Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There is a limited group of pulmonary lesions that one can classify as pseudoneoplastic, but their conditions constitute a significant aggregation in absolute numbers. Some are categorized as malformative or reactive, including pulmonary hamartomas (PHs); selected inflammatory pseudotumors (IPTs) (plasma cell granulomas); tumefactive lymphoid hyperplasias; inflammatory or reparative conditions simulating carcinomas; unusual granulomatous reactions; tumefactive pleural plaques; and florid examples of mesothelial hyperplasia. Other clinical pseudotumors such as rounded atelectasis are confused with neoplasms only by nonpathologists and are not included for discussion here. On the other hand, the variant of IPT now known as inflammatory myofibroblastic tumor (IMT) demonstrates clonal characteristics and can rightly be regarded as a true neoplasm. , Accordingly, it has likewise been omitted from this chapter.
Hamartomas are defined as an abnormal mixture of tissue elements, or an abnormal proportion of a single element, normally present in an organ. Hamartomas can also be defined as an abnormal arrangement of at least two normal mesenchymal tissues that form a discrete mass lesion. Pulmonary hamartomas (PHs) are benign slow growing masses or nodules composed of mature but disordered hyaline cartilage, fibroadipose tissue, and smooth muscle, with entrapped benign respiratory epithelium that forms clefts between the lobules of cartilage and other mesenchymal tissue. The consensus now favors that rather than being merely developmental abnormalities, PHs are benign neoplasms derived from peribronchial mesenchyme (see below). Alternative names include hamartochondroma, chondromatous hamartoma, and mesenchymoma.
PH is the most common form of benign lung tumor with an incidence of between 0.025% and 0.32%. , PHs are usually found in adults with a peak incidence in the sixth decade and there is a male preponderance with the male:female ratio being 2:1 to 3:1. PHs are often asymptomatic and are typically discovered as an incidental coin lesion on routine chest radiograph; indeed, they account for 7% to 14% of all pulmonary coin lesions. They occur in all regions of the lung. Although they usually present in the peripheral parenchyma (∼90%), they can occasionally be found in the bronchial wall at the hilum. They are slow growing and tend to remain asymptomatic and harmless in peripheral locations but can cause coughing, obstruction, hemoptysis, and dyspnea at the endobronchial location. More than one hamartoma in the same patient should raise the suspicion of Carney’s triad (pulmonary chondromas, gastric epithelioid tumors, and extra-adrenal paragangliomas). ,
PHs are thought to arise from primitive peribronchial mesenchymal tissue. Supporting the hypothesis that PH is a true neoplasm, a cytogenetic analysis of PH found an abnormal karyotype revealing recombinations between chromosomal bands 6p21 and 14q24.
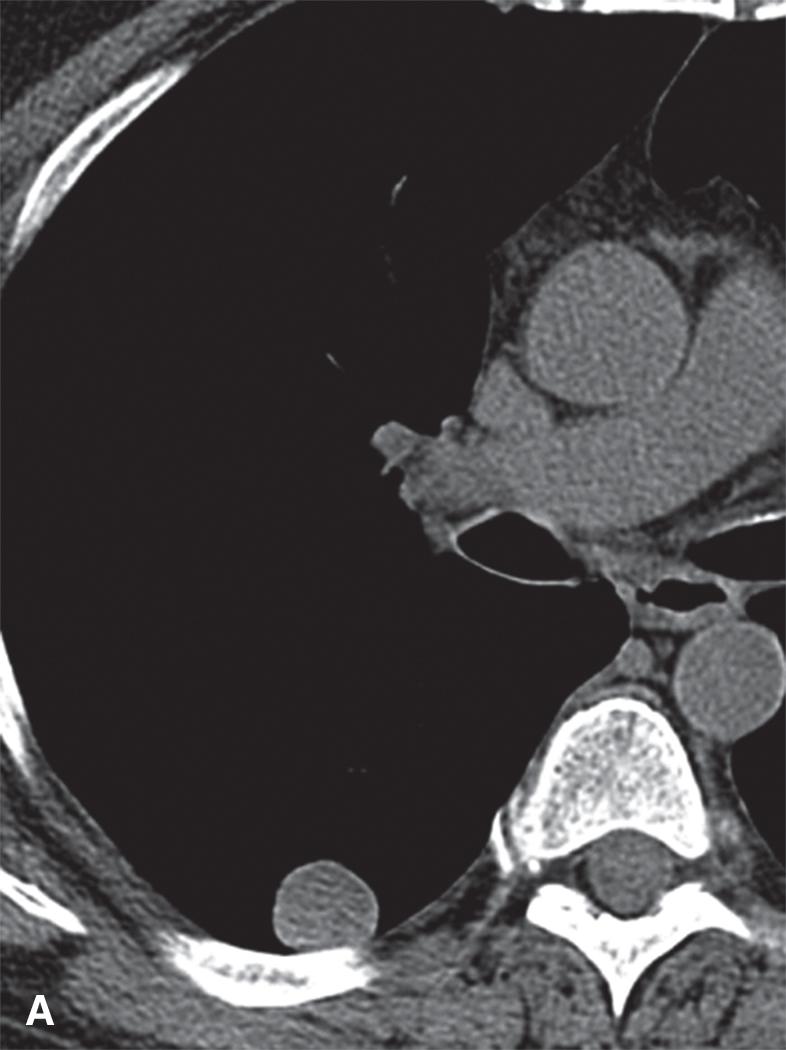
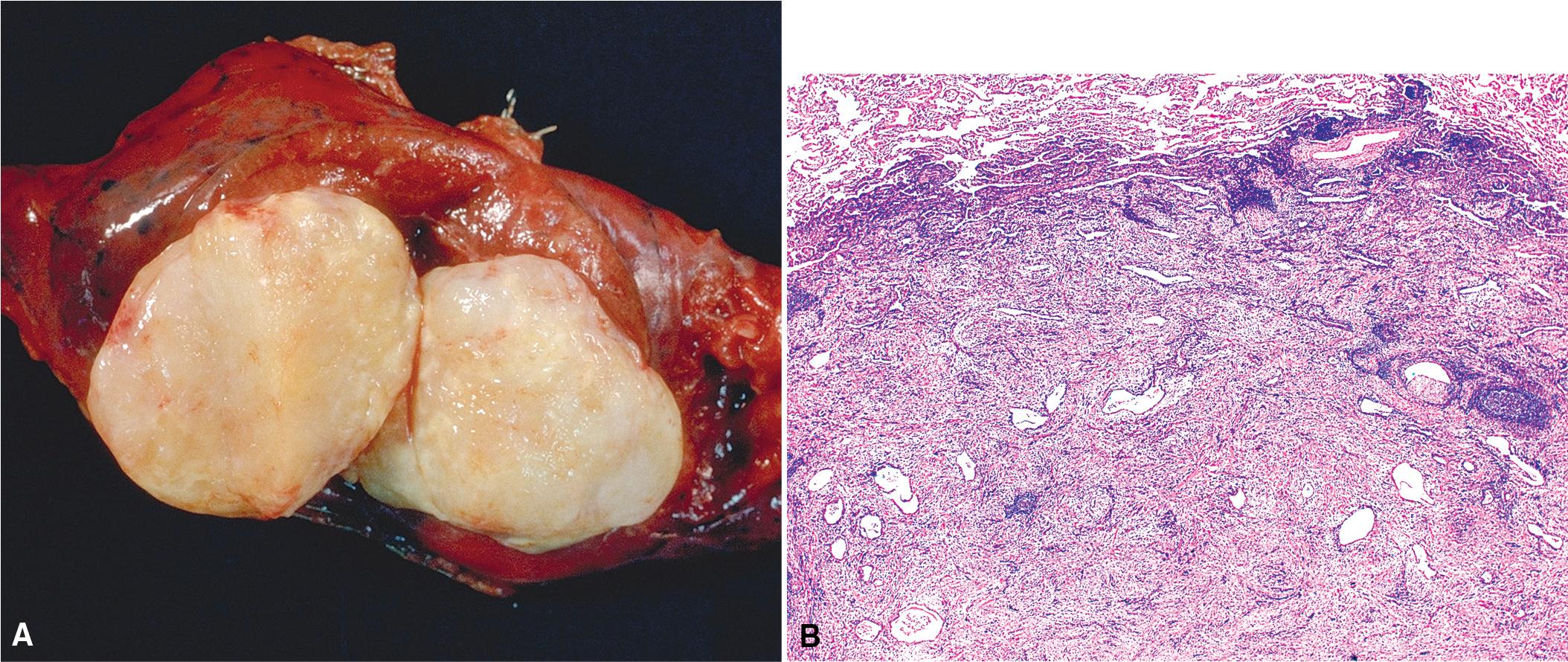
Grossly, PHs typically range in size from 1 to 4 cm; however, they can be as large as 9 cm. They are usually well circumscribed and firm, and the cut surface may be clefted with ossification in some areas. The outer surface is slightly nodular and has a cerebriform quality. The cut surface may have a yellow tone if there is appreciable fat within the lesion ( Fig. 19.1 A).
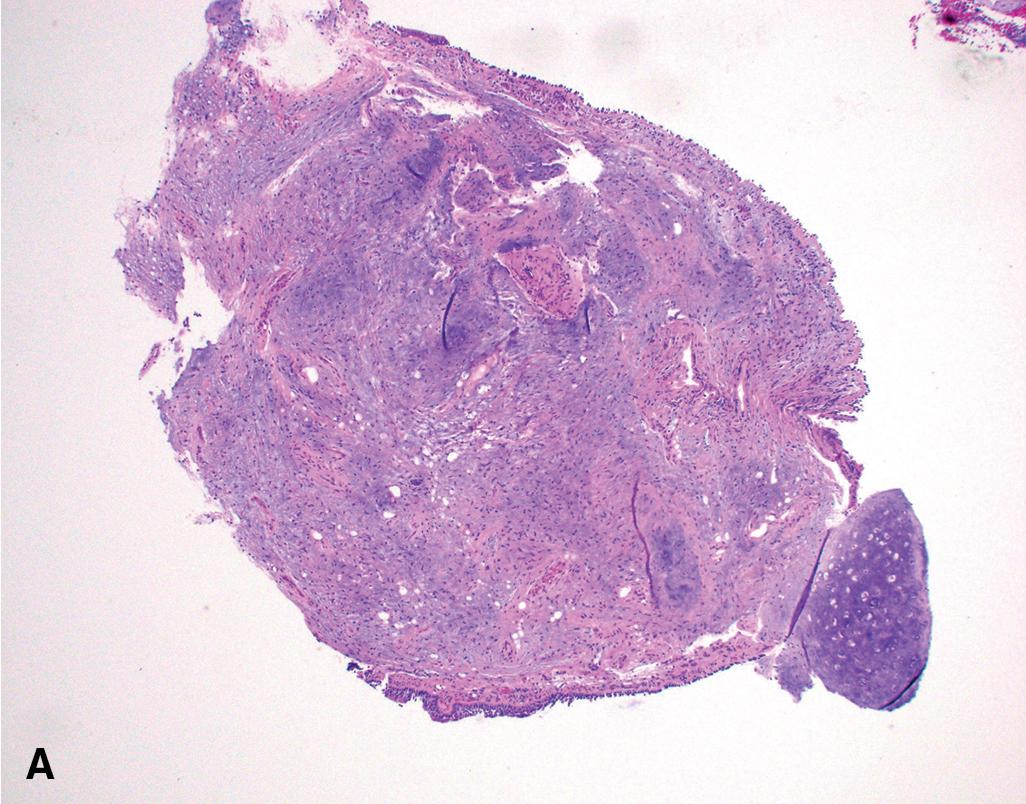
The light microscopic appearance of PH depends on the constituent parts. The most common tissue is cartilage, and the lesions usually have lobular architecture made up of islands of mature cartilage with intervening clefts lined by benign ciliated respiratory epithelium. When mature bone is present, it is due to ossification of the cartilage and is a minor component only. There is, invariably, admixed adipose tissue and myxoid fibroconnective tissue with associated fibroblasts. Smooth muscle can also be found. The proportions and distribution of these constituents vary depending on the site of the hamartomas; there tends to be more adipose tissue and less chondroid material in the endobronchial lesions in comparison to the peripheral lesions. All the tissue components should be cytologically bland ( Fig. 19.2 ). , ,
Immunohistochemical analysis is usually not required in the diagnosis of PH. The constituent tissues will stain exactly as do their nonhamartomatous mature counterparts. Chondromas, lipomas, and fibromas can be excluded on the presence of more than one tissue being present in the resection specimen. A difficulty may arise in the context of resected post-chemotherapy metastatic teratoma; however, this can be resolved with discovery of nonnormal constituent tissue and greatly aided with appropriate clinical information and correlation. Cytologic atypia and cellular crowding in metastatic sarcomas should enable the pathologist to separate them in the differential diagnosis. Grade I chondrosarcoma may have very mild atypia and have similar appearance to hamartoma, but these lesions only very rarely metastasize and are exceedingly rare in the lung.
As these are benign lesions, treatment is only needed to resolve symptoms, which are usually associated with bronchial PH. Surgical enucleation or wedge resection is the treatment of choice. In some circumstances, a lobectomy is required due to the central location or if the lung has become nonfunctioning because of recurrent bouts of pneumonia or infection. Surgical resection may also be required to resolve any diagnostic uncertainty in some cases. The outcome of hamartomas is dependent on location and complications; however, recurrences are very infrequent and malignant transformation is extremely rare.
Many processes can induce cellular injury and reactive changes in lung parenchyma. These include infection, radiation, trauma, infarction, drug-reaction, aspiration, and autoimmune/auto-inflammatory processes. In some settings, these can manifest as discrete lesions that can be concerning for neoplasia on imaging studies. When a biopsy is performed, a common challenge for the interpreting pathologist is distinguishing between reactive atypia induced by a post-infectious/inflammatory process and dysplasia associated with a neoplastic process. Inflammatory-induced atypia can have worrisome cytologic features; however, there are usually several clues that will aid the pathologist in making the correct interpretation. The challenge often involves interpretation of small biopsy material, including cytology specimens. In this setting, the radiologic and clinical information can be crucial and can relieve much of the burden from the pathologist’s shoulders if the biopsy findings are inconclusive. The pathologist may suggest repeat biopsy sampling, but the more appropriate next action may be repeat imaging studies in order to demonstrate growth (concerning for neoplasia) or shrinkage (favoring reactive/nonneoplastic). Discussing the findings with the radiologist and treating clinician can be very helpful in developing an appropriate diagnostic comment for the interpreting pathologist.
Post-inflammatory changes can lead to squamous metaplasia, peribronchiolar metaplasia, pneumocyte atypia, and reactive stromal changes (see discussion below). In some cases, an infection can result in an abscess leading to fibrosis and chronic inflammation. A biopsy of an old/organized abscess can reveal mixed inflammation including plasma cells, foam cells, and prominent spindle cells within a fibrotic stroma. If the biopsy is fortunate to sample the central nidus containing necrotic debris and neutrophils, the correct infectious diagnosis may be discoverable ( Fig. 19.3 ). Otherwise, distinction with other lesions that contain numerous plasma cells and spindle cells, such as plasma cell granuloma, inflammatory myofibroblastic tumor or IgG4-related disease, may require additional diagnostic workup and can be challenging. ,
Other clues to indicate the reactive nature of a post infectious/inflammatory lesion include normal N:C ratio, uniform atypia, gradual transition from normal cellular morphology to cellular atypia, presence of foreign material, viral inclusions, concurrent organizing pneumonia, and/or acute inflammation. Organizing pneumonia is composed of young intra-alveolar fibrotic buds (Masson bodies) and may actually resemble desmoplastic stroma; however, no frankly invasive tumor will be present. A pan-keratin immunohistochemistry stain may reveal the noninvasive epithelial growth pattern lining the tissue and help to confirm a reactive process. Importantly, none of these features are confirmatory for benignity, but in the appropriate context they can be indicative of a nondysplastic process (see Fig. 19.4 ).
Caution should be applied to the interpretation if the biopsy material is very small, if the lesion may not have been adequately sampled, or if the radiologic features are very concerning for a malignancy and the biopsy findings do not provide a definitive answer to explain the image findings (e.g., a necrotizing granuloma with identification of organisms on special stains). Again, in this setting, discussion with the radiologist may prevent discordance and help guide the pathologist in developing a prudent interpretation.
Inflammatory pseudotumor (IPT) is a term that has historically been used to describe several different mass-forming, seemingly nonneoplastic entities. With the benefit of an improved understanding of the pathogenesis of some of the lesions that had been called IPT, some have been split off from the IPT umbrella term and now have well-defined diagnostic criteria with specific names. For example, a distinctive pattern of IPT composed of myofibroblasts with a fibrotic stroma and accompanied by plasma cell-rich chronic inflammation has been discovered to frequently show clonal rearrangement of the ALK gene at 2p23 in the myofibroblasts. This is now recognized to be a neoplastic entity that is called inflammatory myofibroblastic tumor (IMT), and it should not be classified as an IPT. Another entity that had previously been grouped with IPT is IgG4-related disease. Likewise, this also has well defined diagnostic criteria with distinctive pathologic and clinical features (see below). ,
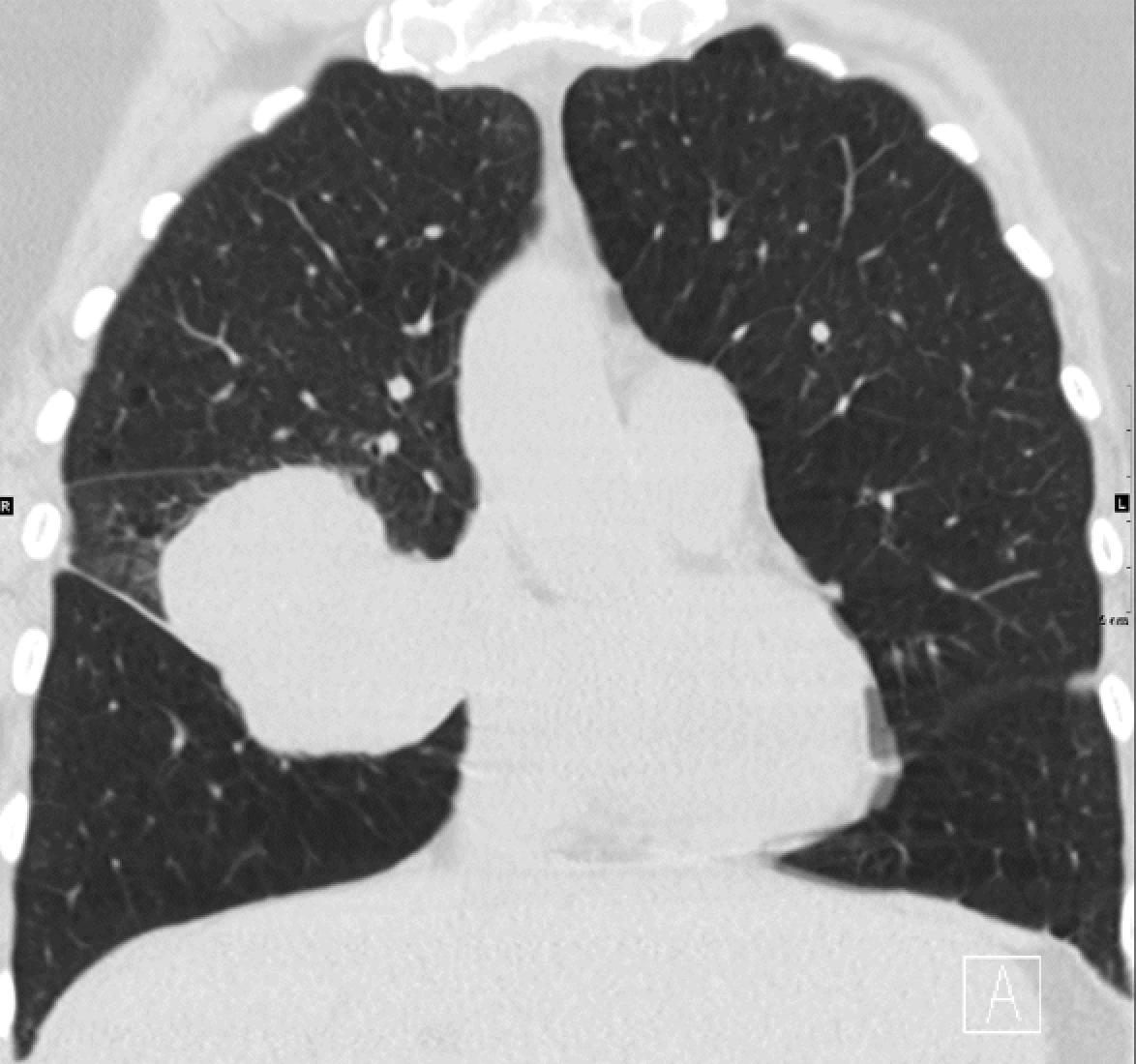
Nevertheless, there remains a nonneoplastic and non-IgG4-related IPT, also known as plasma cell granuloma, which is an amorphous entity with variable degrees of fibrosis and chronic inflammation and which likely represents the common fibroinflammatory destination of many different etiologic processes. As a nonneoplastic entity, the behavior ranges from incidental discovery on imaging studies to symptoms of coughing, dyspnea, and hemoptysis. Imaging studies reveal a lesion that can range from 0.5 cm to 30 cm in size. IPT typically has a rounded shape with well-demarcated borders; some lesions can show cavitation ( Fig. 19.5 ). IPT has equal sex predilection and can be found at any age.
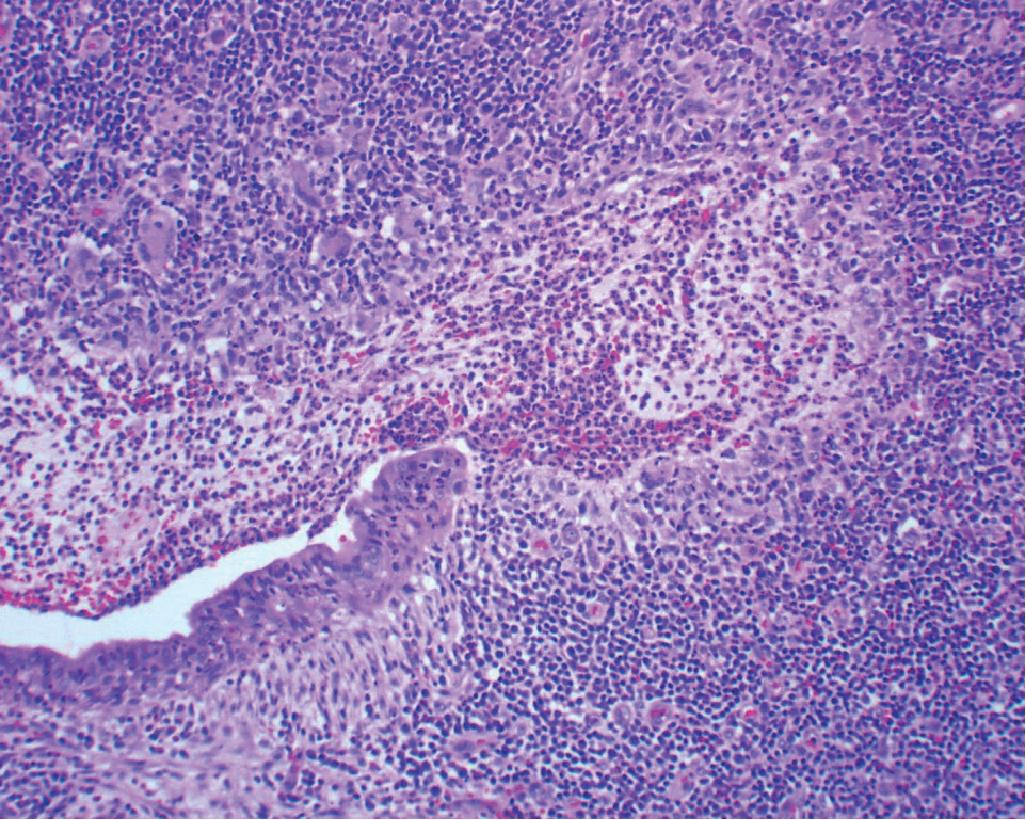
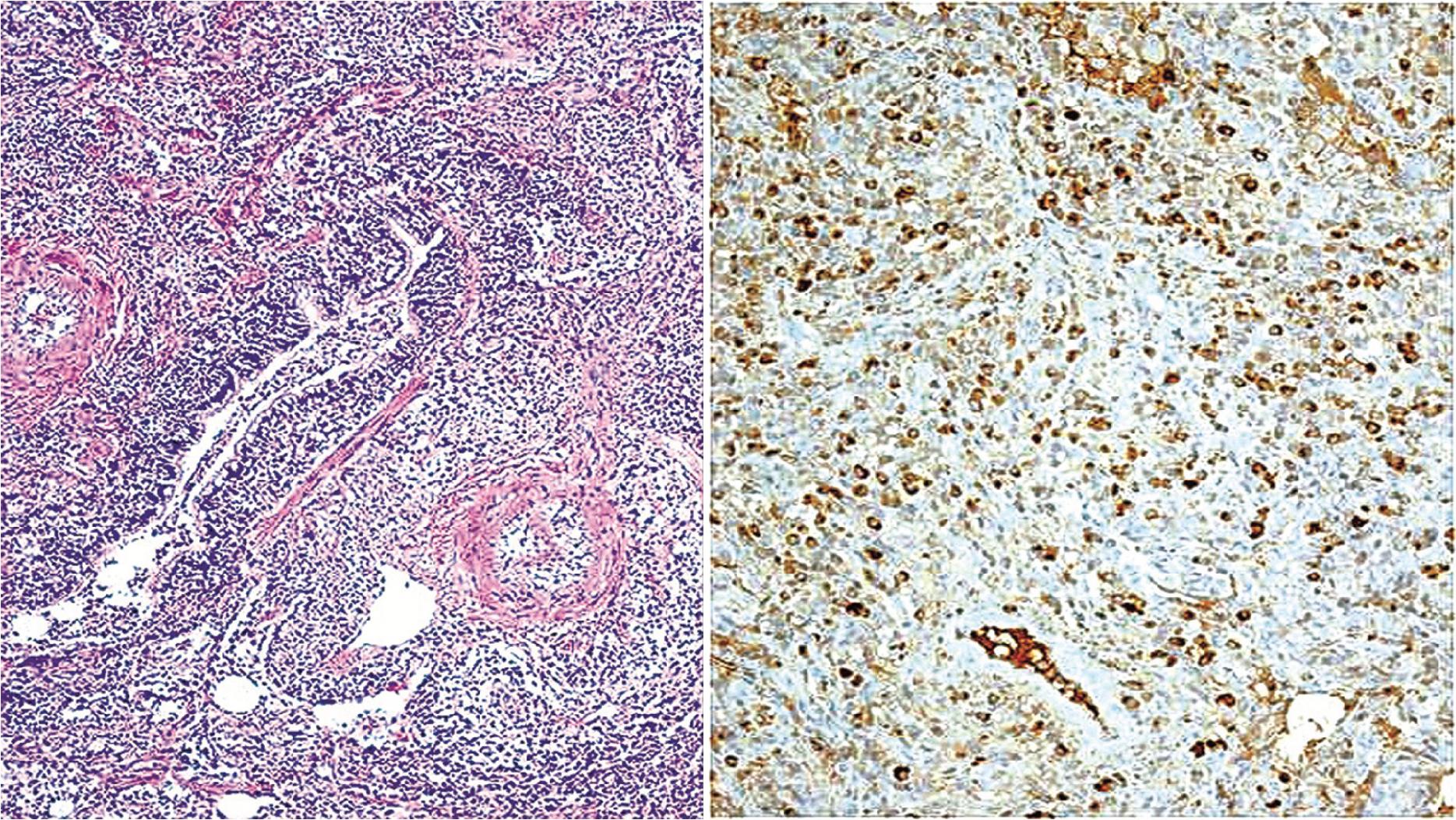
The key and defining histologic features of IPT is the destruction of the underlying lung parenchyma and replacement by a fibroinflammatory lesion without evidence of a clonal population or IgG4 increase. If the lung architecture is preserved within the lesion, this may be an incipient IPT but should be classified as another post infectious/inflammatory lesion such as acute or subacute pneumonia. The microscopic features of IPT vary from mild to dense chronic inflammation in a background of variably cellular fibrosis admixed with areas of xanthomatous change and often accompanied by organizing pneumonia at the periphery of the lesion ( Fig. 19.6 ). Older lesions may have calcifications and evidence of old hemorrhage with hemosiderin deposition.
In each case of presumed IPT, etiologic clues should be sought by the pathologist. If necrosis or epithelioid granulomas are discovered, infectious stains for organisms should be performed. , If the lesion consists of sheets of chronic inflammatory cells, a lymphoplasmacytic process such as low-grade B-cell lymphoma or IgG4-related disease should be considered and investigated with appropriate immunohistochemical or molecular methods. If the lesion shows abundant lymphoid follicles with intervening plasma cell-rich fibrosis, it may be more appropriately classified as nodular lymphoid hyperplasia. If a central nidus of necro-inflammatory debris is present, the etiology may be an organizing abscess. If the lesion is in the right lower or right middle lobe and foreign material is present, the underlying etiology may be aspiration pneumonia with subsequent formation of the IPT.
Surgical resection is curative and would resolve any diagnostic dilemma.
Pulmonary hyalinizing granulomas (PHG) are distinctive circumscribed rounded lesions that are composed of dense, paucicellular collagen and are reactive in nature. The word “granuloma” is a misnomer in this context as these lesions are not composed of histiocytes and epithelioid histiocytic granulomas are absent or rare. PHG are rare, slow growing lesions with fewer than 150 cases reported in the literature. Patients have a wide age range of 15 to 83 years with a mean age of 45 years; there is male predominance. Most cases that come to medical attention are symptomatic and may cause coughing, chest pain, hemoptysis, and/or dyspnea, but approximately 25% of cases are discovered incidentally. Most lesions are multiple with no lobar predilection and approximately 30% of cases are solitary. The lesions may be calcified and approximately 10% of cases show calcifications on imaging. Interestingly, despite the paucicellular and bland microscopic appearance, one study found 60% of cases showed hypermetabolism on PET scan.
Grossly, PHG range in size from less than 1 to 10 cm, and they are distinctive in being rounded with a firm, pale-gray whorled and bulging cut surface. If grossly friable tissue is present, this may represent caseating necrosis due to infection and appropriate personal protective equipment is indicated for the grossing laboratory personnel.
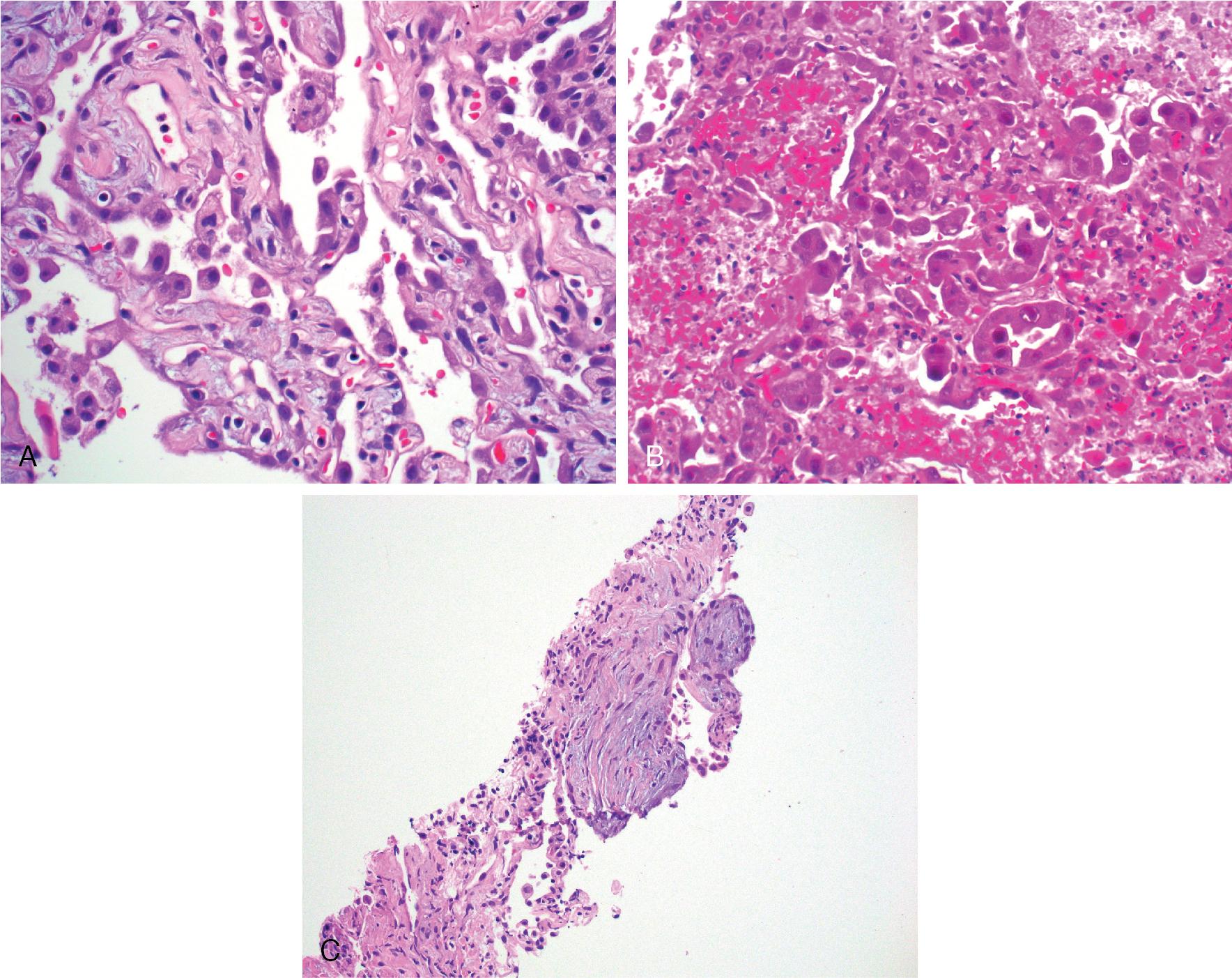
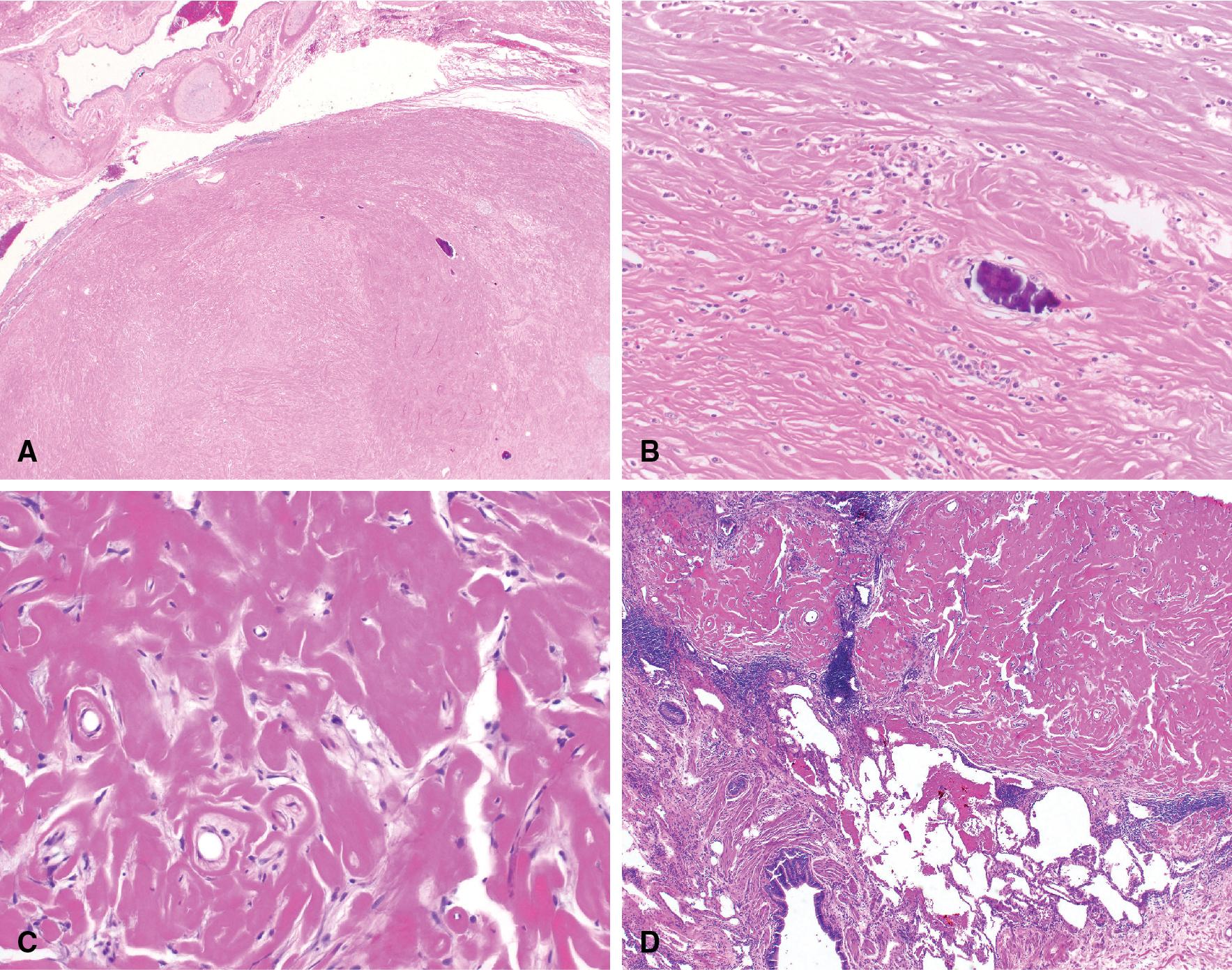
The microscopic features of PHG are quite bland but distinctive. The lesion is composed of dense paucicellular hyalinized fibrous tissue resembling a keloid scar. Chronic inflammation is sparse and, when present, is typically at the periphery of the lesion ( Fig. 19.7 ). Most cases do not have granulomas or necrosis; when present, they should prompt evaluation for infectious organisms. Due to the paucity of cellular infiltrate, special histochemical stains or immunohistochemical staining do not have a significant role in identifying PHG but can sometimes be used to exclude other entities (e.g., IMT, amyloidosis, or IgG4-related disease, see below). The most challenging aspect of establishing the diagnosis may be the small size of the biopsy material. The microscopic features are not specific when the lesion is only partially sampled, and it may be impossible to exclude all other diagnostic considerations. ,
The underlying etiology of PHG is thought to be the result of an exuberant immune reaction to an infectious or noninfectious inflammatory stimulus. There is a strong association to other sclerosing diseases such as sclerosing mediastinitis, systemic fibrosis, or retroperitoneal fibrosis; and some patients have been found to have elevated serologic levels of IgG4 suggesting in these cases that the process is an advanced or “burned out” nodular lesion of IgG4-related disease (see below). Other associations include Castleman disease, rheumatoid factor, anti-smooth muscle antibodies and prior granulomatous infections but as noted above most cases of PHG lack epithelioid granulomas. ,
If the entire specimen has been excised, review of the specimen can usually confirm the diagnosis without ambiguity. On smaller biopsies, if the extracellular matrix is amorphous or appears “cracked,” amyloidosis can be in the differential diagnosis, which can be excluded with a negative Congo red stain. IgG4-related disease can be in the differential diagnosis, especially if there are clusters of plasma cells present. Inflammatory myofibroblastic tumor can also have fibrosis and plasma cells, but the inflammation is usually much more pronounced and diffuse than seen in PHG. Both IgG4-related disease and IMT can be accurately diagnosed with immunohistochemistry (IHC) stains. Other entities associated with chronic inflammation and fibrosis include infections and some forms of lymphoma, particularly nodular sclerosis Hodgkin lymphoma. Special stains to evaluate for organisms are not needed in every case unless there are granulomas or any area of necrosis present.
As reactive, nonneoplastic lesions, there are no reports of deaths attributable to PHG. Symptomatic cases may be treated with immunosuppression with reports of 80% response. If not treated, some reports have shown slow growth of lesions over many years. Surgical resection is rarely necessary unless the lesion is symptomatic or evaluation of the fully resected specimen is needed to resolve any diagnostic uncertainty. ,
Spindle-cell pseudotumors that are reactions to mycobacterial infection have been documented in several organ sites in immunosuppressed patients. These proliferations show a close histologic resemblance to histoid leprosy, , and most reports have documented numerous intralesional mycobacteria (see Chapter 7 ). Only one case of mycobacterial pseudotumor (MP) has been reported in the lung, although we have anecdotally encountered another example in a 41-year-old male patient with acquired immunodeficiency syndrome (AIDS).
Grossly, the lesions appear as yellow-gray nodules. They may show a predilection for small airways. Microscopically, the lesions comprise aggregates of spindle cells with a fascicular growth pattern and without significant atypia or mitoses. Scattered lymphocytes and plasma cells may be present, but overt granulomas are lacking. The cytoplasm of the spindle cells is “foamy” but may contain hemosiderin focally. The lesional cells are immunoreactive for lysozyme, with no labeling for S100 protein, keratin, actin, desmin, or von Willebrand factor. Ziehl-Neelsen staining shows innumerable acid-fast bacilli in the fusiform cells ( Figs. 19.8–19.10 ). Most examples of MP in other anatomic locations have been related to Mycobacterium avium–intracellulare or Mycobacterium kansasii .

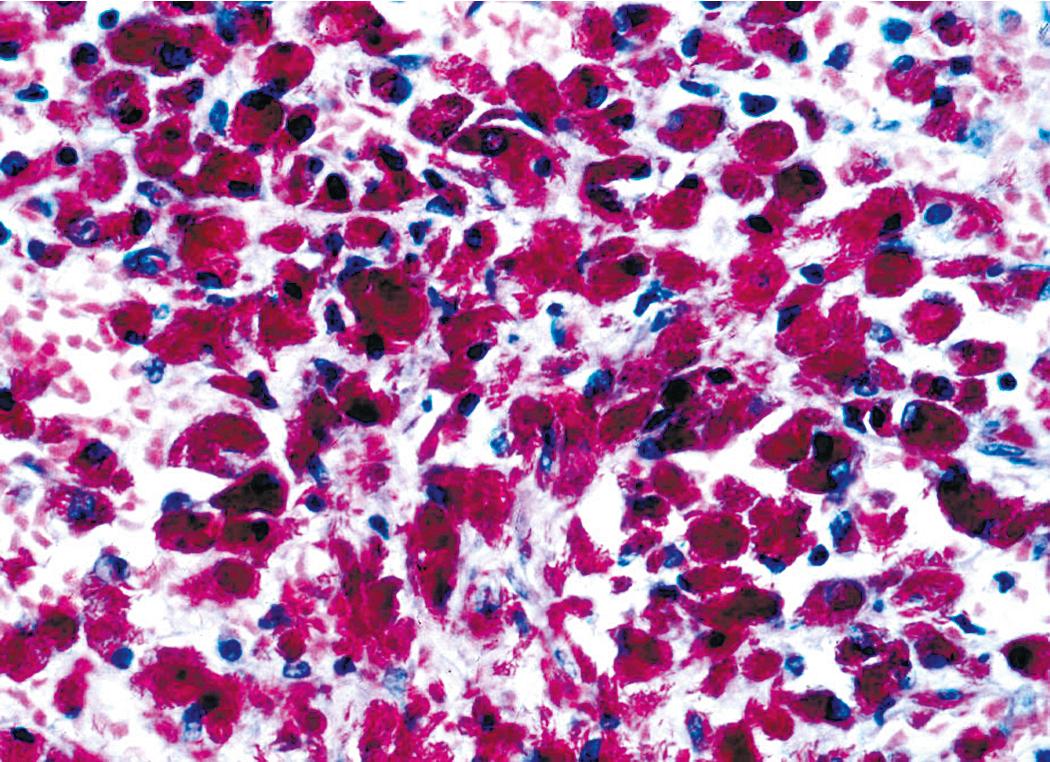
Another reported feature of MPs in other sites is a possible source of diagnostic error. That is, a reproducible cross-reaction has been seen with mycobacterial antigens using certain desmin antibodies, spuriously suggesting the presence of a myogenous proliferation. This observation is especially troublesome in the setting being discussed here because smooth muscle or myofibroblastic tumors enter prominently into the differential diagnosis of MPs. However, a documented lack of immunoreactivity for actin and electron microscopic attributes that support histiocytic differentiation in MP argue against those alternative interpretations.
Other lesions that must be separated from pulmonary MP include Kaposi sarcoma, spindle-cell melanoma, and neural proliferations. Obviously, acid-fast stains should be done in all spindle-cell lesions from immunocompromised individuals, and these consistently confirm mycobacterial causation. In some instances, the nature of MP is more obvious because of overtly granulomatous foci in the lung tissue around the spindle-cell lesion. Characteristics of malignancy such as necrosis, nuclear atypia, and pathologic mitoses are absent in MP. Thus diagnoses of pulmonary sarcomatoid carcinoma or sarcomas would be unlikely.
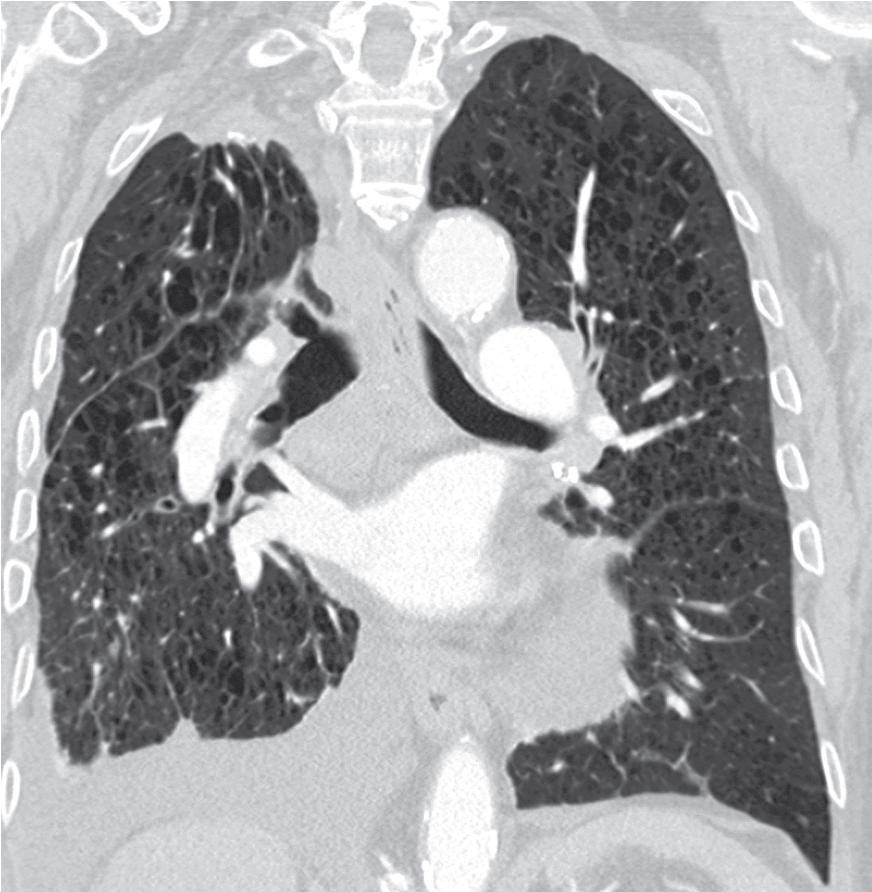
The apical cap (AC) is a frequently encountered entity that is usually found at the apices of the upper lobes as well as the lower lobes. They tend to be discovered incidentally on routine imaging studies but some patients have reported hemoptysis. The etiology is speculative and ranges from ischemic injury to “burned out” or fibrotic infectious lesions, smoking, or to scarring produced by the gravitational pull of the lungs from the negative-pressure pleural cavity at the point of maximum strain. One meta-analysis study found the prevalence to be 11.2% in unilateral cases and 12.2% in bilateral cases. ACs have been reported to range in size from less than 1.0 cm to 5.0 cm. When large, they may be radiographically concerning for an apical malignancy, leading to biopsy ( Fig. 19.11 ).
Grossly, AC appear as pale gray fibrotic scars at the apical pleural surface, but they can often be discolored by anthracotic pigment. On cut section, they are generally flat, often extending less than 0.5 cm into the underlying lung parenchyma. The microscopic features consist of subpleural paucicellular fibroelastosis. Adjacent pneumocytes may show hyperplasia without, or with minimal, cellular atypia. The vessels in and around the lesion can be ectatic or thick walled ( Fig 19.12 ). There should be very little, if any, cellular inflammation and granulomas are not a feature of apical caps; if granulomas are present, this should raise concern for an infection, such as tuberculosis or fungal disease, or for sarcoidosis. The surrounding alveolar parenchyma often has emphysematous changes, anthracosis, and pigmented macrophages. ,
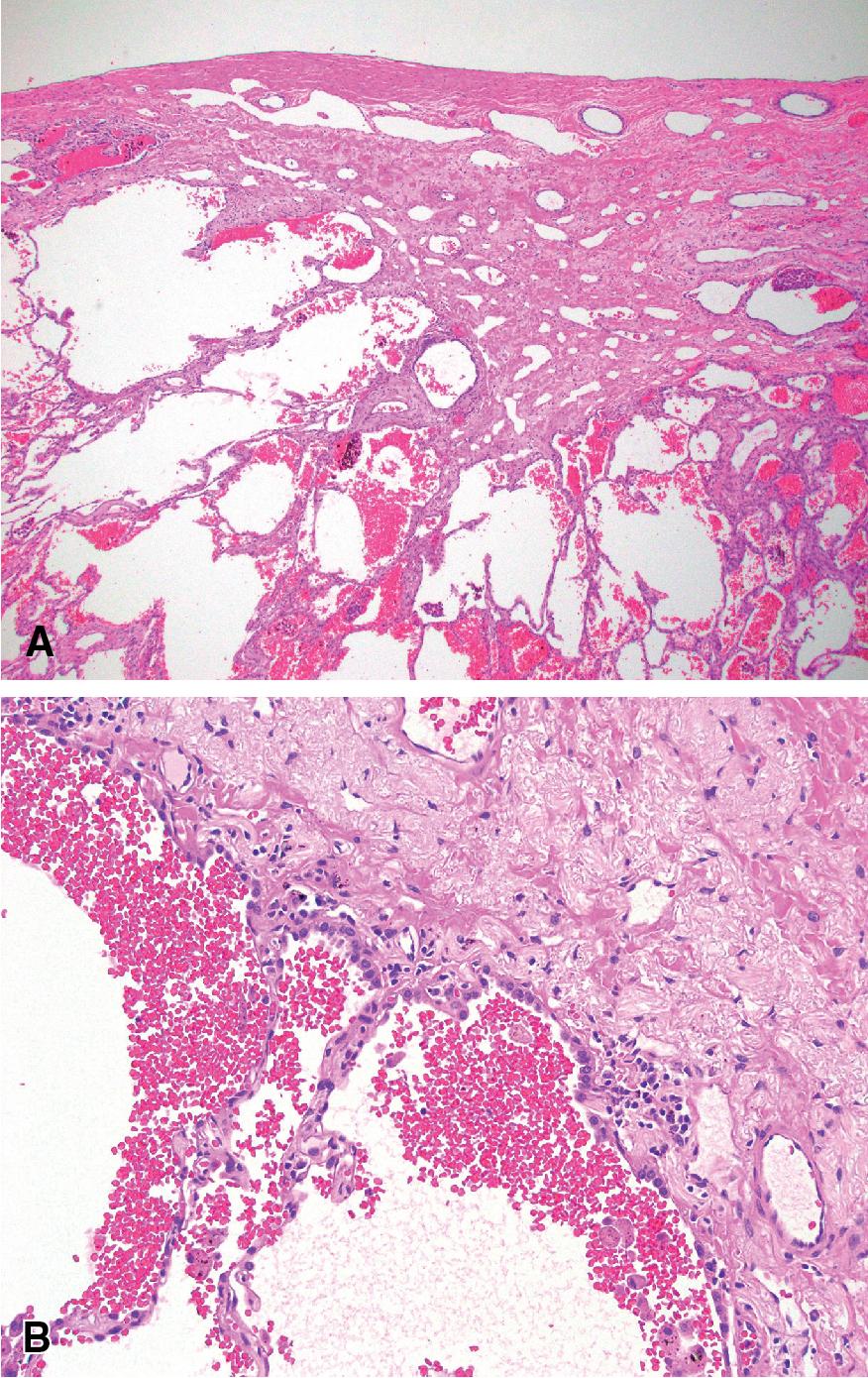
Microscopically, the features on a biopsy are very similar to pleuroparenchymal fibroelastosis (PPFE). However, the clinical presentation of PPFE is very different in that it presents with typical symptoms of interstitial lung disease, such as progressive shortness of breath and cough, and with upper lobe predominant fibroelastosis, whereas apical cap usually presents as an asymptomatic ≤ 5.0 cm lesion in the lung apices. If the full clinical picture is known, PPFE should not be in the differential diagnosis. Asbestosis, old infections and radiation injury can cause apical cap-like scarring. If evidence of an active infection, such as tuberculosis is discovered, the lesion should not be called an apical cap to avoid diagnostic confusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here