Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Brain arteriovenous malformations (AVMs) are rare, congenital anomalies of blood vessels with an estimated incidence and prevalence of 1.12 to 1.34 and 10 to 50 cases per 100,000, respectively. They are typically a tangle of dysplastic vessels within the brain parenchyma, composed of a central nidus that is fed by arteries and drained by veins without an intervening capillary bed. Hemodynamically, there is a high-flow, low-resistance shunt between the arterial and venous systems, which exposes the vessels to abnormal hemodynamic forces and predisposes the nidus to rupture and resultant hemorrhage. , Since the findings of higher symptomatic stroke and death rates among patients in the intervention cohorts of the A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA) and the Scottish Audit of Intracranial Vascular Malformations prospective AVM cohort study, stringent patient selection and treatment risk-to-benefit assessment have become even more important considerations in AVM therapy. , Consequently, decisions in modern-day AVM management rest upon the morbidity and mortality of intervention versus that of its natural history. The goal of AVM intervention remains complete AVM obliteration, and current modalities include microsurgical resection, endovascular embolization, stereotactic radiosurgery (SRS), or a combination of these. In recent years, SRS has emerged as a popular treatment modality for brain AVMs, largely due to its minimally invasive approach, relatively low risk-to-benefit profile, and convenience as an outpatient procedure. ,
SRS, first introduced for AVM treatment in the 1970s, is characterized by the delivery of high-dose, focused radiation to the AVM nidus using a multiheaded cobalt-based unit (Gamma Knife, Elekta AB, Stockholm, Sweden), linear particle accelerator (LINAC), or proton beam unit. Complete angiographic obliteration of the nidus remains the primary goal of SRS, while secondary treatment goals include seizure reduction, vascular steal symptom improvement, and edema-associated neurologic deficit alleviation. , Regardless of the SRS modality used, the rates of nidal obliteration as seen on digital subtraction angiography (DSA) or magnetic resonance imaging (MRI) have been consistently reported to range between 70% and 80% after 3 to 5 years of follow-up in large, unselected cohorts. , Although the risk of hemorrhage during the latency period after SRS remains an important treatment consideration, comparisons of the post-SRS hemorrhage risk with the risk incurred by the AVM’s natural history suggest that SRS may be associated with a partial degree of protection prior to complete obliteration. , This has been postulated to occur by mechanisms of radiation-induced vessel wall thickening and consequent reductions in wall tension.
Prior to SRS delivery for brain AVMs, the patient’s calvarium is affixed within a head frame under local or monitored anesthesia to permit precise targeting of the radiation dose. Subsequently, the angioarchitecture and spatial anatomy of the AVM nidus are delineated on DSA and thin-slice (slice width, 1 to 2 mm) MRI with contrast, or computed tomographic angiography when MRI is contraindicated. , In cases where frameless SRS is used, fiducials are implanted into the patient’s skull, enabling angiography to be undertaken without head-frame placement. A separate radiograph of the localizer box is then merged with the patient’s imaging, thereby yielding a precise treatment plan. Computerized dose planning is undertaken by a multidisciplinary team comprising a neurosurgeon, radiation oncologist, and medical physicist. SRS treatment variables comprise margin dose, maximum dose, and number of isocenters. , Although we do not recommend that prophylactic anticonvulsants be routinely given prior to SRS, if the patient has a seizure history and has been treated with anticonvulsants, therapeutic levels should be established prior to treatment.
Radiation changes to the vascular endothelium are thought to induce proliferation of smooth muscle cells and accumulation of extracellular collagen. AVM obliteration occurs through progressive intimal thickening, thrombosis of irradiated vessels, and eventual occlusion of the vascular lumen. A sigmoid dose-response relationship, with obliteration rates of 50%, 70%, 90%, and 95% corresponding to margin doses of 13, 16, 20, and 25 Gy, was observed by Flickinger et al. Although the investigators highlighted a relationship between in-field obliteration and marginal dose ( P = .04), a subsequent analysis revealed smaller AVM volume to be the sole independent predictor of complete obliteration ( P < .001). , Incomplete angiographic definition of the nidus was the most frequent factor associated with SRS failure. , Therefore this treatment-volume effect has been hypothesized to result from challenges in target delineation and dose definition for larger AVMs.
During SRS dose planning, the achievement of successful obliteration is dependent upon the radiation dose delivered in conjunction with accurate identification of the AVM nidus. Current AVM delineation for SRS planning uses the combination of stereotactic MRI and DSA. Margin doses greater than or equal to 17 Gy at the 50% to 80% isodose line have been associated with satisfactory obliteration rates without significant risks to the adjacent parenchyma. , , Starke et al. observed that younger age ( P < .001), smaller nidus volume ( P < .001), longer duration after SRS ( P < .001), and noneloquent location ( P = .042) were additional predictors of obliteration. A recent comparison of patients who underwent SRS between 1990 and 1997 with those treated between 1997 and 2009 reported higher rates of obliteration ( P < .001) and permanent radiation-induced changes (RICs; P = .02) in the earlier treated cohort. These findings underscore the challenges associated with appropriately dosing the AVM nidus, while minimizing the exposure of adjacent structures to unwanted radiation. Investigations in dose conformality, staged SRS, and adjuvant pharmacologic agents are ongoing as methods to augment SRS-induced AVM obliteration. ,
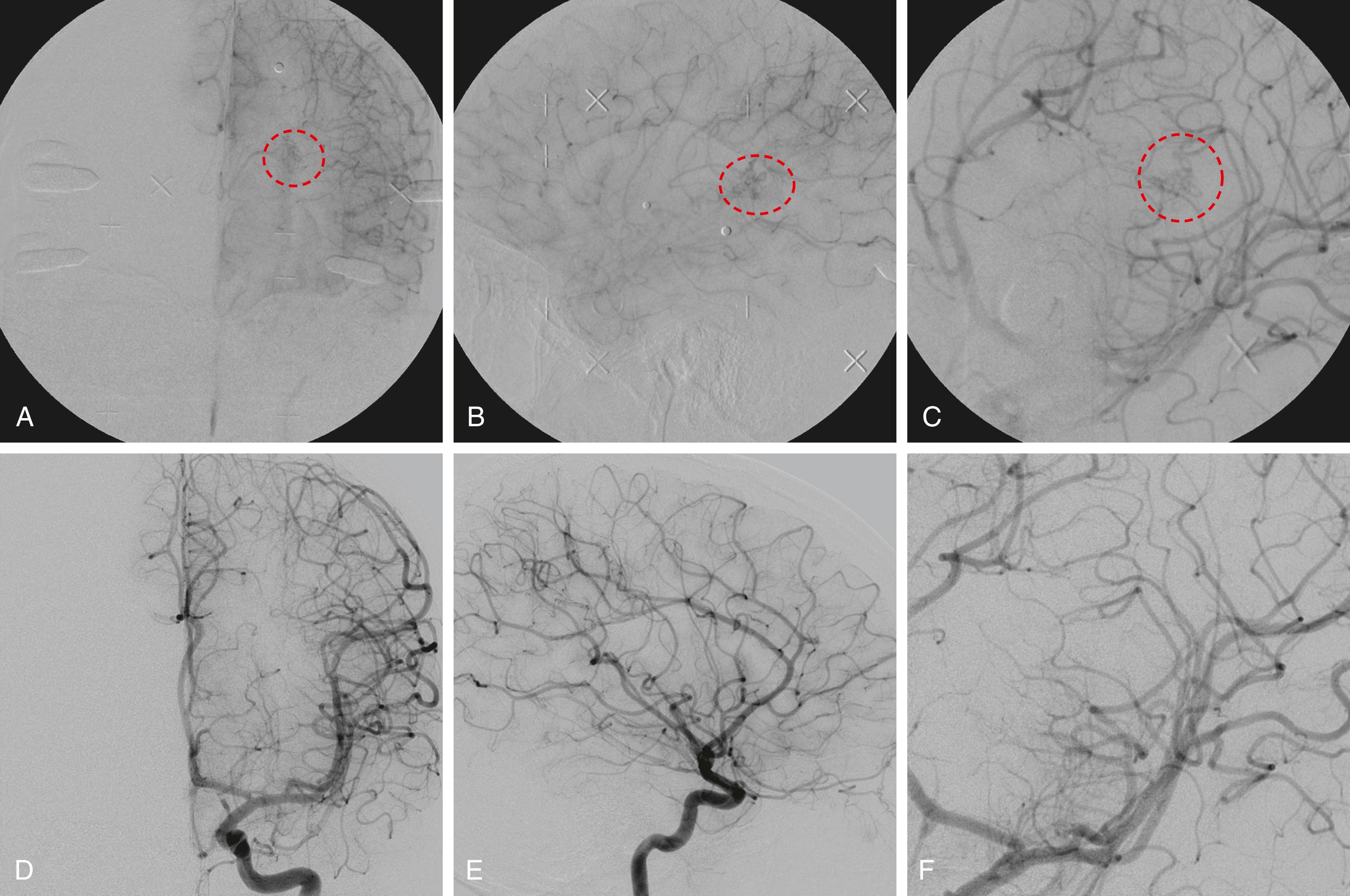
In light of established natural history differences between ruptured and unruptured AVMs, their respective responses to and outcomes after SRS have been investigated. In particular, safety and efficacy of the treatment modality are of critical importance in unruptured AVM treatment decision. In a study comprising 938 unruptured AVMs treated with SRS by Ding et al., a 65% obliteration rate was observed at a median follow-up duration of 71 months (range: 12 to 276 months). The investigators reported a margin dose of 20 Gyor greater to be associated with a higher rate of favorable outcome (70% vs. 36%; P < .001), which was defined as the achievement of obliteration without radiosurgical-induced complications. In a subsequent multicenter study comprising 509 ARUBA-eligible patients with unruptured AVMs, a 75% obliteration rate was observed. Smaller AVM maximal diameter ( P = .012) and higher margin dose ( P = .001) were found to be independent predictors of obliteration in this study. Although a matched comparison of 270 unruptured and 270 ruptured AVMs observed similar obliteration rates (76% vs. 73%, P = .592) after SRS, the unruptured cohort had a higher incidence of post-SRS hemorrhage (2.3% vs. 1.1% [per year?], P = .025) and a lower rate of symptomatic RICs (30.4% vs. 48.9%, P < .001) compared with the ruptured cohort. Currently, longer follow-ups are necessary to demonstrate benefits of SRS for unruptured AVMs when compared with its natural history, thus warranting further investigations in prospective, registry-based studies. An illustrative case of a ruptured AVM treated with SRS is presented in Fig. 96.1 .
DSA is the “gold standard” for the evaluation of post-SRS obliteration. However, its invasive method carries associated procedural risks. Therefore MRI with magnetic resonance angiography (MRA), which has an estimated sensitivity and specificity of 80% and 90%, respectively, can be effectively used as an alternative method for routine follow-up imaging. Imaging follow-up with MRI/MRA at 6-month intervals for the first 2 years after SRS. Then MRI/MRA can be performed annually thereafter, and DSA is recommended once obliteration is achieved on MRI/MRA to confirm the absence of residual nidus. AVMs that are not completely obliterated during the latency interval after SRS pose an ongoing management dilemma due to their associated risk of hemorrhage if left untreated. Microsurgical resection or repeat SRS for persistently patent nidi at 3 to 4 years after the initial SRS procedure is recommended. However, AVM treatment with repeat SRS has been associated with slightly lower obliteration rate (67% vs. 79%, P = .04) and a higher rate of latency period hemorrhage (3.1% vs. 2.6%, P = .04).
Eloquent AVMs associated with sensorimotor and language functions carry a high risk of microsurgical morbidity. They may also be associated with a lower likelihood of treatment success with SRS, owing to dose limitations in sparing eloquent parenchyma from the unwanted effects of radiation. The effect of SRS on the radiographic and clinical outcomes of patients with primary motor and somatosensory cortex (PMSC) AVMs was investigated by Ding et al. in a single-center cohort of 134 patients. The overall obliteration rate of 63% at a median follow-up duration of 64 months (range: 6 to 224 months) was consistent with the 61% to 70% obliteration rates reported in prior smaller series. Subgroup analysis by AVM size revealed obliteration rates of 80% to 87% and 50% to 56% for PMSC AVMs less than 3 cm 3 and greater than 3 cm 3 in volume, respectively. Although a comparison of margin dose between the subgroups was not undertaken, these observations highlight the potential significance of AVM volume, and its relative effect on radiosurgical dosing, in determining treatment efficacy for PMSC AVMs.
A matched comparison of 120 PMSC AVMs versus 120 noneloquent lobar AVMs revealed similar rates of obliteration ( P = .623) and permanent radiosurgery-related morbidity ( P = .820) between the two cohorts. In contrast, eloquent AVMs treated with microsurgical resection were associated with a higher risk of surgical morbidity when compared with noneloquent AVMs. Given the lack of negative prognostic effect of eloquent location on SRS outcomes, SRS may be considered the preferred treatment modality for small (<3 cm 3 ) eloquent AVMs, affording the ability to achieve acceptable obliteration rates with minimal treatment-related morbidity. , Furthermore, seizures are the most common clinical presentation of eloquent AVMs, occurring in 40% to 82% of patients. , Reductions in seizure frequency after SRS have been observed in 61% to 71% of patients, whereas new-onset seizures occur in 0.7% to 2.6% of patients. , , , Therefore SRS offers a reasonable risk-to-benefit profile in the treatment of these challenging lesions.
For deep-seated AVMs of the thalamus and basal ganglia (BG), the 10% annual hemorrhage risk and adjacent critical neural pathways both contribute toward their aggressive natural history. , As a result, intervention is often advocated, even in the absence of prior hemorrhage. However, their deep locations and narrow operative corridors in approaches to thalamic and BG AVMs resection pose significant challenges. Consequently, microsurgical resections for thalamic and BG AVMs have been associated with low rates of complete obliteration and high rates of morbidity. , , Therefore SRS has emerged as an attractive therapeutic option for small- to medium-sized thalamic and BG AVMs, offering a minimally invasive approach with acceptable therapeutic outcomes.
In a multicenter cohort comprising 160 BG and 203 thalamic AVMs, Chen et al. observed a 64.8% obliteration rate at a mean follow-up duration of 86.5 months. Favorable outcome, which was defined as AVM obliteration without post-SRS hemorrhage or permanent symptomatic RIC, was achieved in 58% of the patients. Predictors of favorable outcome included lack of prior AVM embolization ( P = .011), higher margin dose ( P = .008), and fewer isocenters ( P = .044). In a single-center cohort comprising 182 thalamic and BG AVMs, Cheng et al. reported an obliteration rate of 58%, and Kano et al., in a single-center cohort of 133 thalamic and BG AVMs, reported actuarial obliteration rates of 57% and 72% at 3 and 5 years, respectively. , Both Cheng et al. and Kano et al. found a higher margin dose and smaller nidus volume to be independent predictors of obliteration. , Although nidus volume did not predict outcomes in the analysis by Chen et al., the investigators highlighted that smaller, less diffuse, and anatomically regular nidi were treated with fewer isocenters, which may have given rise to the observed relationship between isocenter number and favorable outcomes. Therefore SRS may be particularly effective in patients with morphologically compact thalamic and BG AVMs. Use of a higher margin dose and avoidance of neoadjuvant nidal embolization is recommended during treatment planning for these patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here