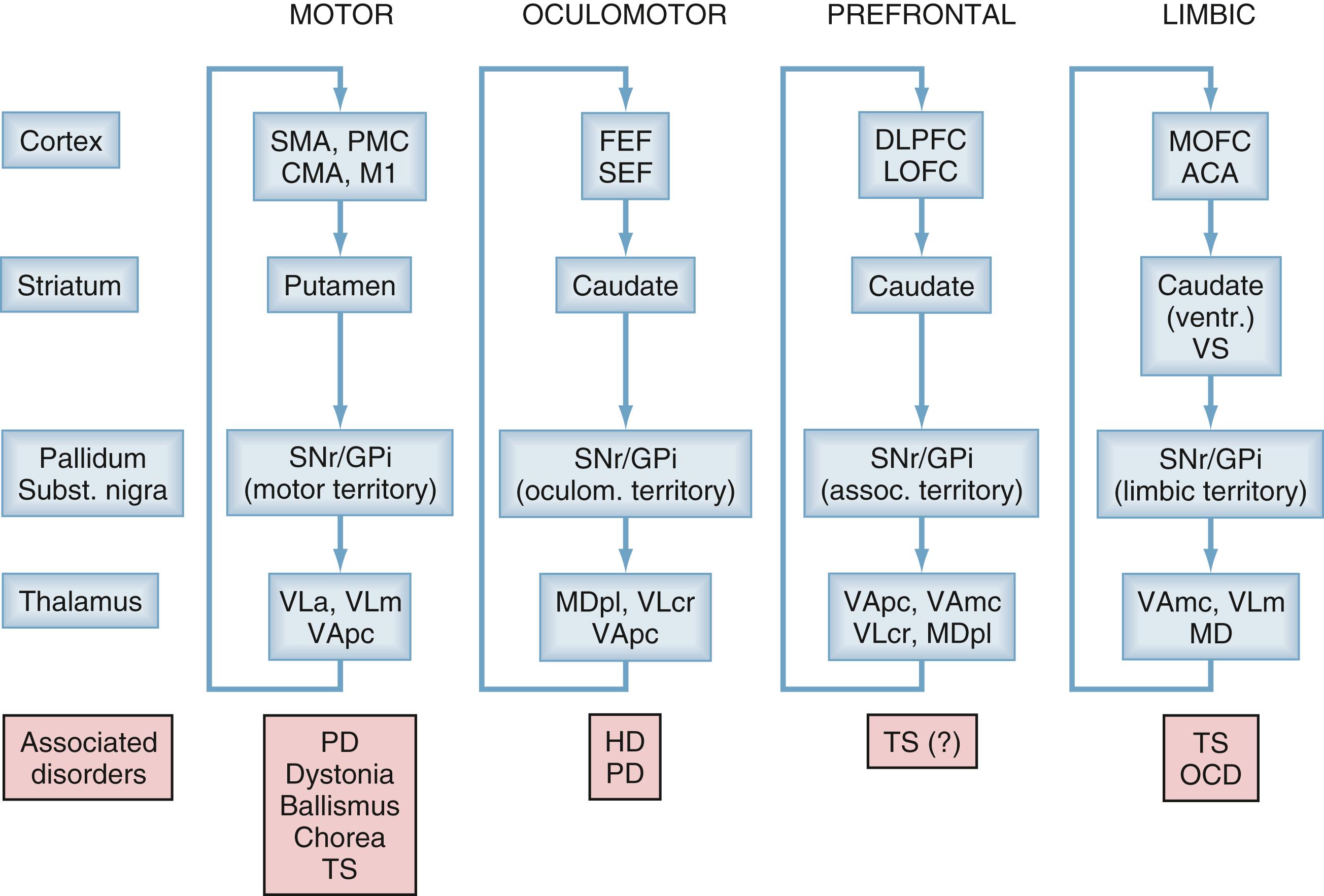
As depicted in the circuit diagrams in Fig. 103.2 , the basal ganglia comprise the neostriatum (caudate nucleus and putamen), the ventral striatum, the external globus pallidus (GPe) and GPi, the STN, and the substantia nigra pars reticulata and pars compacta (SNr and SNc, respectively). The striatum and the STN are the main entry points for cortical and thalamic inputs to the basal ganglia, and the GPi and SNr are the major output nuclei of the basal ganglia, sending projections to the brainstem and thalamus. The corticostriatal, cortico-STN, and other basal ganglia projections are highly topographically organized, which accounts for the presence of segregated circuits for motor, oculomotor, associative, and limbic functions and the preservation of neuronal specificity and somatotopic organization throughout the subnuclei of the motor circuit (see later).
Fig. 103.1 illustrates that dysfunction within individual circuits appears to be associated with specific signs and symptoms of diseases. Many decades of research have demonstrated that the major hypokinetic and hyperkinetic movement disorders (e.g., PD, dystonia, hemiballismus) arise from functional or structural abnormalities within the basal ganglia motor circuit and that interventions targeting the output nodes of the motor circuit in the basal ganglia (GPi and STN) ameliorate the signs and symptoms of these disorders.
The anatomy of the motor circuit is shown in Fig. 103.2 . Topographically arranged projections from several motor cortical areas terminate in the postcommissural putamen and in the STN, defining the motor portions of these structures. For example, in primates, inputs from the primary motor cortex (M1) innervate the dorsolateral STN, while the supplementary motor area, the premotor cortex, and the cingulate motor area project to the dorsomedial STN. The motor portions of the putamen and STN project in turn to motor areas in the basal ganglia output nuclei, the GPi and SNr. The corticosubthalamic pathway allows cortical inputs to reach the output structures of the basal ganglia, the GPi and SNr, with shorter latencies than the transstriatal projections, a feature that may be important in behavioral situations that require rapid cessation of ongoing movements.
Two separate striatal output systems to the GPi and SNr have been found, the so-called direct and indirect pathways (see Fig. 103.2 ). The direct pathway arises from striatal spiny projection neurons that project monosynaptically to neurons in the GPi and SNr. These neurons were shown to express D 1 dopamine receptors. The indirect pathway arises from striatal spiny projection neurons that project to the GPe. , These neurons were found to express D 2 dopamine receptors. The indirect pathway continues from the GPe to the GPi, both by direct projections and via the intercalated STN. In addition to the prototypical GPe projections to the STN or GPi, arkypallidal projections from the GPe are also sent to the striatum. At least in rodent species, these projections can be distinguished by cellular markers, by their activity patterns and functional interactions with the cerebral cortex, , and by their interactions with the cerebral cortex and their differential involvement in movement disorders, specifically parkinsonism. , ,
The topographic organization and segregation of the corticostriatal projections is maintained throughout the course of the direct and indirect pathways. Thus populations of GPe neurons within specific portions of the sensorimotor territory are reciprocally connected with specific populations of neurons in the same functional territories of the STN. Somatotopically organized and movement-specific neurons in each of these regions in turn innervate the same functional territory of the GPi. , The functional topography is also maintained in the basal ganglia projections to the thalamus and the subsequent thalamocortical projections. Basal ganglia motor circuit projections terminate preferentially in portions of the ventral anterior and ventrolateral thalamic nuclei.
Basal ganglia output projections also collateralize to reach the intralaminar nuclei of the thalamus (the centromedian and parafascicular nuclei [CM/Pf]), as well as brainstem targets. The CM/Pf nuclei projections are part of a system of segregated basal ganglia–thalamostriatal feedback projections. In primates, the CM nucleus receives input from cortex as well as motor areas in GPi/SNr and projects to the motor portions of the putamen and STN, whereas the Pf nucleus input and output are related to associative and limbic territories of the basal ganglia. Other thalamic nuclei including ventral anterior and ventrolateral thalamic nuclei and the cerebellar recipient ventral intermedius nucleus send additional sparse projections to the striatum.
Brainstem collaterals of basal ganglia output neurons terminate in the pedunculopontine nucleus (PPN) and the tectum. Some of these projections are part of a feedback system, engaging PPN neurons that give rise to ascending glutamatergic and cholinergic projections to basal ganglia, thalamus, and basal forebrain. The PPN also gives rise to descending projections to pons, medulla, and spinal cord. , The PPN is a component of the physiologically defined brainstem locomotor region. As mentioned previously, the STN sends projections to pontine nuclei that mediate interactions between the basal ganglia and cerebellum. ,
The behavioral functions of the basal ganglia result from their interactions with other brain regions and networks. However, within each of the diverse basal ganglia circuits they have specific physiologic functions. One of these is that the high tonic activity in the basal ganglia output nuclei (GPi/SNr) provides tonic inhibition of the thalamic and brainstem targets of basal ganglia efferents. Models of basal ganglia function have proposed that phasic activation of direct pathway spiny projection neurons reduces inhibitory basal ganglia output with subsequent disinhibition of related thalamocortical neurons, which acts to facilitate intended movements. , By contrast, activation of spiny projection neurons that give rise to the indirect pathway may reduce movement by increasing the (inhibitory) basal ganglia output. These aspects play into the concepts of action selection and movement initiation and control. Variants of this concept pose that the activation profile of basal ganglia neurons at any given time may not be homogeneous, so that some behaviors, represented by low basal ganglia output, would be favored, while others, represented by high basal ganglia output, would be suppressed. Although this simple concept of regulation of individual movements is outdated, average basal ganglia activity is still considered to be involved in the regulation of the overall amount of movement or its speed (motor “vigor” ).
A second major physiologic feature of the basal ganglia networks is that they provide the substrate for neuromodulatory reshaping of the neuronal activity passing through them. While this may apply to many neuromodulators, this aspect has been best described for the modulatory effects of dopamine. The striatum and extrastriatal areas receive dopaminergic inputs from the SNc and the ventral tegmental area. In the striatum, dopamine facilitates corticostriatal transmission on the direct pathway, while inhibiting corticostriatal transmission on the indirect pathway. The net effect of striatal dopamine release appears to be a reduction of basal ganglia output. In terms of motor control, this may translate into a general facilitation of movement. Dopamine release also plays a prominent role in reinforcement learning. Activation of dopamine receptors on striatal spiny projection neurons is involved in the induction of long-term potentiation and depression at glutamatergic (presumably corticostriatal) synapses, which may also involve adenosine receptors, glutamate receptors, and cannabinoid receptors.
In this section, we summarize the available medical treatments for the three movement disorders most commonly targeted by neurosurgeons—PD, dystonia, and essential tremor (ET). We discuss briefly indications for surgery and provide a summary of the current knowledge of the pathophysiology of these disorders. This knowledge provides a significant rationale for the use of surgery in the treatment of these disorders and has helped to refine the indications for surgical interventions.
Most forms of PD are thought to arise from the accumulation of aggregates of α-synuclein in susceptible brain neurons. The cardinal features of PD (akinesia/bradykinesia, tremor at rest, muscular rigidity) are summarily referred to as parkinsonism . Parkinsonism results from decreased dopaminergic transmission in the motor portions of the basal ganglia, in particular the putamen, owing to progressive loss of dopaminergic neurons in restricted regions of the SNc.
Since the early 1960s, dopamine replacement has been the primary method of treatment of parkinsonism. Dopamine replacement is most effective as a treatment of bradykinesia and rigidity but is only variably effective for tremor. Medically refractory tremor is a common reason to consider neurosurgical treatment of PD.
Although dopamine replacement is highly effective in the first years of the disease, long-term use of this therapy is often complicated by the development of motor fluctuations, a term that refers to the early or unpredictable wearing off of the medication benefit and the appearance of involuntary movements (dyskinesias) during the “on” time. The development of these complications, if significant and poorly responsive to medical management, is another common reason to consider surgical treatment.
While tremor and motor fluctuations are principal reasons to consider surgery for PD, the subsequent development of disease features that are unresponsive to dopamine, such as speech, gait, and balance disturbances, cognitive impairment, and severe autonomic dysfunction, may significantly limit the overall benefits of surgical interventions. For this reason, early use of DBS, before the development of significant levodopa complications, has been advocated to maximize the patient’s period of good quality of life (see also later).
As mentioned earlier, many brain cell types and circuits are affected in patients with PD. It is important to realize that our understanding of the pathophysiology of parkinsonism is methodologically limited, largely owing to the consequences of the loss of dopamine. These dopamine-related changes are pertinent to the topic of neurosurgery nonetheless because the surgical interventions act mostly on basal ganglia networks that are affected by the loss of dopamine.
Studies of the pathophysiology of parkinsonism have heavily relied on the results of studies in animal models of dopamine depletion, specifically rodents treated with the dopaminergic toxin 6-hydroxydopamine and monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). More recent animal models that replicate the pathogenetic mechanisms leading to the demise of dopaminergic pathways are generally not as convincing phenotypically as toxin-based approaches.
Early metabolic and electrophysiologic recording studies in monkeys rendered parkinsonian by treatment with MPTP , showed that the spontaneous neuronal activity was increased in the STN, GPi, and SNr, but decreased in the GPe compared with normal controls. These activity changes ( Fig. 103.3 ) were thought to result directly from the loss of dopamine in the striatum, leading to increased activity of striatal neurons of the indirect pathway. Greater activation of the direct pathway was thought to result in inhibition of the GPe and subsequent disinhibition of the STN leading to increased (inhibitory) GPi/SNr output (see Fig. 103.2B , right) and decreased thalamic and cortical activity. The hypothesis that rate changes in the basal ganglia output underlie the development of parkinsonism (see Fig. 103.2B ) was also supported by the finding that inactivation of the sensorimotor portions of the STN or GPi improves bradykinesia, rigidity, and tremor in parkinsonian subjects and that it increases the metabolic activity in cortical motor areas. More recently, this model has been supported by optogenetic and chemogenetic rodent studies, which showed that general overactivity in the indirect pathway can result in akinesia in rodents. ,
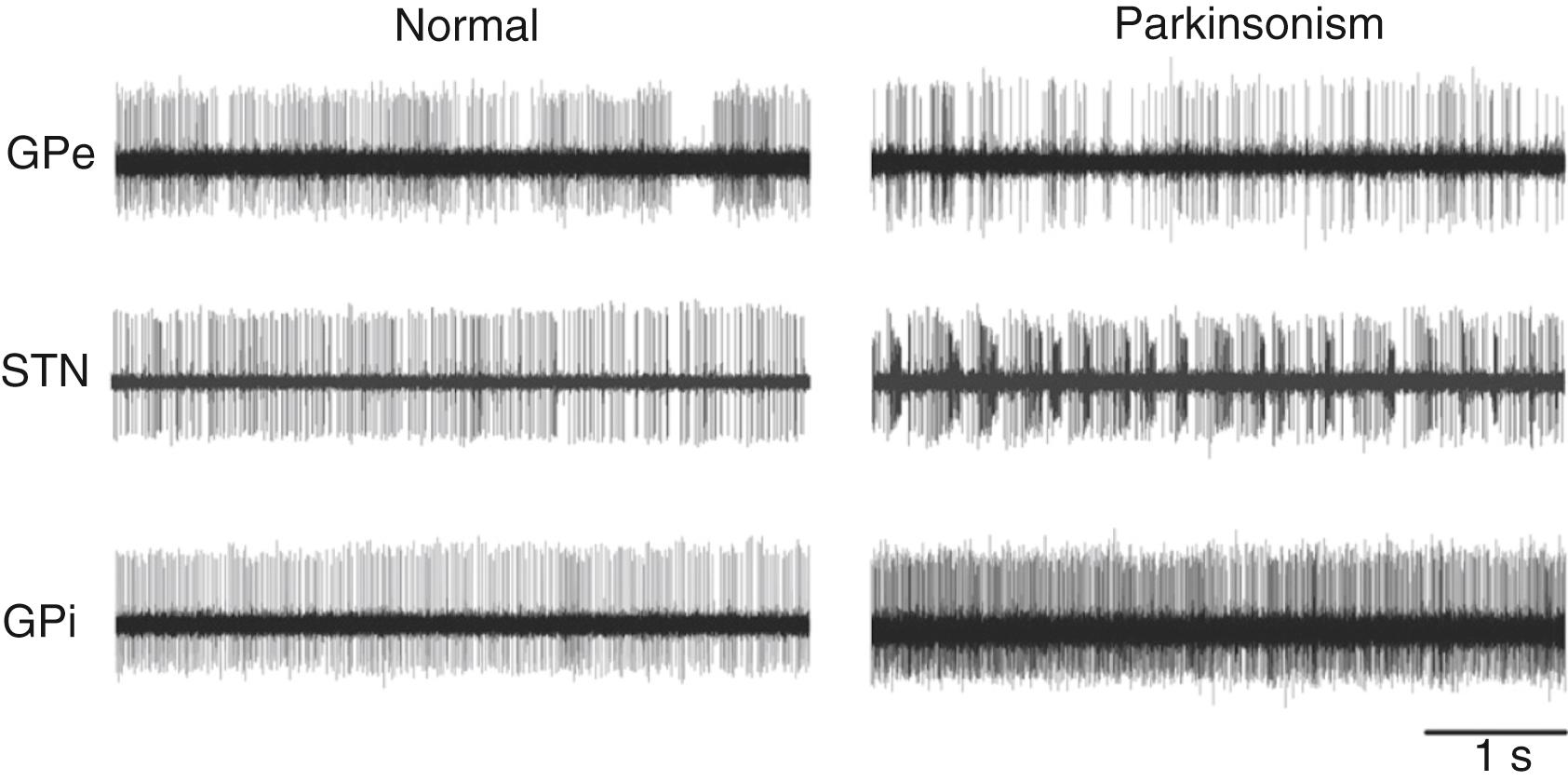
It is now believed that abnormalities of basal ganglia, thalamic, and cortical neuronal activity, other than changes of average firing rates, are more likely to be relevant than mere rate changes to the pathophysiology of parkinsonism. These include the emergence of abnormal oscillatory discharge patterns, burst discharges, and abnormal interneuronal synchronization. Of the identified changes, synchronous oscillatory fluctuations in the discharge rates of neurons in the GPi, SNr, and STN are most often discussed (e.g., see Hammond et al. ). The increased oscillations (usually in the 10- to 25-Hz beta range) have been detected in animal models and patients at multiple nodes of the basal ganglia network including the STN, GPi, and SNr. The abnormal synchronized oscillatory activity can also be identified in patients with PD by using implanted DBS electrodes (e.g., electrodes implanted into the motor areas of the STN or GPi) to record local field potentials.
Interactions between the abnormal beta-band oscillations and gamma-band oscillations may also be important. The amplitude of gamma-band oscillations was found to be excessively coupled to the phase of beta-band oscillations in the primary motor cortex, GPi, and STN in parkinsonian subjects, likely representing synchronized oscillatory bursts of neuronal activity at beta-band frequencies. In addition, the coupling of oscillatory activity between the cerebral cortex and the STN and GPi is strengthened. Other interesting oscillatory activities have also been described, such as the emergence of isolated gamma-band peaks that are characteristic of dyskinetic states in parkinsonian patients, as well as theta-range oscillations that may accompany tremor.
It remains uncertain whether any of the observed electrophysiologic abnormalities are the cause or a consequence of parkinsonian signs and symptoms. While some studies suggest potentially causative links between the electrophysiologic abnormalities and parkinsonism, , others have failed to find such evidence of causation.
The relatively simple idea that the dopamine loss in the basal ganglia is the sole reason for abnormal activity in these (otherwise normal) structures has come under increasing scrutiny in recent years. This arises from the fact that the lack of striatal dopamine has been shown to lead to persistent (secondary) morphologic changes, affecting glutamatergic and GABAergic synapses throughout the basal ganglia–thalamocortical network. In studies in rodents, monkeys, and humans, remodeling of nondopaminergic synaptic interactions has been demonstrated to occur in the striatum, cerebral cortex, STN, , and CM/Pf nuclei. , A detailed review of these changes is beyond the scope of this chapter.
Pathology outside of the basal ganglia may also be relevant for the development of parkinsonian motor signs. For example, dysfunction of the PPN is implicated in gait and balance problems and potentially even akinesia, based on studies in primates using lesioning and stimulation approaches, and dysfunction in the cerebellar output pathways may have a significant role in the expression of tremor in PD, supported by the observation that DBS of the thalamic cerebellar-receiving territory treats parkinsonian tremor and reduces ipsilateral activity. The dopaminergic denervation of the cerebellar receiving territory of the thalamus may also contribute to tremor, and dopaminergic drugs could act directly in the thalamus in such patients. STN-DBS also affects cerebellar function , potentially via the pathways shown in Fig. 103.1 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here