Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Over the past several decades, remarkable advances have been seen in the use of imaging for diagnosing, staging, and following up cases of soft tissue neoplasms. In this chapter we discuss the radiologic evaluation of soft tissue tumors, reviewing the modalities of radiography, ultrasound, computed tomography (CT)–positron emission tomography (PET), magnetic resonance imaging (MRI), and PET-MRI. We also discuss recent advances in technology, with an emphasis on the importance of MRI for the most comprehensive evaluation of soft tissue tumors.
Radiographs have limited utility in the evaluation of soft tissue neoplasms. However, radiographs can be helpful in demonstrating whether there is any bony involvement and may show fatty tumors as lucencies. Also, radiographs of vascular tumors may demonstrate the presence of phleboliths, often the key to identifying these vascular tumors.
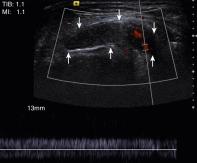
In recent years, ultrasonography (US) has gained popularity for the evaluation of soft tissue neoplasms, particularly in Europe and Asia, where US is the preferred modality for the initial evaluation of palpable soft tissue masses. The advantages of US include its wide availability; low-cost, real-time imaging capabilities; and lack of ionizing radiation. In the United States, US is the diagnostic imaging modality of choice for pediatric patients. For small and superficial lesions, US can help differentiate between cystic and solid masses and can also be used for guidance during biopsy procedures. The drawback to US lies in its operator-dependent nature and its inability to characterize lesions definitively. Many lesions demonstrate a nonspecific appearance on ultrasound images, although several studies have shown that lesions larger than 5 cm with infiltrative margins are almost certainly malignant ( Fig. 3.1 ).
The advent of multidetector CT has allowed for the use of multiplanar reformatted thin-slice imaging and three-dimensional (3D) rendering of images. Faster examination times with CT than with MRI lead to improved patient tolerance and decreased patient motion artifact. Additionally, CT has a high accuracy rate for detecting calcifications and ossification that may be missed on MRI or may be too subtle to be seen on radiographs. CT is also helpful in evaluating the relationship between a soft tissue neoplasm and the adjacent bony cortex or marrow. In most cases, diagnostic CT angiography has replaced conventional angiography for the evaluation of vascular structures because of the less invasive nature of CT angiography. A disadvantage of CT is its exposure of patients to ionizing radiation. In addition, CT has a much lower soft tissue contrast resolution than MRI, which may make fine detail (e.g., tumor’s relationship to neurovascular structures) difficult to ascertain.
Although anatomically based imaging modalities are considered the standard of care for the diagnosis of soft tissue neoplasms, functional imaging such as PET-CT is beginning to play a larger role as an adjuvant option. With PET-CT, a radiotracer is administered, generally fluorine-18 fluorodeoxyglucose ([ 18 F] FDG), which is an analogue of glucose that is more avidly trapped in malignant cells than in noncancerous cells. Standardized uptake value (SUV) is often used to characterize lesions on PET-CT; a higher SUV indicates increased metabolic activity. However, many benign conditions such as insufficiency fractures, infectious or inflammatory conditions, postoperative changes, and heterotopic ossification may demonstrate increased SUV on PET-CT simulating an aggressive process. The primary benefit of PET-CT is whole-body functional evaluation. As such, PET-CT is ideal for staging malignant soft tissue tumors. PET-CT is also helpful in assessing response to therapy and monitoring for residual or recurrent soft tissue masses after treatment. In general, effective therapy leads to decreased tumor vascularity and metabolic activity, which results in less uptake of [ 18 F] FDG in the remaining soft tissues. PET-CT is a relatively safe imaging modality, with only minor incidence of allergic reactions reported.
MRI is indispensable in the evaluation of soft tissue tumors and has become the modality of choice for orthopedic oncologic imaging. MRI can be used to delineate anatomic involvement, define fascial planes, evaluate bony involvement, and demonstrate a lesion’s relationship to nearby neurovascular bundles. MRI also offers better soft tissue contrast than CT. Although in many instances MRI cannot provide a histologic diagnosis, it can provide a reasonable differential based on signal characteristics, morphology, multiplicity, and location of the lesion when this information is considered in combination with the relevant clinical history. The appearance of benign and malignant soft tissue tumors is similar on MRI, although a lesion should not be classified as “benign” unless it can be definitively characterized and named based on specific MR criteria. When a soft tissue mass cannot be definitively named based on MR characteristics, location of the mass, and patient age, it must be classified as an “indeterminate mass” and clinically managed as a malignancy until proved otherwise.
MRI is also useful for surgical planning and follow-up. In patients with sarcoma, MRI can delineate the tumor margins, which allows the surgical team to decide on the appropriate surgical approach. However, the overlap in appearance of tumor recurrence, edema, hemorrhage, and postoperative inflammatory change on MRI may complicate the interpretation of follow-up images.
One major drawback to MRI is its unreliability in detecting calcifications and ossifications. In such situations, radiography and CT are more useful and may provide clinicians with the diagnosis.
Many pulse sequences are used to evaluate soft tissue tumors on MRI. Standard spin echo T1- and T2-weighted images have largely been replaced by faster imaging techniques, usually the fast spin echo (FSE) method, to produce higher-resolution T2-weighted images with reduced examination times. Fat suppression is another important tool in the MR evaluation of soft tissue tumors. Fat suppression can be used to characterize fat-containing tumors, improve the dynamic range for soft tissue contrast display, and more accurately discriminate between signal differences for different tissues on T1- and T2-weighted images. A short inversion time (T1) recovery (STIR) sequence can be used to suppress signal from fat. STIR sequences also eliminate chemical shift artifact at fat-water interfaces, which can lead to improved lesion-background contrast. When fat suppression is used, a nonsuppressed T1-weighted image should also be acquired; this image can be used to differentiate between intralesional fat and hemorrhage and to identify subtle marrow signal changes in the adjacent bones.
The appearance of soft tissue tumors on MRI is fairly consistent. On T1-weighted images, most soft tissue tumors demonstrate signal intensity similar to that of muscle; on T2-weighted images, most soft tissue tumors demonstrate signal intensity higher than that of muscle. In cases where a tumor demonstrates signal characteristics specific to that lesion, these characteristics are a direct reflection of the histologic makeup of the mass. This is most often the case in benign lesions but may occur in a few malignant soft tissue tumors as well.
We do not believe that gadolinium-DTPA enhancement is particularly useful in the evaluation of most soft tissue tumors, and this agent is therefore not routinely used at our institution. In some cases (e.g., vascular and neural lesions), however, using contrast may provide additional diagnostic information. Contrast is used in our practice only to differentiate between cystic and solid masses and to assess the degree of necrosis in a mass.
One area of soft tissue tumor imaging that often poses a conundrum for the interpreting radiologist is the “benign-appearing” mass (i.e., a small, superficial, homogeneous, well-defined lesion without evidence of infiltration); such masses may mislead the clinician. More than 30% of soft tissue sarcomas may be superficial or small (<5 cm). Thus we classify all soft tissue masses that cannot be definitively characterized by MRI as “indeterminate.”
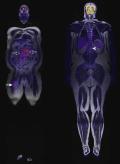
Since its introduction in 2010, PET-MRI has been slowly taking its place in major academic centers around the world. Potential advantages of PET-MRI are similar to those offered by PET-CT; added benefits include a lack of ionizing radiation and higher soft tissue contrast. The lack of ionizing radiation with PET-MRI is particularly important for pediatric patients and young adults, who may require long-term oncologic follow-up and multiple radiologic studies. In soft tissue sarcomas such as myxoid liposarcoma, the whole-body functional imaging offered by PET-MRI may demonstrate both the primary tumor and distant metastases ( Fig. 3.2 ).
Lipomatous tumors are frequently encountered in clinical practice. Lipoma variants are defined as fatty tumors with variable amounts of fibrous tissue, chondroid, or bone matrix. Most institutions reserve the term atypical lipoma for malignant lipomatous tumor found in the superficial soft tissues of the extremities above the fascia, using the term well-differentiated liposarcoma for lesions in deep locations, especially the retroperitoneum. Dedifferentiation of atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDL) is more frequently seen in deep lesions of the trunk and is rare in extremity lesions.
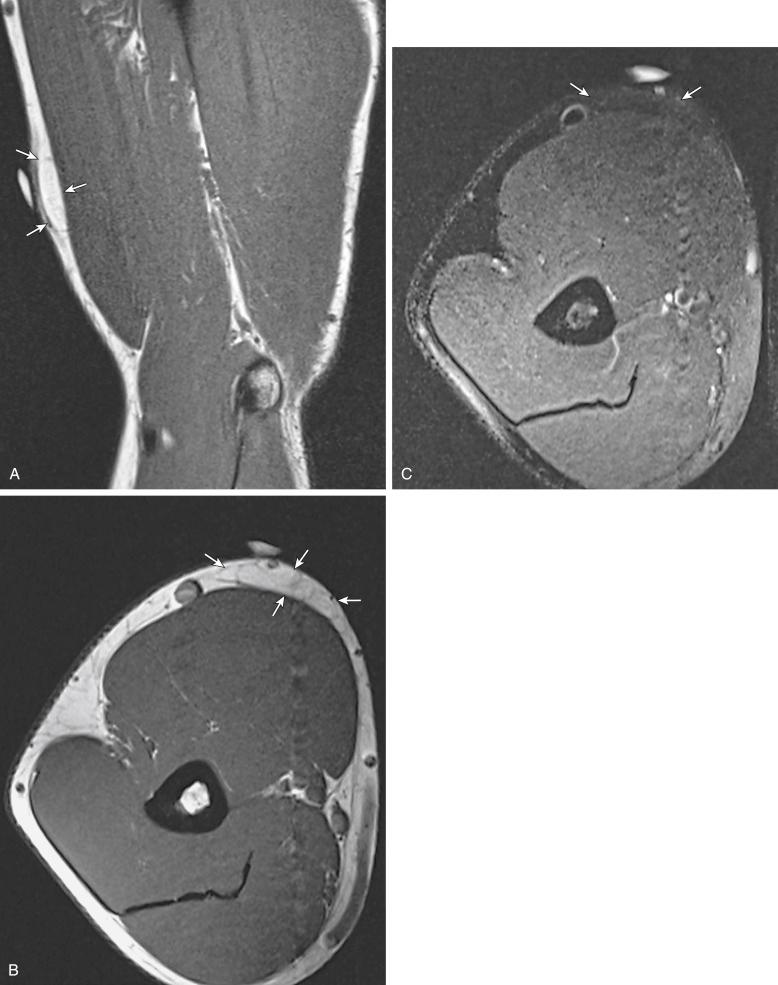
MR findings for lipomatous masses are usually sufficiently characteristic to suggest this diagnosis; however, the distinction among lipoma, lipoma variants, and ALT/WDL is less clear, because of overlap in the MR features of these lesions. On MRI, a typical lipoma will demonstrate homogeneous fat signal intensity on all pulse sequences, an appearance similar to subcutaneous fat, and complete saturation of signal on fat-suppressed sequences ( Fig. 3.3 ). A subtle capsule or pseudocapsule is frequently seen on MR images of superficial lipomas, as are thin septations. Deeper tumors are more likely to have poorly defined and infiltrating margins ( Fig. 3.4 ).
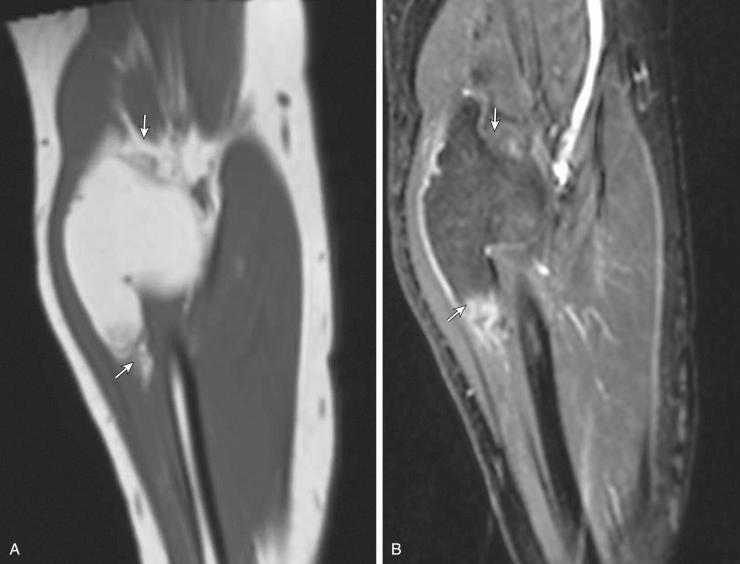
Some authors suggest that certain MR features of ALT/WDL may help to differentiate these lesions from lipomas, such as thickened septa, nodularity, and hyperintensity on fluid-sensitive MR sequences. However, some of these findings are also associated with traumatized benign lipomas with fat necrosis and lipoma variants with nonfatty components ( Fig. 3.5 ). The various amounts of fibrous tissue, chondroid, and bone matrix in lipoma variants produce heterogeneity on MR images. For example, a hibernoma is a lipoma variant in which brown fat predominates; brown fat typically demonstrates a slightly different signal intensity than subcutaneous fat on all sequences, resulting in a unique MR appearance ( Fig. 3.6 ). When attempting to differentiate between lipomas and ALT/WDL, clinicians should consider the location of the lesion on imaging as the most reliable feature; lipomas are generally seen above the fascia, whereas ALT/WDL generally occurs in deep locations.
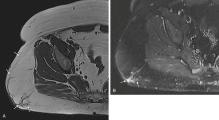
Solitary benign peripheral neurogenic tumors comprise two main groups: schwannoma (neurilemoma) and neurofibroma. Together, these lesions constitute about 10% of all benign soft tissue tumors. Unlike many other soft tissue tumors, neurogenic tumors have characteristic imaging and clinical features that may be helpful for diagnosis.
Schwannomas , which are slightly less common than neurofibromas, are slow-growing tumors arising from the outer sheath of a peripheral nerve, typically eccentric to the nerve fibers, and may be diagnosed prospectively, especially if proximal and distal nerve fibers are visualized on MRI ( Fig. 3.7 ). However, it may be difficult to identify the associated nerve fiber when tumors arise from small nerve branches. These tumors most frequently occur in the flexor surfaces of the extremities (particularly the ulnar and peroneal nerves), mediastinum, retroperitoneum, and head and neck. Long-standing and large lesions known as giant ancient schwannomas may demonstrate cystic changes, calcification, hemorrhage, and fibrosis, which may be mistaken for features of more aggressive tumors on imaging.
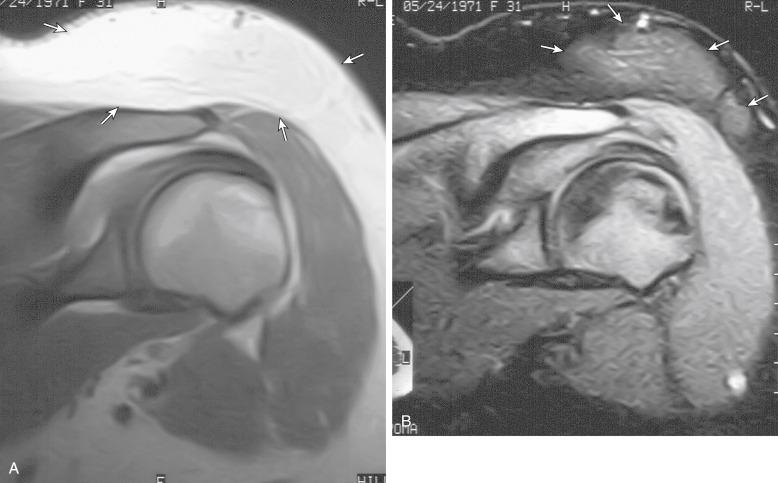
Localized or solitary neurofibromas are also slow-growing lesions with a centrally entering and exiting nerve, giving a fusiform shape to the tumors. Neurofibromas can occur anywhere in the body, including the skin, subcutaneous tissues, and viscera. The growth of neurofibromas is usually slow, but faster growth may be seen during pregnancy and puberty or in cases of malignant transformation. The three types of neurofibromas are localized, diffuse, and plexiform. All three are often associated with neurofibromatosis type 1 (NF1). Localized neurofibroma is most frequently associated with NF1, but plexiform neurofibroma is essentially pathognomonic of NF1 ( Fig. 3.8 ). Plexiform neurofibromas usually develop during childhood and adolescence and can precede the appearance of cutaneous neurofibromas. Because of their large size, plexiform neurofibromas typically extend beyond the epineurium into the surrounding tissue.
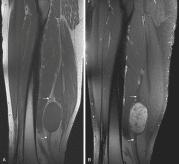
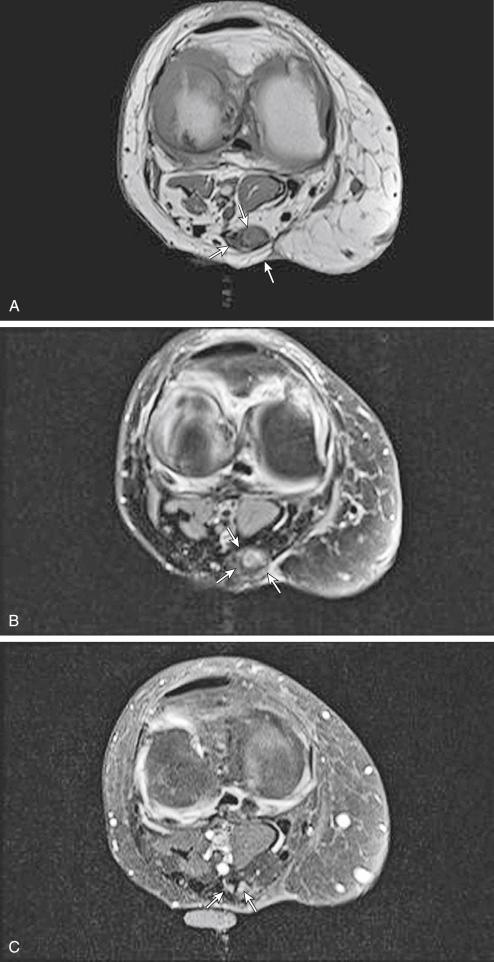
Schwannomas and neurofibromas share many MR features. Both present as well-defined, often elongated masses that rarely exceed 5 cm in diameter. Paraspinal lesions typically have a dumbbell shape, which may enlarge the surrounding neural foramen through pressure erosion over a long time. Continuity with a nerve, the most helpful diagnostic MR feature, is usually evident on images of lesions arising from larger nerves. Differentiation between schwannomas and neurofibromas based on the position of the tumor relative to the nerve (eccentric vs. central) is often difficult, especially when smaller nerves are involved. Intramuscular neurogenic tumors may be surrounded by a thin rim of fat; this creates the “split fat sign” on T1-weighted MR images, especially along the long axis of the extremity, because of slow growth over an extended period ( Fig. 3.9A and B ). Most benign neurogenic tumors are isointense or slightly hyperintense to muscle on T1-weighted images and are markedly hyperintense to fat with a variable degree of heterogeneity on T2-weighted images. On fluid-sensitive sequences, these tumors may exhibit high signal intensity in the periphery and low to intermediate signal intensity in the center. This appearance, the “target sign,” is likely caused by the presence of myxoid material in the periphery and fibrous tissue in the center. Although initially thought to be pathognomonic of neurofibromas, the target sign has also been observed in schwannomas and even in malignant peripheral nerve sheath tumors. MRI of neurogenic tumors may also demonstrate the presence of multiple small, ringlike structures within the tumors; this appearance, called the “fascicular sign,” represents the fascicular bundles.
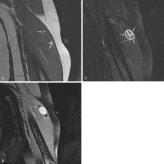
On contrast-enhanced images, small neurogenic tumors often show intense and relatively homogeneous enhancement, whereas large lesions may demonstrate predominantly peripheral, central, or heterogeneous nodular enhancement ( Fig. 3.9C ). Atrophy of innervated muscles distal to the tumor is another imaging finding suggestive of neurogenic tumors.
Several classification systems have been proposed for vascular anomalies. In 1996 the International Society for the Study of Vascular Anomalies adopted and expanded two leading classification systems. Two categories of vascular anomalies are considered in these systems: vascular malformations and vascular tumors (with infantile hemangioma being the most common).
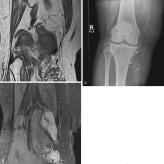
Venous malformations are the most common type of peripheral vascular malformation. Venous malformations are usually septated lesions and are often mistakenly identified as hemangiomas by radiologists. Extremities are the most common location for venous malformations, accounting for almost two-thirds of vascular malformations in these areas. Venous malformations are present at birth, but symptoms usually do not appear until late childhood or early adulthood. These lesions demonstrate intermediate to hypointense signal intensity on T1-weighted images and increased signal intensity on T2-weighted and STIR images. Internal fluid-fluid levels are rare and likely caused by hemorrhage. In cases of thrombosis or hemorrhage, heterogeneous signal intensity can be observed on T1-weighted images ( Fig. 3.10 ). The presence of phleboliths often confirms the diagnosis of venous malformation. Although phleboliths can be difficult to recognize on MRI, appearing as small low-signal-intensity foci on all pulse sequences, radiographs can clearly demonstrate their presence ( Fig. 3.11 ). Venous malformations also frequently have fat interspersed between the blood vessels. When fat becomes the dominant part of the mass, it may be confused with a lipoma variant.
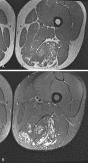
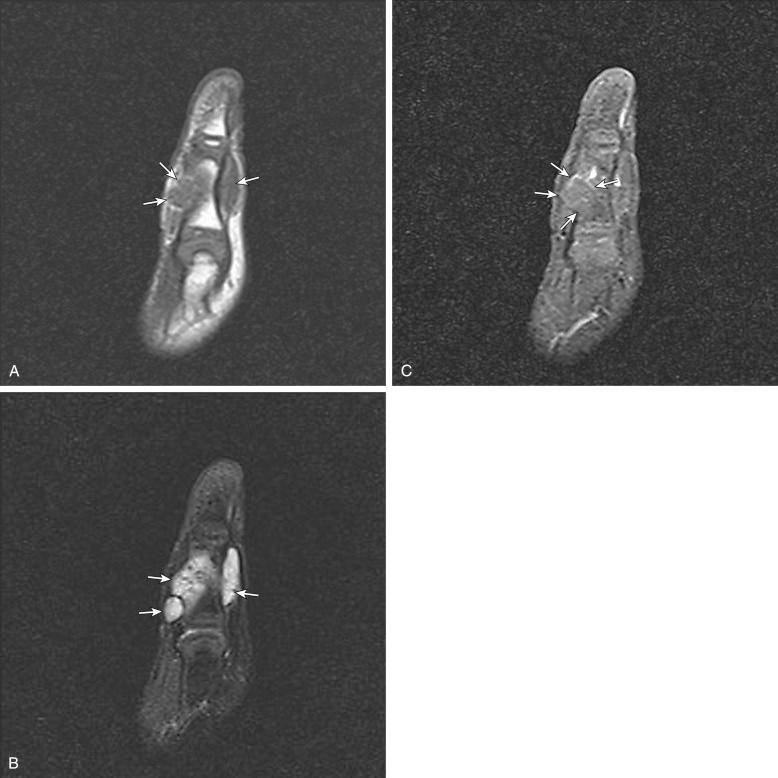
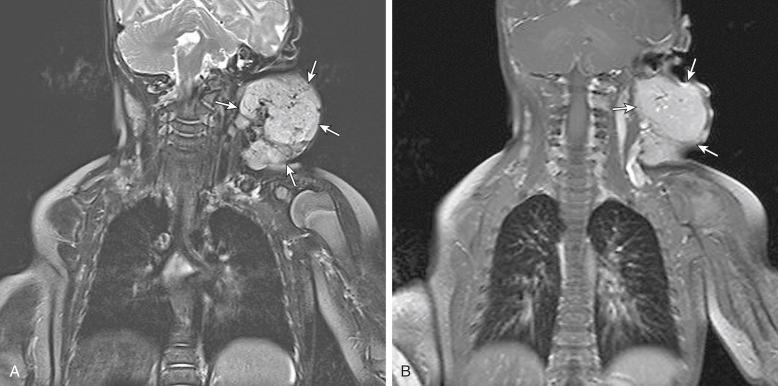
Lymphatic malformations are the second most common type of vascular malformation. They are usually located in the neck and occur rarely in the extremities. On MRI, lymphatic malformations are usually seen as lobulated, septated, solid-appearing masses with intermediate to decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted and STIR images ( Figs. 3.12 and 3.13 ). Internal fluid-fluid levels are common. Lymphatic malformations tend to be infiltrative and involve multiple tissue planes, giving them an aggressive appearance on imaging studies. Contrast-enhanced MRI is helpful in the diagnosis of lymphatic malformations. The microcystic type of malformation usually demonstrates no significant enhancement on imaging ( Fig. 3.12C ), whereas the macrocystic type may demonstrate peripheral and septal enhancement with no enhancement in the center. Central enhancement may be seen with some microcystic malformations ( Fig. 3.13C ); this pattern is typically caused by septal enhancement of the small cysts or enhancement of the venous component in mixed malformations.
Capillary malformations are areas of congenital ectasia of thin-walled, small-caliber vessels of the skin and present in 0.3% of neonates and present as areas of cutaneous red discoloration. They predominantly involve the head and neck region and are typically confined to the dermis or mucous membranes, although they may also be a sign of more complex underlying anomalies such as Sturge-Weber, Klippel-Trenaunay, or Parkes Weber syndrome. Capillary malformations are usually diagnosed based on clinical findings; imaging is unnecessary unless an underlying syndrome is suspected. MR findings of capillary malformations are subtle and nonspecific and may include thickening of overlying skin or subcutaneous tissues if no deeper involvement is present.
Capillary-venous malformations are low-flow malformations formed from dysplastic capillary vessels and enlarged postcapillary vascular spaces. Imaging findings for these malformations are nonspecific and similar to those of venous malformations. On dynamic contrast-enhanced MR studies, capillary-venous malformations typically show early homogeneous enhancement.
High-flow malformations are uncommon, representing approximately 10% of vascular malformations in the extremities. Arteriovenous malformations (AVMs) are complex anomalies consisting of feeding arteries, draining veins, and a nidus composed of multiple dysplastic vascular channels that connect the arteries and veins. Arteriovenous fistulas are much simpler, formed by a single vascular communication between an artery and a vein. MR findings in AVMs include high-flow serpentine and enlarged feeding arteries and draining veins, which appear as large flow voids with absence of an encapsulated mass ( Fig. 3.14 ). Areas of high signal intensity on T1-weighted images may represent areas of hemorrhage, intravascular thrombosis, or flow-related enhancement with contrast. Contrast-enhanced studies (CT or MR angiogram) are useful in demonstrating the feeding arteries and draining veins.
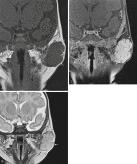
Infantile hemangioma is the most common vascular tumor of infancy, with a prevalence of about 2% to 3% and a higher prevalence of about 10% in premature infants of very low birth weight. Infantile hemangiomas are normally not seen at birth but manifest during the first few weeks as rapidly growing subcutaneous lesions that resemble the surface of a strawberry. These hemangiomas can be diagnosed based on visual inspection without a need for imaging. However, MRI may be useful for assessing deep lesions with no skin manifestations or for guiding therapy. MR features of infantile hemangiomas vary depending on the biologic phase of the tumor. In the proliferating phase , these hemangiomas typically appear as well-defined lobulated masses with high arterial-type flow and enhancement without arteriovenous shunting ( Fig. 3.15 ). After this proliferating phase in infancy, a slow but constant regression ( involuting phase ) can be seen, with the process usually completed by age 7 to 10 years. During the involuting phase, MR appearances are more variable, with increasing amounts of fat replacing the lesion and less enhancement seen on postcontrast studies.
Congenital hemangioma , which is much less common than infantile hemangioma, is fully developed and clinically evident at birth. This lesion has two subtypes: rapidly involuting congenital hemangiomas, which completely regress during the first 2 years of life; and noninvoluting congenital hemangiomas, which demonstrate growth proportional to that of the child without regression. MR findings for these tumors are generally similar to those of infantile hemangiomas, but congenital hemangiomas may also demonstrate evidence of aneurysms, intravascular thrombus formation, and arteriovenous shunting.
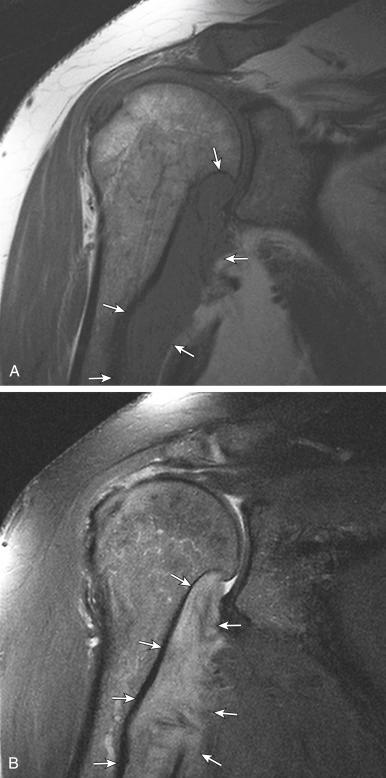
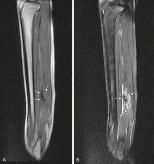
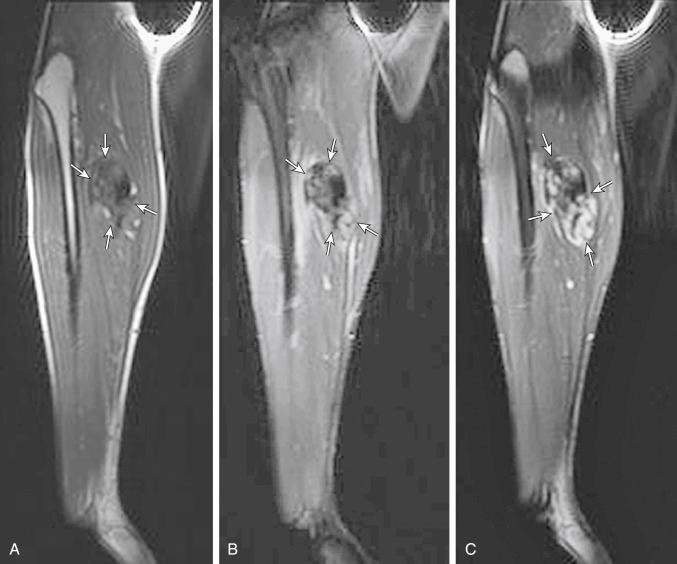
Desmoid tumors (also known as fibromatosis or aggressive fibromatosis ) are rare, locally aggressive lesions and tend to recur, even after complete resection, in nearby tissues. On T1-weighted MRI, desmoid tumors typically show hypointense or isointense signal relative to skeletal muscle. On fluid-sensitive sequences, the typical signal intensity is low, although high signal is seen in more cellular lesions ( Fig. 3.16 ). Contrast enhancement is variable, with absent or mild enhancement seen in most lesions and intense enhancement seen in more cellular lesions. When the lesions are small, they may be difficult to visualize because they have a signal intensity similar to that of the surrounding muscle; this is especially true for small intramuscular or intermuscular lesions ( Fig. 3.17 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here