Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiation therapy (RT) can be used as a primary or adjuvant treatment for many cancers.
The unit of dose measurement is the gray (Gy).
Side-effects are characterized as acute and late toxicities.
Acute toxicities are temporary and reversible.
Late toxicities are permanent.
The primary mechanism of action of radiation is through breakage of DNA strands.
Treatment planning is achieved through use of three-dimensional computed tomography (CT).
Delivery of radiation can be very precise with the use of intensity-modulated radiation therapy (IMRT), particle therapy, such as proton therapy, as well as with image-guided radiation therapy (IGRT).
![]()
![]() Access video and video lecture content for this chapter online at Elsevier eBooks+
Access video and video lecture content for this chapter online at Elsevier eBooks+
Radiation therapy (RT) utilizes ionizing radiation to treat malignant tumors and some benign disorders. Nearly two-thirds of cancer patients receive RT as part or all of their treatment. Radiation oncology is the discipline of medicine that addresses the causes, prevention, and treatment of human cancer with special emphasis on the role of ionizing radiation. Delivery of radiation can be achieved using different modalities, including with photons (kilovoltage, orthovoltage, megavoltage), electrons, protons, neutrons, and carbon ions, and may be through external beam RT or brachytherapy.
Radiation oncologists work in a multiprofessional team with radiation therapists, nurses, dosimetrists, and medical physicists, along with dieticians, social workers, and counselors. Additionally, radiation oncologists collaborate with a multidisciplinary team of surgical and medical oncologists, diagnostic radiologists, and pathologists, and are essential participants at tumor board for decision-making.
Once patients are on treatment, radiation therapists deliver the RT; they spend not only considerable time on the technical components of treatment (treatment delivery, quality assurance, and verification), but also interact with and support cancer patients throughout the duration of their therapy. Outside the immediate team, it is rare for other physicians to know much about the technology, process, rationale, and issues around RT, and even less about the risks and causes of toxicity.
This chapter is designed as a primer or virtual elective in the topic and aims to improve communication and understanding between plastic surgeons and radiation oncologists. This chapter starts with a brief description of the basics of radiation technology, modalities, medical physics, and radiobiology. It then outlines practical applications, with a description of RT planning and process, and clinical treatment issues for select cancers. The final section describes specific toxicity syndromes.
RT consistently relies on technology. Although new technology and innovation continually replaces older technology, some of it remains in effective and efficient use. For example, kilovoltage X-ray therapy can be effectively used for very superficial treatments. It delivers 100% of the prescribed dose at the skin, has rapid fall-off below the skin along with a tight penumbra (edge of the beam). In the 50–150 kV range, X-ray penetration provides useful coverage only to a depth of 5 mm; orthovoltage therapy (150–500 kV) provides better penetration but is not capable of treating much more than 3 cm below the skin. For these reasons, kilovoltage and orthovoltage therapy have been used to treat superficial, flat, skin malignancies and lesions, taking advantage of the higher skin dose with minimal radiation exposure to deeper tissues.
Megavoltage (MV) radiotherapy allows even greater penetration of tissue, resulting in better skin-sparing and improved dose homogeneity. It is not influenced by the atomic number (Z) of the absorbing medium, as Compton scattering predominates in this energy range. Therefore, it can be used to treat deeper-seated tumors, and minimize dosimetric uncertainty with bone or hardware within the treatment field.
In November 1895, Wilhelm Conrad von Roentgen discovered a new type of ray that was generated from a stream of electrons in a cathode gas discharge tube and had the ability to pass through tissues with different degrees of penetration. Because the composition of the rays was unknown, they were called X-rays. His subsequent presentation to the Wurzburg Society on the ability of his rays to generate an image of structures inside the body gave birth to diagnostic radiology. The following year, in France, Henri Becquerel discovered that fluorescent materials made of uranium also emitted constant radiation. Marie and Pierre Curie used ionization to measure becquerel rays and quantify radiation intensity against uranium. Marie Curie first used the word “radioactivity” in 1898. The couple isolated two new radioactive elements from pitchblende, polonium, named for Marie’s native Poland, and radium, Latin for ray. Today, these pioneers’ names are in constant use as measures of radioactivity.
The earliest oncologic brachytherapy procedure was in 1902, when a radium applicator was used to treat a tumor of the palate and pharynx. Within a few years, there were clinical studies of effectiveness, and radium tubes were inserted directly into tumors, and by the 1920s, radium had become the standard of care in oncology, as well as the treatment for many benign conditions. The use of Roentgen’s rays to palliate advanced cancer was first described in 1903, and this was the forerunner of external-beam radiation treatment.
Until the 1950s, most radiation machines were very similar to diagnostic radiology ones, and generated X-rays at potentials of up to 300 kV. Superficial therapy (50–150 kV) could treat to a depth of 5 mm, and thus was useful for superficial skin cancers. Orthovoltage (or deep) therapy (150–500 kV) provided greater penetration but was not capable of treating much more than 3 cm below the skin, with 100% of the dose at the skin. At this range of energy, interaction between radiation and matter is strongly dependent on the atomic number (Z) of the attenuating material – in other words, in this process, known as the photoelectric effect, tissue such as bone, which has a high Z, attenuates more radiation; fat and soft tissue (lower Z) attenuate less; and air (for example, in the lungs) hardly at all, thus producing the contrast necessary for diagnostic imaging. Unfortunately, this higher absorbed dose in bone resulted in many more problems from osteoradionecrosis (ORN).
The development in Canada of the cobalt-60 ( 60 Co) unit in 1951 introduced the new era of megavoltage (MV) radiotherapy to the world, allowing greater penetration of tissue and, with maximal dose not achieved until a depth of 0.5 cm, resulting in better skin-sparing and improved dose homogeneity. Since then, computer-controlled megavoltage linear accelerators have been developed, providing increasing penetration and skin-sparing, and a rapid accumulation of technologies to verify, deliver, and record increasingly precise radiation therapy.
Cobalt units deliver MV radiation and have a 60 Co source that generates gamma (γ) rays with average energy of 1.25 MV. 60 Co has a half-life of 5.26 years; this decay and subsequent drop in output means that the source needs to be replaced every few years. Maximum dose ( D max ) is not achieved until 0.5 cm beyond the surface of the skin and the intrinsic scatter of the beam and ionized particles means that the penumbra, or dose fall-off, at the edge of a beam, is not very tight. Despite this, cobalt is still a prevalent and important external-beam radiotherapy modality, as it still provides an excellent low-maintenance, reliable, safe, and effective alternative in many low- and middle-income countries.
The most commonly used RT modality is photon (X-rays) RT. The photons are generated by a linear accelerator, abbreviated to “linac”, which uses high-frequency electromagnetic waves to accelerate electrons to a very high energy through an accelerator tube and beam transport system. When the accelerated electrons hit a high Z target at the end of this, they produce MV X-ray beams that have sharper edges and can penetrate deep tissue. Typical photon energies produced by linacs are 4, 6, 10, and 18 MV that result in increasing maximum depth doses. This contrasts with the 1.25 MV energy from cobalt units; linacs therefore allow increased skin-sparing and conformality of the radiotherapy dose for tumors in deeper locations, decreasing acute and late toxicities.
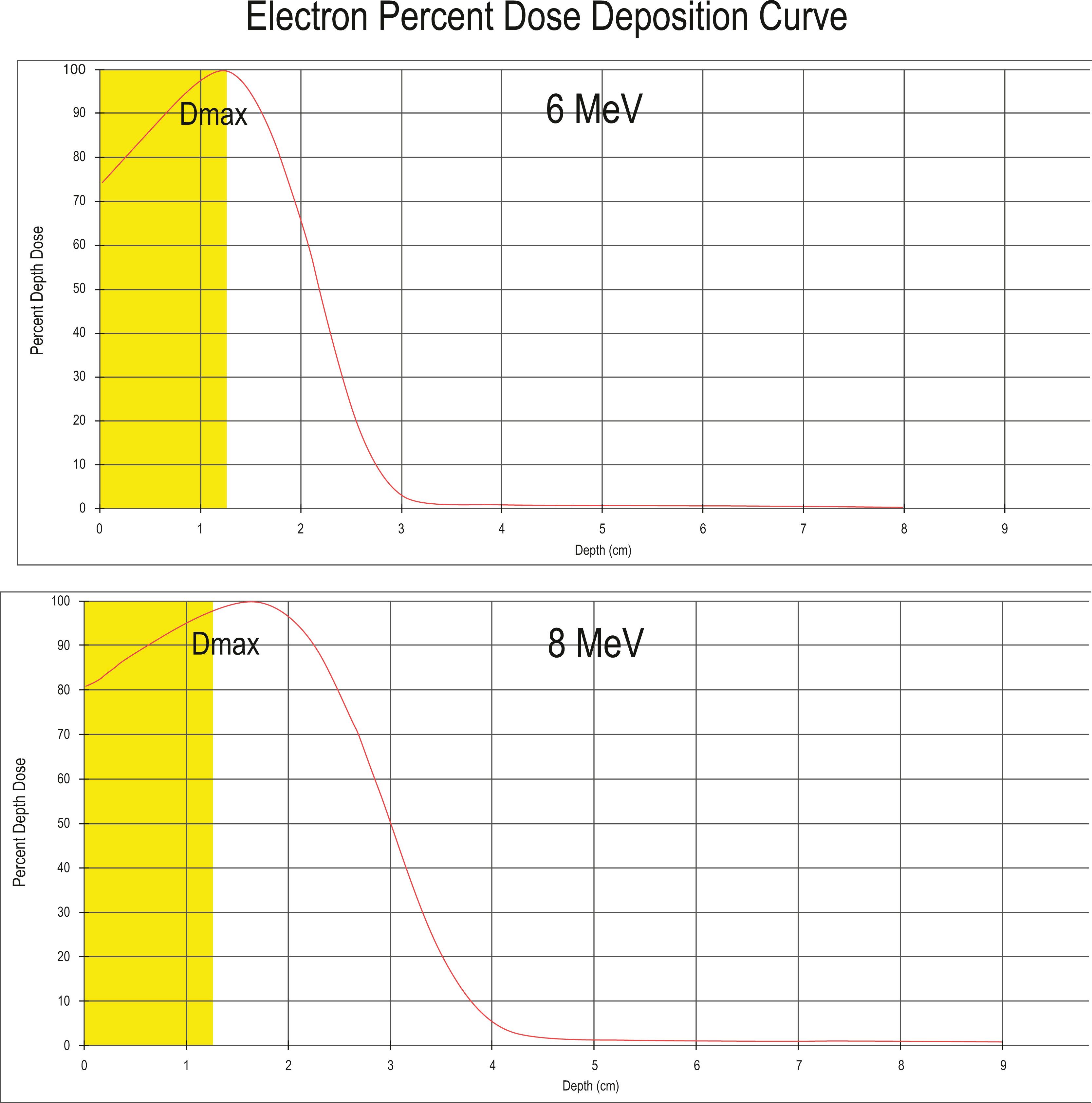
Electrons are the most commonly used particle therapy. Like photons, they are generated by linacs, which generally can produce a range of electron energies from 4 to 17 MeV. They deposit their energy superficially, sparing structures deeper in the body, with fairly steep dose fall-off on distribution curves. Electrons produce more side scatter than photons and have wider penumbras.
Electrons are typically used to treat superficial tumors such as skin cancers and “boosts” to breast tumor cavities, as well as for intraoperative radiotherapy. Fig. 27.1 shows a characteristic electron dose-depth deposition, which illustrates how increasing electron beam energies results in the ability to treat deeper tissues, with an exponential dose fall-off. If full dose is required at the skin surface, such as treating a skin malignancy, then one can add a layer of tissue-equivalent artificial material, called a bolus, which lets dose build up before reaching the skin.
Proton beam facilities are expensive to build and maintain, as they require a cyclotron or a synchrotron to accelerate protons, which have a mass nearly 2000 times that of an electron. While over the past decade we have seen a large expansion in proton centers around the world, many larger geographic regions require patients to travel for this treatment since it is often not readily available in the community.
Protons have similar biologic activity as photons but have an advantage in the unique shape of their dose distribution. They enter the body with low steady-dose deposition until near the end of the clearly defined range when the dose peaks sharply then falls off rapidly to nearly zero. This is the Bragg peak phenomenon, which allows very precise high-dose dose distribution to homogenously cover the target volume, protection of critical structures beyond the Bragg peak, and dose intensification. For this reason, one typically selects 1 to 4 beam angles maximum to provide robust dose distribution for the desired target, but to provide as much sparing of the low integral dose seen with highly conformal photon (X-ray) radiotherapy plans.
Proton therapy is delivered via different techniques. Historically, to produce a clinically useful bream, a spread-out Bragg peak (SOBP) was created using energy range modulators to produce a flat dose distribution at the proximal and distal ends of the target. Proton therapy treatment techniques have evolved from single and double passive scatter to the more advanced techniques of uniform scanning and pencil beam therapy. Uniform scanning uses a SOBP to vary the beam energy to treat layer by layer and uses a magnet to scan the beam. The most precise form of proton therapy is pencil beam therapy, where a “spot” only a few millimeters wide scans the tumor to treat complex shapes and distributions, sometimes using volumetric repainting of the target to minimize setup and motion uncertainties. Pencil beam therapy is the preferred proton modality of choice if proximal target conformality would be clinically meaningful, leading to sparing critical organs-at-risk and modest skin-sparing compared to uniform scanning.
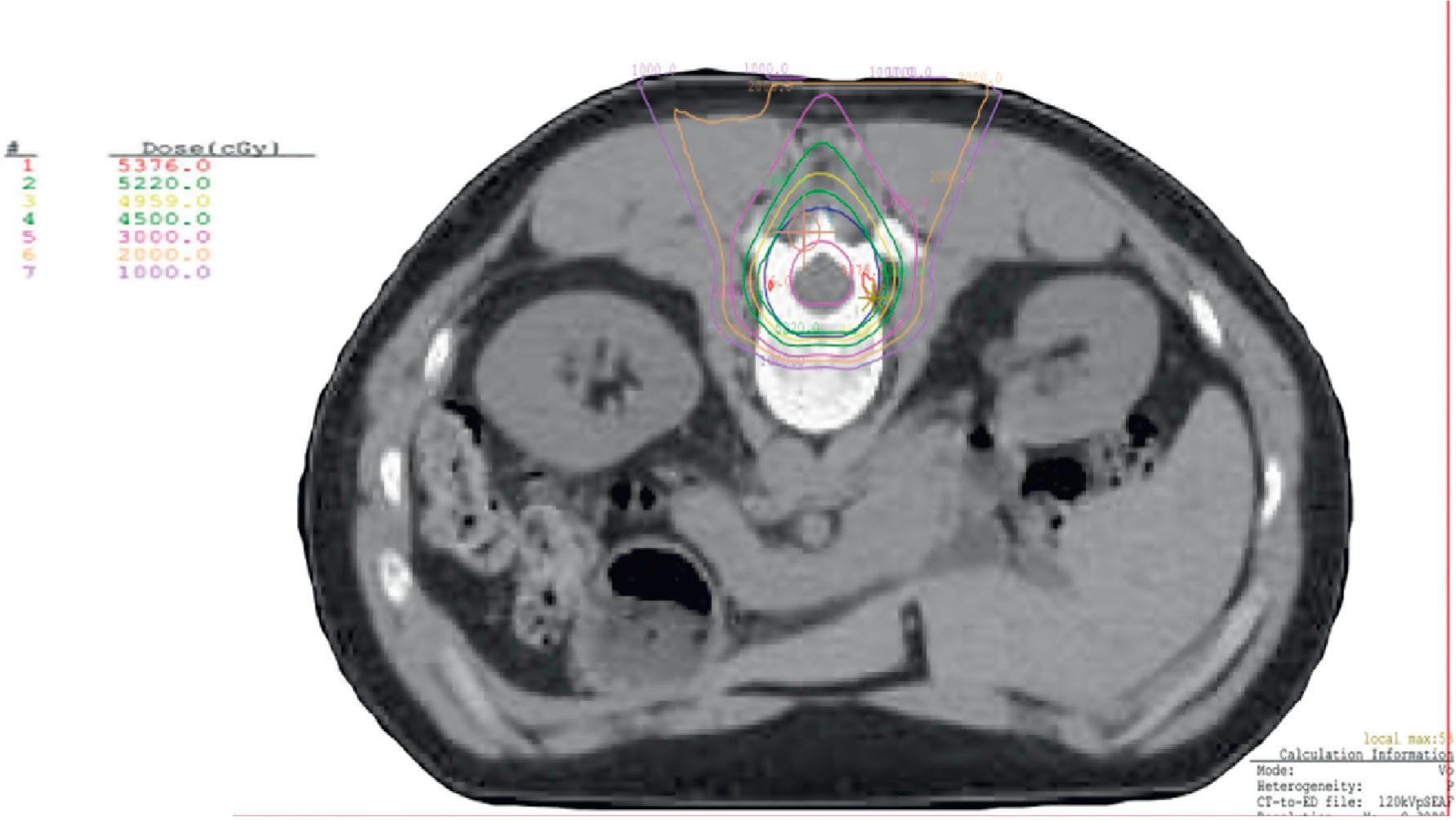
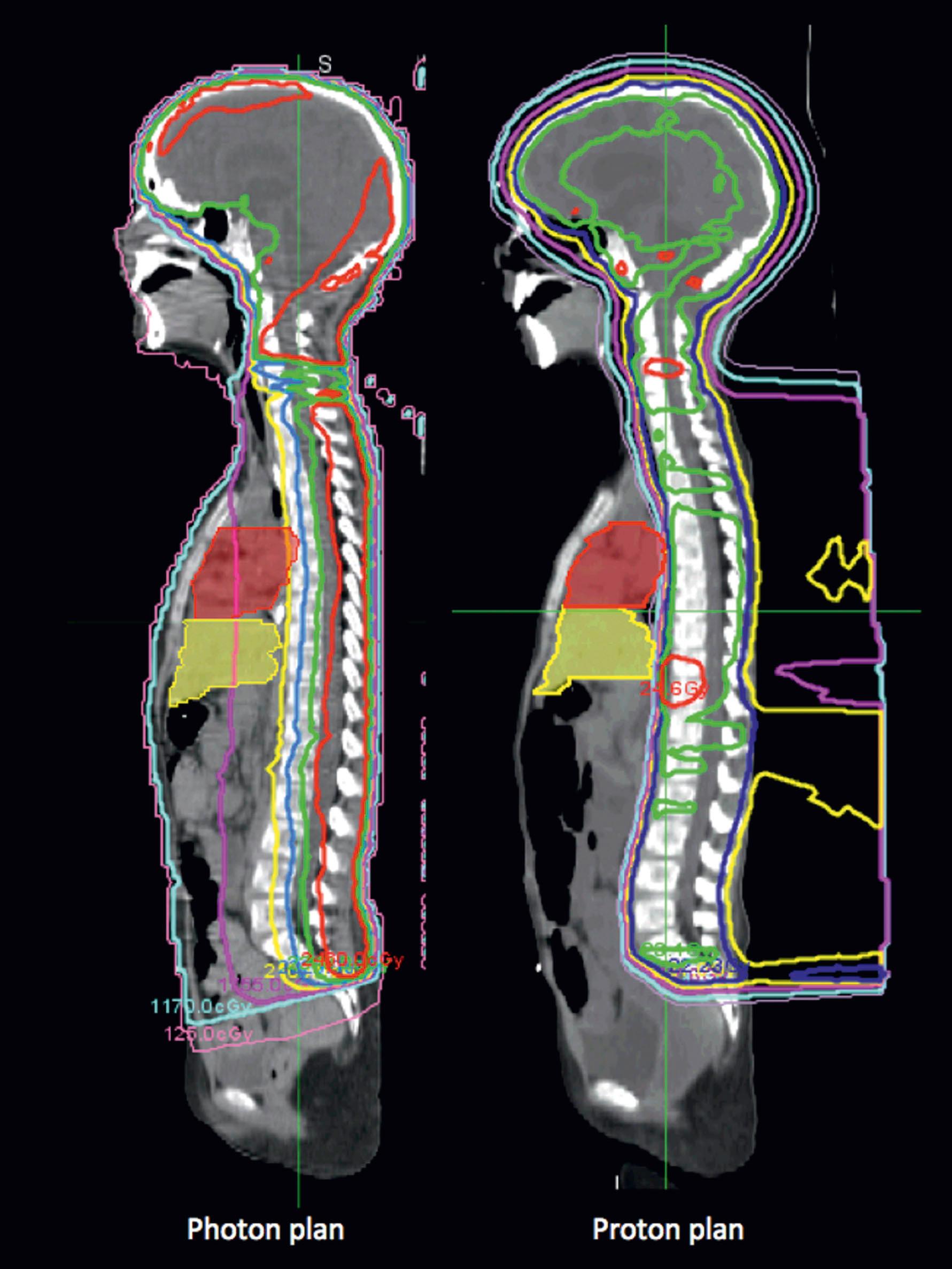
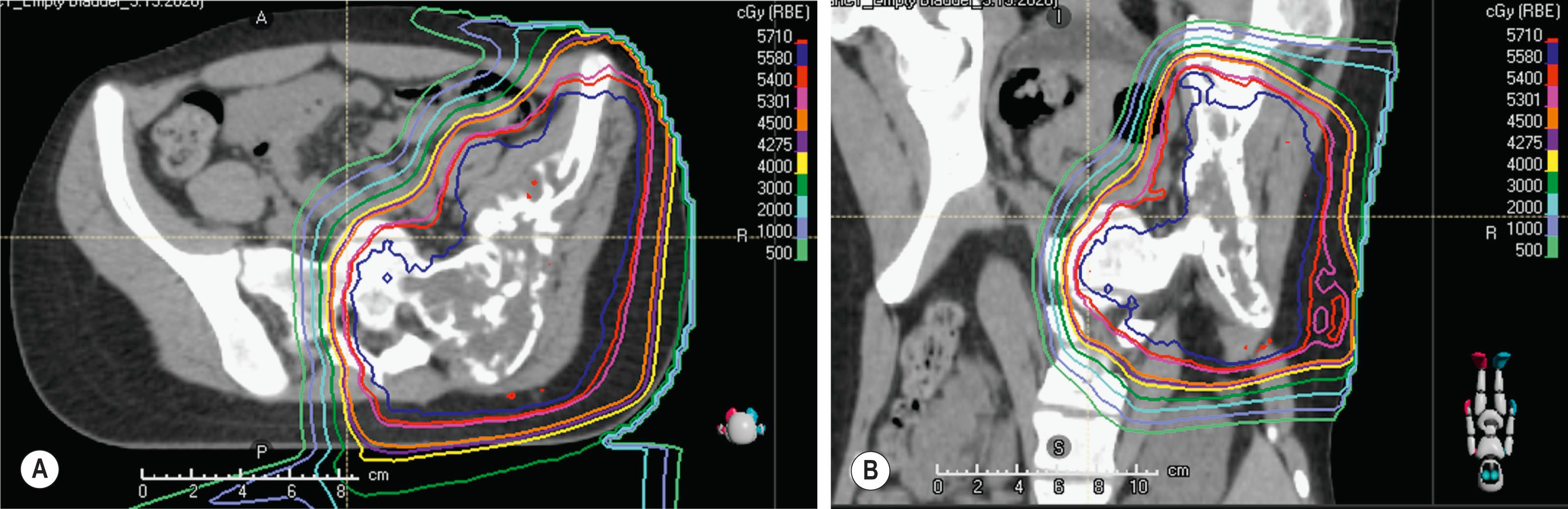
Protons are ideal for treating structures in the base of the skull, such as the clivus, especially for chordomas or chondrosarcomas, where the necessary dose is far above the tolerance of the adjacent optic chiasm and other sensitive structures in that region, and would otherwise cause severe toxicity. Spinal cord tumors, such as ependymoma ( Fig. 27.2 ) also benefit from this modality, as well as craniospinal irradiation for pediatric cancers such as medulloblastoma, because the proton dose distribution significantly reduces the exit dose to anterior structures (heart, kidneys, bowel, lungs, pelvic organs such as uterus and ovaries) when compared to conventional photon therapy ( Fig. 27.3 ). When treating patients with sarcomas of the pelvis, proton beam therapy has been shown to minimize irradiation of gonadal organs (uterus, ovaries, testes), which can result in maintenance of fertility for children and young adult patients ( Fig. 27.4 ).
In other disease sites, there are several ongoing randomized trials evaluating the role of protons versus photons in respect to survival, toxicity, and quality of life outcomes (PARTIQoL for prostate cancer, RADCOMP for breast cancer, RTOG 1308 for lung cancer). Proton beam radiotherapy is also useful for re-irradiation, as it often can reduce dose to critical structures that have already been exposed to prior radiation (NRG06 for esophageal cancer, BN003 for low to intermediate grade gliomas).
Clinically, we observe that the skin reaction from proton therapy can be more marked than with photon (X-ray)-based therapy. This is because protons therapy does have less skin-sparing properties than photons and deposit a somewhat higher dose at the surface. It also uses fewer beam angles in order to maximize internal organ sparing, thus making the entry dose higher. Therefore, techniques to increase the skin dose such as using bolus (artificial tissue-equivalent) are not recommended for proton therapy, particularly for breast cancer treatment. Conversely, sometimes boluses may be used with protons to provide further separation and “dose fall-off” – so that the artificial substrate rather than a critical organ at risk absorbs dose, for example, creating extra “tissue” over testicles moved to the contralateral leg when treating sarcoma in an inguinal region.
Proton beam therapy does have some limitations. There can be some uncertainty in dosing because changes in tissue heterogeneity can affect where the particle stops, as well as the position of the Bragg peak. Dose distribution of protons can be sensitive to changes in air cavities (e.g., sinus congestion), postoperative changes (tumor bed post-surgical edema), bowel gas, and weight changes. Traditional high-Z material hardware such as titanium cages or hip prostheses cause significant dose shadowing within the treatment field, and likely better managed with photon (X-ray)-based radiotherapy. However, carbon fiber hardware is becoming increasingly available as a solution since it has the same Z properties as the human body and can allow for confident delivery of the desired proton therapy dose along with improved post-treatment surveillance imaging with less metallic susceptibility artifact. Other newer proton capabilities are evolving rapidly.
Neutron RT, first used for cancer therapy in the 1930s, offers the advantage of high linear energy transfer, a property that allows the delivery of 20–100 times more energy along their path than photons, thus being biologically more effective in treating radioresistant tumors such as sarcomas and salivary gland tumors. Their use requires a careful balance of risks and benefits as they can lead to more severe late toxicities.
Carbon, neon, and heavier ions hold promise as they combine the radiobiological advantages of neutrons with the dose distribution and Bragg peak of protons but are only available in select centers. Like neutron therapy, carbon ion therapy has a higher biologic effective dose which can be useful for radioresistant tumors, but unlike neutron therapy it has the increased precision of proton beam therapy.
A current area of intense research is FLASH radiotherapy, which involves the delivery of photon or proton radiation at dose rates several orders of magnitude higher than currently used clinically. In preclinical studies, FLASH radiotherapy reduces the rate of toxicity while maintaining local tumor control, and this was recapitulated when a patient with cutaneous T-cell lymphoma was treated in 2019. This effect needs to be confirmed in further clinical studies but has the potential to revolutionize therapy in oncology.
Brachytherapy, meaning “short distance” in Greek, is a method of delivering radiation treatment near or within a target using radioactive sealed sources. There are three main ways of delivering this type of radiation: (1) an applicator can be used to deliver dose to the surface of a thin tumor, for example, an eye plaque used to treat ocular melanoma; (2) intracavitary – inserting the sealed source into the cavity of an organ, e.g., in the treatment of cervical cancer, a combination (tandem) apparatus is used consisting of a cylindrical rod inserted into the cervical os and through the cervical canal into the uterus, with two ovoids placed in the lateral fornices; and (3) interstitial, when the source is either implanted directly into the tumor or postoperative bed, for example, for postoperative treatment of sarcoma, or when radioactive gold seeds are implanted directly into an organ, e.g., in the treatment of early-stage prostate cancer. Brachytherapy is often used in conjunction with external beam, usually to “boost” the total dose to a smaller volume at the site of greatest risk of recurrence, for example, in the postoperative management of cervical cancer.
Following the work done by Marie Curie and others, radium needles were initially used, until the importance of radioprotection was realized. In the modern era, artificially produced radionuclides are used, most commonly those derived from cesium ( 137 Cs), iridium ( 192 Ir), iodine ( 131 I), palladium ( 103 Pd), and gold ( 198 Au). The activity of these radionuclides is still calculated relative to that of radium. To protect health professionals and the patient, the process of using high-energy sources, such as 137 Cs and 192 Ir, has evolved considerably, most notably with the use of after-loading. Empty cylinders (for intracavity) or catheters and trocars (for interstitial brachytherapy) are inserted and loaded with inert metal “dummy” sources that are visualized on radiologic imaging; this is done to verify the position and calculate the dose distribution. The patient is treated in a shielded room, either as an inpatient over several days, or in an outpatient brachytherapy suite, for shorter high-dose rate treatments. The radioactive sources are housed in a shielded safe in a remote-controlled after-loading unit, which looks very much like a small heating furnace. After a final verification of positioning, the applicator, catheters, cylinders, or trocars are connected to the after-loading unit. After all personnel have left the room, the active sources, which look like small beads, are remotely loaded through a pressurized system. The unit is programmed to control the position of the sources and the duration of the sources in any position to achieve the prescribed dose in a homogeneous fashion over the three-dimensional (3D) dose distribution. The unit can be paused, and the active sources retract temporarily into the safe within the after-loading unit, making it possible for personnel to enter the room to provide care.
Low-energy radionuclides such as 131 I, 103 Pd, and 198 Au do not pose the same concerns for radioprotection, and thus are used as permanent implants in the form of seeds, most commonly for prostate cancer.
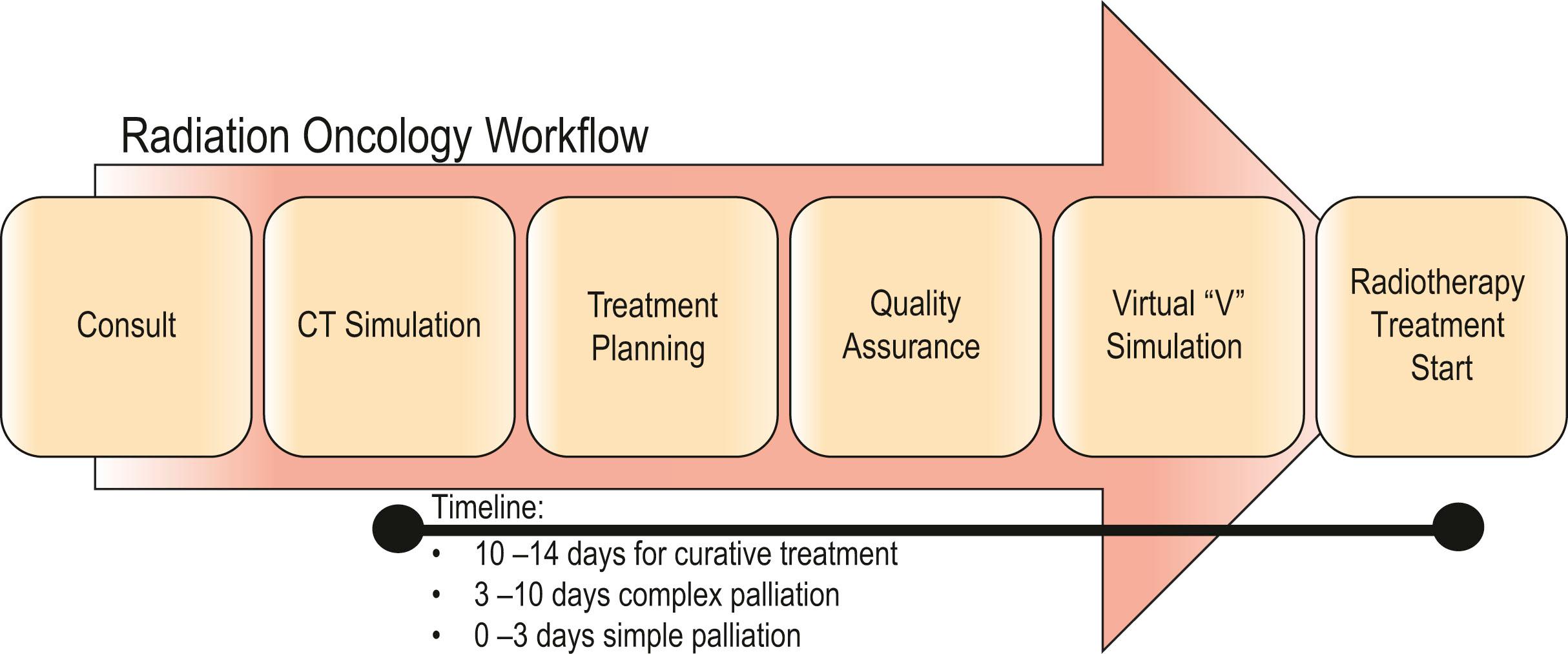
The preparation for radiation treatment, or planning, starts with a decision on the treatment intention – curative, palliative, or optimization of local control (complex palliation) – and the probable dose prescription ( Fig. 27.15 ). Following informed consent, the patient is simulated for the treatment. ![]() highlights the simulation process and immobilization techniques to understand this entire process from start to finish. Simulation is the radiation oncologist’s equivalent to the operative day, allowing dedicated time to accurately map out the areas for treatment. Two-dimensional planning with fluoroscopy has now been mostly replaced by a three-dimensional approach, using diagnostic quality CT scan with contrast (intravenous and oral as necessary). The scanners are equipped with a flat, as opposed to concave, couch, and the bore is often extra-wide, to accommodate some of the positions, such as arms above the head, or legs separated in a frog-leg position, to accommodate the proposed treatment position. To achieve a reproducible position, immobilization devices are made. The type of device depends very much on the treatment site. To treat the head and neck region, a mesh acrylic mask, similar to the type of mask worn by burn patients requiring compression treatment, is formed exactly to the patient’s face and head with eye, nares, and mouth openings ( Fig. 27.16 ). This not only allows reproducible positioning but also obviates the need for tattoos or other skin markings in visible areas. Extremities are immobilized using casts of the same material and body immobilization is achieved with the use of vacuum splints and bags. All of these devices are referenced to the couch position. Once the position is confirmed, immobilized, and referenced, the patient then has a CT scan in this position and goes home.
highlights the simulation process and immobilization techniques to understand this entire process from start to finish. Simulation is the radiation oncologist’s equivalent to the operative day, allowing dedicated time to accurately map out the areas for treatment. Two-dimensional planning with fluoroscopy has now been mostly replaced by a three-dimensional approach, using diagnostic quality CT scan with contrast (intravenous and oral as necessary). The scanners are equipped with a flat, as opposed to concave, couch, and the bore is often extra-wide, to accommodate some of the positions, such as arms above the head, or legs separated in a frog-leg position, to accommodate the proposed treatment position. To achieve a reproducible position, immobilization devices are made. The type of device depends very much on the treatment site. To treat the head and neck region, a mesh acrylic mask, similar to the type of mask worn by burn patients requiring compression treatment, is formed exactly to the patient’s face and head with eye, nares, and mouth openings ( Fig. 27.16 ). This not only allows reproducible positioning but also obviates the need for tattoos or other skin markings in visible areas. Extremities are immobilized using casts of the same material and body immobilization is achieved with the use of vacuum splints and bags. All of these devices are referenced to the couch position. Once the position is confirmed, immobilized, and referenced, the patient then has a CT scan in this position and goes home.
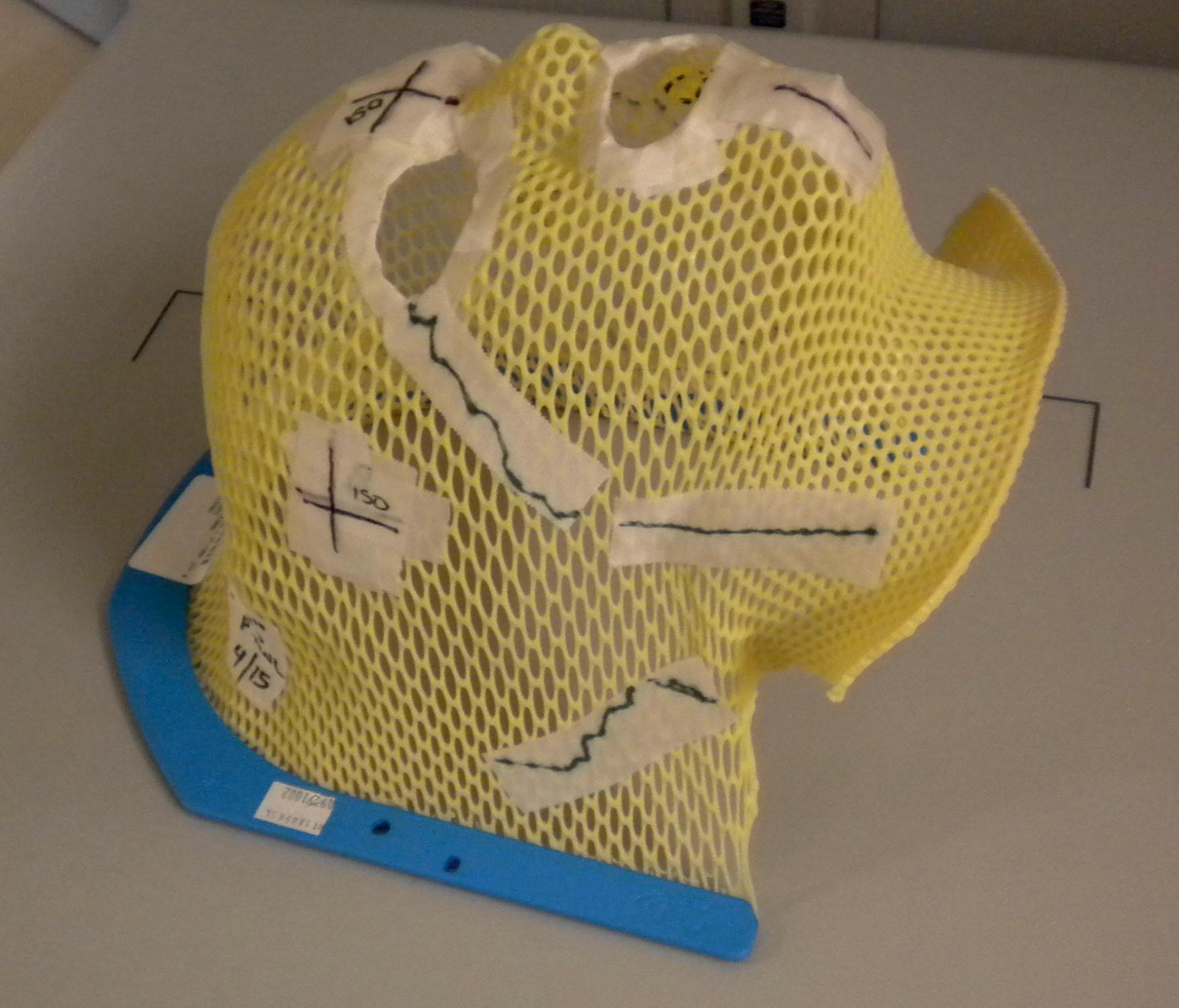
Three localizing tattoos (usually anterior, posterior and laterally) are placed on the skin to identify the isocenter (center of the treatment field where the beams converge) or CT origin (surrogate for isocenter until treatment-planning occurs, then daily shifts are applied from this location to isocenter) to facilitate daily external alignment prior to verifying internal alignment with imaging. Many centers now employ surface-guided image alignment where a 3D rendering on the skin is applied to align to a skin rendering generated at the time of CT simulation, which has enabled some centers to move toward tattooless treatment approaches, such as for breast cancer.
These images are imported into a radiation treatment-planning system. Using the original diagnostic scans, and often fusing them with the planning CT, the radiation oncologist starts delineating all the structures of interest on every CT slice as it is displayed on the workstation. Any visible tumor is delineated as one or several gross tumor volumes (GTV). These are then encompassed by one or more clinical target volumes (CTV), which include normal-looking tissue considered by the radiation oncologist to be at high risk of containing tumor cells. Defining this volume, and deciding on its dose, require judgment of risks and benefits, and are central to the discipline of radiation oncology. It is possible to have several CTVs that require different doses, depending on the likelihood of tumor burden. The CTV is then expanded to a form a planning target volume (PTV) that accounts for measured movement of the tumor or organs, for example, to account for a lung mass moving with respiration, as well as other calculated variations.
The next step is to identify and contour all organs-at-risk (OAR), that is, those normal tissues that are sensitive to radiation – examples include kidneys, spinal cord, and femoral head. Each of these sensitive structures is identified with a dose constraint. The result is a 3D digital representation of the patient, tumor, and organs at risk. Working with dosimetrists, a treatment technique is decided by placing multiple beams through a series of protocols to determine the optimal beam arrangement to cover the target volumes homogeneously to the prescribed dose while sparing critical structures. Dose-volume histograms calculate constraints for the OAR, for example, in the thorax, the V 20 refers to the percentage of cancer-free lung (i.e., without the CTV) lung that receives >20 Gy; if this is above 35% it is strongly predictive for radiation pneumonitis. This aspect of radiation treatment-planning, rather than just positioning the patient on the CT bed, is akin to the surgical procedure itself. ![]() describes the process of plan review, including comparing different treatment techniques.
describes the process of plan review, including comparing different treatment techniques.
Once the radiation treatment plan has been designed, it is checked by a physicist for quality assurance to ensure that the plan is technically feasible on the treatment unit, and that no further beam modification is necessary. The patient returns for a dry run of the plan, and verification of set up. During this session the patient is repositioned in the immobilization device on the treatment machine, and, using light beams, the geometric parameters are checked. Verification includes comparison of the portal imaging to the digitally reconstructed radiographs (DRR) from simulation to ensure that the isocenter of the fields, the field shapes, and the shielding are consistent with the plan. If there are any problems, adjustments are made before the patient returns the next day for the start of actual treatment. Further verification is done using a cone beam CT, and adjustments, or shifts, are made as necessary. This verification is usually repeated on a daily basis. This process is image-guided radiation therapy (IGRT) and verifies that highly conformal plans are delivered with precision.
An explanation of RT techniques and dosimetry requires a basic understanding of physics. The photons generated by the linac are very high-energy X-rays that do not have any charged particles in it. When a photon beam enters the body, it uses the Compton process to deposit energy. This means that it interacts with tissue by colliding with an atom’s loosely bound outer shell electron ( Fig. 27.5 ). The photon continues along its path, but the electron is knocked off, like a billiard ball, at an angle that depends on the speed and angle with which it was hit. The electron travels (scatters) a distance that is proportional to the energy with which it was hit, meaning that higher photon energies propel electrons more in a forward direction than lower energies can do, before the electron is deposited, and the dose absorbed into the tissues. This triggers downstream free radical interactions, and these can cause DNA single-strand or double-strand breaks, or other injuries, including damage to base pairs and protein cross-links. The chromosomal aberrations that result from double-strand breaks mean that the cancerous cell cannot repair or duplicate at the end of its life cycle, leading to the principal cause of cell death.
If all these electron energy deposits are added up, then a distribution curve showing absorbed dose and depth can be plotted ( Fig. 27.6A ) that shows that skin absorbed dose is less than 40%, and 100% or D max occurs some distance into the skin at a depth that is consistent with the energy of the photon beam. The most common energy used is 6 MV which has a D max of 1.5 cm beyond skin. The dose then falls off at a steady rate that is just less than 5%/cm, meaning that 50% of the dose has been deposited approximately 11 cm into the body. If a tumor is situated centrally, this means that there is too much of a gradient of dose across it. To overcome this heterogeneity within the target volume, a second beam can enter the body (assuming a diameter of 20 cm) from the opposite side, again depositing its maximal energy at 1.5 cm, then attenuating at <5%/cm. The energy deposits from each side are added, a homogeneous distribution is achieved across the target volume ( Fig. 27.6B ), and the skin does not receive a therapeutic dose, unless bolus is used ( Fig. 27.6C ). The addition of right and left lateral beams (four-field box technique) would give an even tighter homogeneous distribution to the target volume, and, since the entrance and exit doses are shared between four beams, further skin dose reduction. This is the basis for using multiple fields, or beams from different angles, to “focus” (a misleading term, since the beam does not focus on a spot, as light does through a lens) and deposit maximal dose to a target.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here